Abstract
Spermatozoa have a highly complex RNA profile. Several of these transcripts are suggested as biomarkers for male infertility and contribute to early development. To analyze the differences between sperm RNA quantity and expression of protamine (PRM1 and PRM2) and testis-specific histone 2B (TH2B) genes, spermatozoa from 33 patients who enrolled in assisted reproduction treatment (ART) program were analyzed. Sperm RNA of teratozoospermic (T), oligoteratozoospermic (OT), and normozoospermic (N) samples was extracted, and the differences in transcript levels among the study groups were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). The correlations of total RNA per spermatozoon and the expression of the transcripts were evaluated in relation to sperm characteristics and preimplantation embryo development. The mean (±standard deviation) RNA amount per spermatozoon was 28.48 (±23.03) femtogram in the overall group and was significantly higher in the OT group than that in N and T groups. Total sperm RNA and gene expression of PRM1 and PRM2 genes were related to preimplantation embryo development and developmental arrest. Specific sperm characteristics were correlated with the expressions of PRM1, PRM2, or TH2B genes. We conclude that the sperm RNA amount and composition are important factors and might influence early embryonic development and also differ in different cases of male infertility.
Keywords: embryonic development, PRM1, PRM2, RNA, spermatozoa, TH2B
INTRODUCTION
The mammalian spermatozoon is a highly differentiated unique cell with its condensed chromatin, a small amount of cytoplasm, and the ability to move. The male gametes contain essential transcripts required for the maturation steps in the testicular tissue where they share their cytoplasmic contents via cytoplasmic bridges.1 As a result of chromatin condensation, spermatids become transcriptionally inactive during spermiogenesis.2 However, ejaculated spermatozoa still contain a highly complex RNA profile that is helpful as a diagnostic tool for male infertility and that contributes to early embryonic development.3,4,5,6,7,8,9
Protamines are the most abundant nuclear proteins in human spermatozoa, and during spermiogenesis, they maintain the condensation of the paternal genome to a high degree.10 Packaging of sperm DNA with protamines protects the paternal DNA from endogenous or exogenous agents such as nucleases or potential mutagens and allows the cells to progress through the genital tract.10,11 During formation of the nucleosome–protamine complex, histones are firstly replaced by transition proteins and then by protamines.10 However, in humans, approximately 15% of sperm DNA remains complexed with histones in sequence-specific regions and is not packaged with protamines.12,13 These persistent histone-bound regions of the sperm DNA have been proposed to function in the delivery of the imprinting messages to the embryo and suggested to take part in fertilization and early embryo development.14,15,16 Abnormal persistence of histones complexed with sperm DNA,17 and an altered protamine 1 to protamine 2 (PRM1:PRM2) ratio at both mRNA and protein levels have been demonstrated in spermatozoa of infertile men.11,18,19 Insufficient DNA compaction and altered expression of protamine are also associated with poor embryo development. 14,20,21,22 Thus, the RNA content of the spermatozoon and the proper protamination of its DNA are crucial for the maintenance of the reproductive potential of men.
In previous studies, a differentiated sperm RNA profile was demonstrated in infertility and associated with different sperm characteristics.4,16,23,24 This study aimed to analyze the differences in RNA quantity and the expression of protamine (PRM1 and PRM2) and testis-specific histone 2B (TH2B) genes in spermatozoa of infertile patients attending an assisted reproduction treatment (ART) program. The possible influence of these transcript-level alterations on sperm characteristics and preimplantation embryo development was evaluated in the study group.
PARTICIPANTS AND METHODS
Study population and patient selection
This noninterventional, prospective study enrolled 42 male partners of the couples who were treated for infertility at the IVF Unit of Istanbul Faculty of Medicine, Istanbul University (Istanbul, Türkiye) between September 2011 and September 2014. After sperm RNA isolation, eight males were excluded from the study, since the total RNA yields were insufficient for analysis, and one sample with positive gene expression for a leukocyte cell marker (protein tyrosine phosphatase, receptor type c [CD45]) was excluded. Finally, 33 male partners were included. The indications for infertility were either unexplained or male factor. The study subjects were classified into three groups with normozoospermia (N; n=11), teratozoospermia (T; n = 15), and oligoteratozoospermia (OT; n = 7). Couples with (1) a history of accompanying female indication, (2) semen samples with >1×106 round cells per ml, or (3) the usage of any medications that might affect spermatogenesis were excluded from the study. Smoking status and frequency were recorded. The study was approved by the Ethics Committee of the Istanbul Faculty of Medicine (Istanbul, Türkiye; Approval No. 1360) and informed consent was obtained from all participants.
Controlled ovarian hyperstimulation and ART
The treatment of the couples was with standard gonadotropin-releasing hormone (GnRH) antagonist ovulation induction (with cetrorelix acetate; Merck Serono, Darmstadt, Germany), oocyte retrieval, embryo culture, and embryo transfer protocols. Autologous oocytes were used in all ART cycles. Fertilization was by intracytoplasmic sperm injection (ICSI) and the embryos obtained were cultured in a sequential medium system (Vitrolife, Gothenburg, Sweden). Pronucleus (PN) scoring and embryo grading were performed on the 1st, 2nd, and 3rd days of the development after fertilization (D1, D2, and D3) by two European Society of Reproductive Medicine and Embryology (ESHRE)-certified clinical embryologists. In the laboratory grading protocol, which was modified from Alpha Scientists in Reproductive Medicine (ALPHA) and ESHRE Guidelines,25 Grade I (GI) and GIII embryos represented the best-quality and the worst-quality embryos, respectively. According to the legislation held by the Republic of Türkiye Ministry of Health, elective-single embryo transfer was performed, except for the two embryo transfers in five patients who were above 35 years old (one female in the N group, three in the T group, and one in OT group). Embryos were transferred on D3 under ultrasound visualization, and implantation was confirmed when a positive β-human chorionic gonadotropin (β-hCG) level (>10 IU ml−1) was detected on day 12 after transfer. Implantation and pregnancy outcomes were recorded. No birth defects were reported in the self-report questionnaire of the patients at the routine follow-up.
Preparation of semen samples
Semen samples were obtained by masturbation after 3–5 days of abstinence. Following liquefaction, semen volume, total sperm count, sperm concentration, sperm motility, and sperm normal morphology were recorded according to the World Health Organization 5th guideline.26
Sperm preparation was performed as a part of the ART before ICSI, and samples were prepared by a two-layer discontinuous density gradient (90%/45%, v/v) method. Briefly, 1 ml 90% Percoll solution (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) was added to the bottom of the conical centrifuge tube, and 1 ml 45% Percoll solution was carefully layered on the upper surface. Then, 1 ml semen was layered on the Percoll solutions and centrifuged at 400g for 15 min (Labofuge 400R; Heraeus, Hanau, Germany). The sperm pellet obtained was resuspended with 5 ml sperm washing medium and centrifuged at 400g for 5 min. The resulting sperm pellet was resuspended in culture medium. After this initial sperm preparation as a part of ART, half of the sperm suspension was processed again by the same method, in order to eliminate epithelial cells, lymphocytes, and residual particles as previously described.3,27 Next, the samples were washed by centrifugation in 5 ml phosphate-buffered saline (PBS) at 600g for 10 min at 4°C and then suspended in PBS. Contamination of somatic cells was evaluated microscopically (BX53; Olympus, Tokyo, Japan). Epithelial lysis solution was not applied for further purification of the sperm samples because of its reported alteration of transcription profiles.28 Sperm counts of each sample were recorded for the calculation of RNA quantity per spermatozoon. Sperm pellets were snap-frozen in liquid nitrogen and stored at −80°C until RNA isolation.
RNA isolation and complementary DNA (cDNA) synthesis
Total RNA was isolated with a commercial kit according to the manufacturer’s recommendations (RNeasy Mini Kit; Qiagen, Hilden, Germany) with minor modifications as previously described.29 The frozen sperm pellet was thawed on ice and resuspended in denaturation buffer supplemented with β-mercaptoethanol (10 μl ml−1). Samples were mechanically homogenized by passing through a 22G injection needle and then incubated at 65°C for 30 min. Following these additional steps, isolation was performed according to the commercial kit manual. The quantity and quality of the sperm total RNA samples were assessed using an RNA 6000 Nano Kit and Bioanalyzer 2100 Instrument (Agilent Technologies, Santa Clara, CA, USA). RNA integrity number (RIN) obtained from the Bioanalyzer was recorded for all samples. cDNA synthesis was performed as described by the commercial kit (QuantiTect Reverse Transcription Kit, Qiagen), and 100 ng total RNA was used for the reverse transcription reaction of each sample.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Expression of PRM1 (NM_002761.3), PRM2 (NM_001286356.2), and TH2B (HIST1H2BA, NM_170610.3) genes was analyzed by qRT-PCR performed in the LightCycler 480 Instrument (Roche, Mannheim, Germany) and the 480 SYBR Green I Master Kit (Roche) according to the manufacturer’s recommendations. From our previous work and other studies, beta actin (ACTB, NM_001101.5), which has a stable gene expression in spermatozoa, was selected as endogenous control gene.27,30,31 The specificity of all primer pairs was confirmed by the Sanger sequence analyses of the amplicons.
Gene-specific qRT-PCR primers were designed manually with the OligoAnalyzer 3.1 (IDT Technologies, Coralville, IA, USA) algorithm. The sequence homology of the primers was confirmed from the BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi, last accessed on January 04, 2023) database. Primer sequences are presented in Table 1.
Table 1.
Gene-specific primers
| Gene | Primer sequence | Size of PCR product (bp) | Tm (°C) |
|---|---|---|---|
| PRM1 | 151 | ||
| Forward | 5’- GGA GCA GAT ATT ACC GCC A-3’ | 54.3 | |
| Reverse | 5’- GAG TTT GGT GGA TGT GCT A-3’ | 54.3 | |
| PRM2 | 214 | ||
| Forward | 5’- CAA GAG GAG CAA GGG CTG A-3’ | 57.2 | |
| Reverse | 5’- GCC TTC TGC ATG TTC TCT TCC TG-3’ | 58.1 | |
| TH2B | 106 | ||
| Forward | 5’- GAG AGC TGG CTA AAC ATG CTG TG-3’ | 58.2 | |
| Reverse | 5’- AGA GCC TTT GGG TTA GAA ATG ACG-3’ | 57.2 | |
| ACTB | 204 | ||
| Forward | 5’- CTT CCT GGG CAT GGA GTC-3’ | 55.4 | |
| Reverse | 5’- GGA GGA GCA ATG ATC TTG-3’ | 54.1 |
PCR: polymerase chain reaction; PRM: protamine; TH2B: histone cluster 1 H2B family member a; ACTB: beta actin; bp: base pair; Tm: melting temperature of the primers
Relative quantification (RQ) of gene expression was calculated by the comparative Ct (threshold cycle) method and the fold-change (Fc) results were expressed as RQ values.32 The specificity of the PCR amplicons was confirmed by melting curve analysis. The Ct value of the ACTB gene was used to normalize the expression data. Delta Ct values of a pool of cDNA sample (standard cDNA) prepared from seven individuals within the study reflecting the study population (three normozoospermic, three teratozoospermic, and one oligoteratozoospermic) were used to calibrate the data. All samples were studied in triplicate. The ratios of PRM1 to PRM2 (PRM1:PRM2) and TH2B to total protamine (TH2B:[PRM1 + PRM2]) were calculated as Fc values.
In the qRT-PCR experiments, technical and biological positive/negative controls were used. RNA isolated from three testicular biopsy specimens obtained from severe male infertility patients was used as positive controls. The presence of spermatozoa in the biopsy specimens was confirmed in an inverted microscope (IX73; Olympus) and the expression of the protamine genes was tested in qRT-PCR experiments as that in our previous work.27 For negative controls, RNA isolated from three testicular biopsy specimens was used and the absence of spermatozoa was confirmed in an inverted microscope. In these three samples, the absence of protamine gene expression and the presence of housekeeping gene expression (ACTB) were confirmed.27 Accordingly, no template controls were run on each plate.
Confirmation of somatic cell contamination
In addition to microscopic examination, the purity of the sperm samples was confirmed by the absence of an amplification product of somatic cell markers by qRT-PCR (Supplementary Figure 1 (47KB, tif) ). The studied genes were germ cell marker, c-kit proto-oncogene (C-KIT; forward primer: 5’-CCA GAA ATC CTG ACT TAC GA-3’; reverse primer: 5’-TCC ACT GTA CTT CAT ACA TG-3’), epithelial cell marker, E-cadherin (CHD1; forward primer: 5’-GGT TCA GAT CAA ATC CAA CA-3’; reverse primer: 5’-GGG AAA CTC TCT CGG TCC A-3’), and leukocyte cell marker (CD45; 5’-TCA GTG TGG TAA TAT GAT A-3’; reverse primer: 5’-ACC TGG CTT GGA GGA GCA-3’).
Statistical analyses
The power analysis of the study was performed with G*Power version 3.1.9.733 (Heinrich Heine University Düsseldorf, Germany). RNA quantity per spermatozoon among the study groups was analyzed by independent-samples Kruskal–Wallis test. Significance values were adjusted by the Bonferroni correction. The mean Fc values of gene expression levels between the study groups were evaluated with the nonparametric Mann–Whitney U test (two-tailed). One-way analyses of variance (ANOVA) were used to analyze the association of Fc values with the related covariates such as semen volume, sperm motility, percentage of normal morphology, fertilization rate, number of good-quality embryos, and number of arrested embryos. Categorical variations such as smoking status, implantation, and pregnancy rates were analyzed with the Chi-squared test. Post hoc Tukey’s analyses were performed for the comparison of the study groups for the analyzed variables. Linear correlations were tested with the Spearman’s correlation test. P < 0.05 was considered statistically significant. The values were presented as mean ± standard deviation (s.d.). All statistical analyses were performed using SPSS version 21.0 software (IBM Corp., Armonk, NY, USA).
RESULTS
Demographic characteristics of the study group
RNA/spermatozoon and expressions of PRM1, PRM2, and TH2B genes in N, T, and OT samples from 33 individuals undergoing ART were analyzed. The power of the study group was calculated as 84.0%. In the overall study population, the age of the female partner ranged from 24 years to 38 years (mean ± s.d.: 30.8 ± 4.6 years) and the male partner from 27 years to 48 years (mean ± s.d.: 34.1 ± 4.7 years). There were no statistically significant differences between the groups in male and female ages, female basal hormone levels, fertilization rate, the number of arrested embryos on D2 or D3, and ART outcomes (all P > 0.05; Table 2). The sperm characteristics of the study groups are presented in Table 3. Although no differences were observed between the three groups in head, neck, or tail morphology (all P > 0.05; Table 3), there were significant differences in strict morphology according to Kruger’s criteria in the N, T, and OT groups (P < 0.0001). Similarly, sperm concentration and total sperm count were significantly different between the groups (P = 0.03 and P = 0.002, respectively), reflecting the characteristics of the sperm parameters of the N, T, and OT samples.
Table 2.
Characteristics of the male and female patients attending the assisted reproduction treatment program and outcome of the treatment
| Characteristic | Normozoospermia (n=11) | Teratozoospermia (n=15) | OT (n=7) | P |
|---|---|---|---|---|
| Male age (year), mean±s.d. | 34.4±5.8 | 35.0±4.0 | 31.9±4.3 | NS |
| Female age (year), mean±s.d. | 31.3±4.6 | 31.0±4.8 | 29.9±4.7 | NS |
| Female FSH (mIU ml−1), mean±s.d. | 7.0±1.6 | 6.6±2.3 | 7.2±2.2 | NS |
| Female LH (IU ml−1), mean±s.d. | 6.0±3.8 | 6.4±3.2 | 5.2±1.9 | NS |
| Female E2, (pg ml−1), mean±s.d. | 42.4±17.4 | 49.5±21.4 | 44.1±27.6 | NS |
| Female PRL (ng ml−1), mean±s.d. | 13.7±5.9 | 20.3±9.8 | 20.6±8.9 | NS |
| MII (n), mean±s.d. | 9.0±6.5 | 8.6±5.1 | 9.1±5.8 | NS |
| GnRH antagonista (mg), mean±s.d. | 2146.3±1034.3 | 2365.2±800.0 | 1955.4±534.9 | NS |
| Fertilization (%), mean±s.d. | 75.6±32.4 | 61.5±22.0 | 64.5±26.3 | NS |
| D2 high-quality embryos (n), mean±s.d. | 4.3±3.7 | 4.5±4.0 | 4.7±2.6 | NS |
| D2 low-quality embryos (n), mean±s.d. | 0.4±0.7 | 0.5±0.9 | 0.9±1.6 | NS |
| D2 arrest embryos (n), mean±s.d. | 0.1±0.3 | 0.4±0.5 | 0.1±0.4 | NS |
| D3 high-quality embryos (n), mean±s.d. | 2.2±3.0 | 2.93±4.0 | 3.14±2.6 | NS |
| D3 low-quality embryos (n), mean±s.d. | 0.8±1.4 | 0.2±0.6 | 1.1±2.0 | NS |
| D3 arrest embryos (n), mean±s.d. | 0.2±0.4 | 0.2±0.4 | 0.6±0.9 | NS |
| Implantation (yes, %) | 22.2 | 33.3 | 14.3 | NS |
| Pregnancy (yes, %) | 22.2 | 13.3 | 14.3 | NS |
| Smoking status (no, %) | 44.4 | 69.2 | 33.3 | NS |
| RNA/sperm quantity (fg), mean±s.d. | 20.1±17.5 | 26.2±20.2 | 53.4±27.8 | 0.02 |
| PRM1 (Fc), mean±s.d. | 0.3±0.7 | 2.2±5.0 | 0.5±1.3 | NS |
| PRM2 (Fc), mean±s.d. | 0.1±0.1 | 2.1±3.5 | 0.2±0.2 | NS |
| TH2B (Fc), mean±s.d. | 1.1±0.6 | 2.1±2.0 | 1.2±0.7 | NS |
aTotal dose of GnRH antagonist used for ovulation induction in ART treatment. Gene expression levels are given as Fc values. ANOVA test was used for the comparison of the mean values of continuous variables and the Chi-squared test was used for categorical variables. ART: assisted reproduction treatment; s.d.: standard deviation; FSH: follicle-stimulating hormone; LH: luteinizing hormone; E2: estradiol; PRL: prolactine; MII: metaphase II oocytes; GnRH: gonadotropin releasing hormone; D2: day 2 of embryo development; D3: day 3 of embryo development; fg: femtogram; ANOVA: analyses of variance; NS: not significant; Fc: fold-change
Table 3.
The semen characteristics of the study groups
| Characteristics | Normozoospermia (n=11) | Teratozoospermia (n=15) | OT (n=7) | P |
|---|---|---|---|---|
| Semen volume (ml), mean±s.d. | 2.3±1.0 | 2.9±1.5 | 1.3±0.9 | 0.04 |
| Sperm concentration (×106 ml−1), mean±s.d. | 85.0±80.7 | 55.1±19.5 | 16.2±15.7 | 0.03 |
| Total sperm count (×106 per ejaculate), mean±s.d. | 157.1±100.8 | 151.7±82.07 | 15.5±9.39 | 0.002 |
| Sperm motility (%), mean±s.d. | 62.1±20.3 | 52.5±12.1 | 55.3±22.0 | NS |
| Sperm nonprogressive motility (%), mean±s.d. | 5.1±5.3 | 4.4±7.3 | 3.0±2.4 | NS |
| Sperm normal morphologya (%), mean±s.d. | 4.3±0.5 | 1.2±0.7 | 0.6±0.8 | <0.001 |
| Head (%), mean±s.d. | 82.7±6.3 | 87.5±8.4 | 91.2±4.1 | NS |
| Neck (%), mean±s.d. | 44.3±11.9 | 48.2±10.9 | 53.0±8.2 | NS |
| Tail (%), mean±s.d. | 22.3±8.7 | 28.4±10.8 | 31.2±8.2 | NS |
| TZI (%), mean±s.d. | 1.5±0.2 | 1.7±0.2 | 1.7±0.1 | NS |
aMorphology was evaluated by Kruger’s strict criteria. Parameters were analyzed according to the World Health Organization criteria.26 The ANOVA test was used for the comparison of means. TZI: teratozoospermia index; s.d.: standard deviation; ANOVA: analyses of variance; NS: not significant; OT: oligoteratozoospermia
In the overall study group, no correlation was observed between RNA/spermatozoon and age (r = 0.02, P = 0.93) or smoking status (r = −0.15, P = 0.47) of the male. Higher expression levels of PRM1 were correlated with increasing male age (r = 0.38, P = 0.03). On the other hand, no influence of the smoking status of the male partner on the expression levels of PRM1 or PRM2 levels or the PRM1:PRM2 ratio was observed. Although RNA/spermatozoon (r = −0.15, P = 0.47) and gene expressions (PRM1: r = −0.17, P = 0.38; PRM2: r = −0.06, P = 0.79; and TH2B: r = −0.33, P = 0.08; Table 4) were negatively correlated with the smoking status of the male partner, these relations were not statistically significant. However, smoking status itself was negatively correlated with teratozoospermia index (TZI; r = −0.45, P = 0.04) and implantation rates (r = −0.40, P = 0.04).
Table 4.
The correlation analysis of the overall study group
| Variable | RNA/spermatozoon, r (P) | PRM1:PRM2, r (P) | PRM1, r (P) | PRM2, r (P) | TH2B, r (P) |
|---|---|---|---|---|---|
| Overall study group | 0.42 (0.02)* | 0.17 (NS) | −0.04 (NS) | −0.05 (NS) | 0.15 (NS) |
| Male age | 0.02 (NS) | 0.05 (NS) | 0.38 (0.03) | 0.30 (NS) | 0.08 (NS) |
| Male BMI | −0.24 (NS) | 0.06 (NS) | 0.43 (NS) | 0.36 (NS) | 0.25 (NS) |
| Smoking status | −0.15 (NS) | −0.15 (NS) | −0.17 (NS) | −0.06 (NS) | −0.33 (NS) |
| Semen volume | −0.55 (0.002)** | −0.34 (0.060) | −0.06 (NS) | 0.17 (NS) | 0.07 (NS) |
| Total sperm count | −0.65 (<0.0001)** | −0.21 (NS) | 0.17 (NS) | 0.29 (NS) | 0.05 (NS) |
| Sperm concentration | −0.05 (0.004)** | −0.11 (NS) | 0.27 (NS) | 0.32 (NS) | 0.06 (NS) |
| Sperm motility | −0.20 (NS) | −0.16 (NS) | −0.11 (NS) | 0.09 (NS) | −0.33 (0.07) |
| Sperm nonprogressive motility | −0.18 (NS) | 0.13 (NS) | −0.29 (NS) | −0.41 (0.04)* | 0.30 (NS) |
| Sperm morphology | −0.21 (NS) | −0.14 (NS) | 0.09 (NS) | 0.05 (NS) | −0.31 (NS) |
| Fertilization | 0.08 (NS) | −0.04 (NS) | −0.16 (NS) | −0.16 (NS) | 0.10 (NS) |
| D2 good-quality embryos | 0.25 (NS) | −0.05 (NS) | 0.03 (NS) | 0.07 (NS) | 0.21 (NS) |
| D2 low-quality embryos | −0.16 (NS) | −0.19 (NS) | 0.02 (NS) | 0.26 (NS) | −0.05 (NS) |
| D3 good-quality embryos | 0.27 (NS) | 0.29 (NS) | 0.16 (NS) | 0.06 (NS) | 0.09 (NS) |
| D3 low-quality embryos | 0.05 (NS) | 0.02 (NS) | 0.01 (NS) | 0.06 (NS) | −0.02 (NS) |
| D2 arrest | −0.07 (NS) | 0.17 (NS) | −0.35 (0.06) | −0.46 (0.02)* | −0.12 (NS) |
| D3 arrest | −0.57 (0.02)* | 0.26 (NS) | −0.28 (NS) | −0.19 (NS) | −0.09 (NS) |
| Implantation | 0.01 (NS) | −0.06 (NS) | 0.25 (NS) | 0.31 (NS) | 0.21 (NS) |
| Abortus | 0.25 (NS) | 0.13 (NS) | 0.50 (NS) | 0.38 (NS) | 0.38 (NS) |
| Birth | 0.22 (NS) | 0.02 (NS) | 0.01 (NS) | −0.09 (NS) | 0.05 (NS) |
*P<0.05, **P<0.01, correlation is significant (two-tailed). Correlations were analyzed by the Spearman nonparametric correlation test. Correlations between RNA quantity per spermatozoon and gene expressions and male age, smoking status and sperm parameters and embryonic development are presented. r: Spearman correlation value; OT: oligoteratozoospermia; PRM1: protamine 1; PRM2: protamine 2; TH2B: testis-specific histone 2B; BMI: body mass index; D2: day 2 of embryo development; D3: day 3 of embryo development; NS: not significant
RNA amount and expression of PRM1, PRM2, and TH2B genes in the study groups
In our study, the mean total RNA yield was 55.7 (s.d.: 53.8, range: 9.0–282.3) ng ml−1. The mean RNA/spermatozoon value, derived from the total RNA yield and sperm count, was 28.5 (s.d.: 23.0, range: 1.5–95.4) femtogram (fg). RIN values were under 3 and electrophoretograms showed the degradation of 28S ribosomal RNA (rRNA) and partially degraded of 18S rRNA. Full degradation of 28S rRNA34 and no amplification of the somatic cell markers after qRT-PCR were considered as the independent confirmations of the purity of RNA in addition to the microscopic evaluation of samples for somatic cells.
In the pairwise comparisons (Kruskal–Wallis test), the mean RNA/spermatozoon (mean ± s.d.) in the OT group was significantly higher than that in the N group (OT: 53.4 ± 27.8 vs N: 20.1 ± 17.5; P = 0.02) and but not the T group (OT: 53.4 ± 27.8 vs T: 26.2 ± 20.2; P = 0.05). On the other hand, the RNA/spermatozoon level was not comparable between N and T groups (P = 0.35; Figure 1). The RNA/spermatozoon range in the N group was more tightly distributed than the other two groups (Figure 1).
Figure 1.
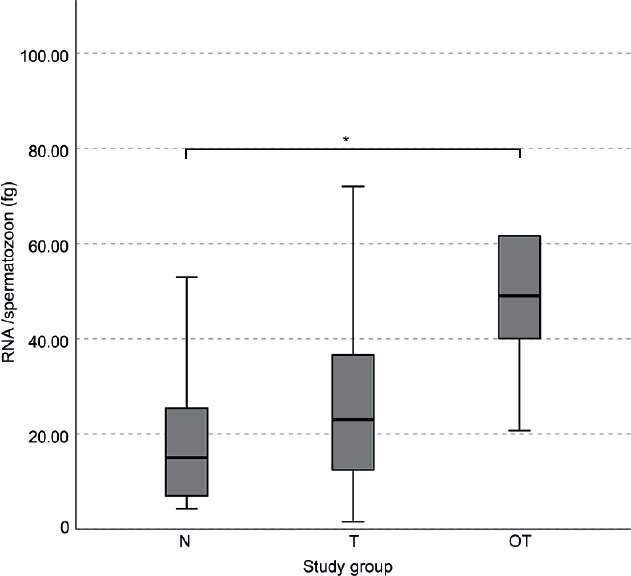
The RNA per spermatozoon values in the normozoospermia (N), teratozoospermia (T), and oligoteratozoospermia (OT) groups. The graph shows the median value (thick lines) and the range with upper and lower extremes (whiskers) of the RNA quantity per spermatozoon. P values were obtained by the independent-samples Kruskal–Wallis test. *P < 0.05. fg: femtogram.
The expression of PRM1 was strongly correlated with PRM2 in the overall study group (r = 0.89, P < 0.0001) as well as in N group (r = 0.81, P = 0.005), T group (r = 0.93, P < 0.0001), but not OT group (r = 0.08, P = 0.05) groups. In the pairwise comparisons, PRM2 expression (mean ± s.d.) in the T group was higher than that in the OT group (T: 2.1±3.5 vs OT: 0.2 ± 0.2; P = 0.03), but not the N group (T: 2.1±3.5 vs N: 0.1 ± 0.1; P = 0.07). PRM1:PRM2 ratio (mean ± s.d.) was significantly different between T and OT groups (T: 1.0±2.0 vs OT: 3.1±3.2; P = 0.04). The gene expression levels, especially PRM1 in the T group, were more widely distributed than that in the other two groups (Figure 2). On the other hand, PRM1 and PRM2 gene expressions or PRM1:PRM2 were not correlated with TH2B expression.
Figure 2.

Expression levels of PRM1, PRM2, and TH2B genes and PRM1:PRM2 ratio in the normozoospermia (N), teratozoospermia (T), and oligoteratozoospermia (OT) groups. Gene expression levels are given as fold change (mean ± s.d.). P values were obtained by the pairwise comparisons of the groups by the Mann–Whitney U test. *P < 0.05. PRM: protamine; TH2B: histone cluster 1 H2B family member a; s.d.: standard deviation.
Relationship to sperm characteristics
Although the RNA/spermatozoon level was significantly different among the study groups, it was correlated neither with sperm motility (r = −0.20, P = 0.29) nor normal morphology (r = −0.21, P = 0.30; Table 4). When the study groups were analyzed individually, there were no correlations between RNA/spermatozoon and sperm parameters either (N: motility [r = −0.51, P = 0.11] and morphology [r = −0.28, P = 0.54]; T: motility [r = 0.12, P = 0.66] and morphology [r = 0.38, P = 0.20]; and OT: motility [r = −0.10, P = 0.87] and morphology [r = 0.29, P = 0.64]).
PRM1 expression was significantly correlated with normal morphology (r = 0.84, P = 0.02) and negatively correlated with the abnormal morphologies of the sperm head (r = −0.90, P = 0.04) only in OT group. PRM2 expression was inversely correlated with the sperm nonprogressive motility in the overall study group (r = −0.41, P = 0.04) and the N group (r = −0.76, P = 0.01), however, not in T or OT groups. On the other hand, a higher PRM1 level was correlated with decreased sperm motility only in the N group (r = −0.55, P = 0.08), though not statistically significantly. There were no correlations between PRM1:PRM2 and sperm characteristics. In addition, male BMI had no correlation with the expression of the genes in the overall study or the individual study groups (all P > 0.05; Table 4).
Increased expression of TH2B was positively correlated with the percentage of morphological anomalies of the sperm tail (r = 0.40, P = 0.05) in the overall group. When the groups were analyzed individually, TH2B expression was not correlated with sperm tail abnormalities.
Relationship to embryo development
RNA/spermatozoon was not correlated with fertilization rate (r = 0.08, P = 0.67), PN score (r = 0.12, P = 0.55), number of good-quality embryos on D2 (r = 0.25, P = 0.19) or D3 (r = 0.27, P = 0.14), or implantation rate (r = 0.01, P = 0.96) in the overall study (Table 4). On the other hand, increased RNA/spermatozoon was negatively correlated with the number of arrested embryos on D3 in the overall group (r = −0.57, P = 0.02). After the data were stratified according to the study groups, RNA/spermatozoon was not correlated with fertilization rate (r = 0.59, P = 0.06), but with PN score (r = 0.78, P = 0.004), the number of good-quality embryos on D2 (r = 0.64, P = 0.03) and D3 (r = 0.73, P = 0.01) in the N group. Increased RNA/spermatozoon was not significantly correlated with the number of arrested embryos on D2 (r = −0.50, P = 0.07) and D3 (r = −0.63, P = 0.09) in the T group.
There were no correlations between PRM1 and embryo development in the overall study group. When the data were compared among the study groups, higher PRM1 level was correlated with the increased number of good-quality embryos on D2 (r = 0.86, P = 0.01) only in the OT group. On the other hand, PRM2 expression was negatively correlated with the number of arrested embryos on D2 in the overall study group (r = −0.46, P = 0.02) and the T group (r = −0.64, P = 0.05), as shown in Table 4. PRM2 was correlated with the number of low-quality embryos on D2 (r = 0.76, P = 0.05) and D3 (r = 0.76, P = 0.05) only in the OT group, and an increasing PRM1:PRM2 ratio was positively correlated with the increasing number of arrested embryos on D3 in the T group (r = 0.73, P = 0.03) but not in the other groups or the overall study groups.
In this study, there was no direct correlation between TH2B expression levels or TH2B:(PRM1+PRM2) ratio with embryo development up to D3 in the overall study group and the individual study groups, and no correlation between the studied gene expression levels and implantation rates was observed.
DISCUSSION
The complex RNA composition of the mature male gamete can be helpful as a biomarker for the diagnosis of male infertility and has been suggested to contribute to early embryonic development.3,4,5,6,9 In our study, we compared the RNA quantity per spermatozoon in a group of normozoospermic, teratozoospermic, and oligoteratozoospermic patients and observed that it was comparable among the study groups, especially in the normozoospermic and oligoteratozoospermic samples. It was shown that RNA quantity per spermatozoon and the gene expression of PRM1 and PRM2 was related to in vitro embryo development and that specific sperm characteristics were correlated with the expression of PRM1, PRM2, and TH2B genes.
It has been proposed that the RNA content of ejaculated spermatozoa reflects the fingerprint of their testicular development, involving transcripts retained in mature gamete.3,35 On the other hand, it has been reported in recent studies that epididymosomes (epididymal exosomes) contribute to epididymal sperm maturation by their protein and noncoding RNA cargo.41 Thus, during the epididymal maturation of the spermatozoa, their RNA load might be altered by the activity of small RNA molecules and other epigenetic mechanisms.16 From the evidence on testicular development and epididymal maturation, differences in sperm RNA quantity might be caused by these two mechanisms. In one scenario, the higher RNA load could arise from gene expression regulation defects during spermatogenesis, leading to abundant expression of certain transcripts. In another scenario, higher RNA load could result from posttranscriptional defects inhibiting protein synthesis in later maturational steps and leading to their persistence in mature spermatozoa. This hypothesis should further be tested by the comparison of transcript profiles of semen samples with different characteristics arising from testicular and epididymal maturation steps. Aoki et al.36 suggested that abnormal protein translation was associated with aberrant mRNA retention and the defects in translational regulation might contribute to protamine deficiency in infertile men. Carrell et al.37 suggested that abnormal protamine expression could be the result of defective overall mRNA storage or translation mechanisms. Owing to the nature of the male gamete, uncoupled transcription and translation act in the maturation of spermatozoa.38 The majority of genes are transcribed in diploid round spermatids and translation takes place in haploid stages later; it is proposed that the mature spermatozoon is incapable of transcribing novel RNA molecules.39 However, in certain in vitro conditions, sperm RNA can be translated by mitochondrial machinery,40 though such studies are contradictory.41 All these observations demonstrate that sperm transcripts are prone to micro-environmental changes and under specific genetic regulation.
Previous studies have reported that human mature spermatozoa contain 10–400 fg of total RNA.6,42,43 Our data are similar with a mean value of 28.5 fg RNA per spermatozoon in the overall study population, with different mean levels in the OT group (2.7- and 2.0-fold higher than that in N and T groups, respectively). With the significantly higher RNA load, it is clear that the testicular and epididymal history during sperm production and maturation have marked effects in oligoteratozoospermia, normozoospermia, and teratozoospermia. On the other hand, although we observed that RNA/spermatozoon value differed significantly between study groups, there was no correlation with sperm motility or normal morphology. This suggests that the different RNA content of spermatozoa is related to functions of the male gamete other than morphology and motility, which are mainly used to characterize the semen samples.
Although sperm RNA quantity was not correlated with sperm characteristics in this study, our findings suggest that the sperm RNA cargo may influence early embryo development. Increased sperm RNA was correlated with the higher quality of D3 embryos, especially in normozoospermia, and decreased rate of D2 and D3 embryonic arrest in teratozoospermia. On the other hand, since the embryo transfer is routinely held on D3, we have no data on further embryo development and blastocyst formation. The link between teratozoospermia and embryonic developmental arrest has previously been suggested.44 Additionally, Platts et al.4 in their elegant work demonstrated that sperm RNA profiles in normozoospermia and teratozoospermia were differently distributed, with ubiquitin pathway transcripts in both spermatocytes and spermatids being especially underrepresented in the latter condition. These findings suggest that the RNA composition of spermatozoa developed in a healthy testicular environment is more likely to support embryonic development and is important in the maintenance of competent embryos.
The evaluation of sperm protein and mRNA levels of protamines has revealed that abnormal protamine protein levels are usually accompanied by an increased protamine transcript. The decreased protamine expression results in decreased sperm and embryo quality, leading to implantation failure in ART programs.11,45,46 Although it has been reported that PRM1 and PRM2 mRNA copy numbers are higher in normozoospermic than those in teratozoospermic men,47 we observed no differences in PRM1 levels among the N, T, and OT samples. On the contrary, the PRM2 level was significantly higher in T than that in OT and incomparable between N and OT samples in our study.
Lambard et al.48 reported that spermatozoa with low motility retained higher PRM1 mRNA than those from higher motility spermatozoa from the same ejaculate. Another study, however, reported that the PRM1 mRNA in normal-motility spermatozoa was higher than in that in low-motility cells.49 This conflicting evidence implies that PRM1 expression is somehow related to sperm motility. On the other hand, Carrell and Liu50 demonstrated that decreased PRM2 protein levels were correlated with lower sperm counts, lower motility, and morphological abnormalities. Additionally, in Prm2-null mice generated by clustered regularly interspaced palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) technology, complete loss of sperm motility and abnormal sperm head morphology were observed.51 In our overall study group, PRM1 levels were specially correlated with normal sperm morphology, whereas PRM2 expression was correlated with nonprogressive motility. However, the present study was designed to evaluate the RNA/spermatozoon and gene expression differences in teratozoospermia, oligoteratozoospermia, and normozoospermia. Since the asthenozoospermic samples were not analyzed, it was not possible to evaluate the relationship with sperm motility.
In spermatozoa, the relative proportion of both mRNA and protein levels of PRM1 to PRM2 is around 1:1.52 Aberrant PRM1:PRM2 ratios in testicular tissue and ejaculated spermatozoa of infertile men are associated with aberrant chromatin condensation and increased DNA fragmentation. These ratios are suggested to be a biomarker for the abnormal spermiogenesis,46,52 as well as a prognostic factor for sperm fertilizing capacity.36 In our study, the PRM1:PRM2 ratio was higher in the T and OT groups than that in N group, although we observed no correlation between PRM1:PRM2 ratio and sperm characteristics. On the other hand, an increase in the PRM1:PRM2 ratio and a decrease in PRM2 expression were correlated with an increased number of D3 arrested embryos in the T group. Additionally, higher PRM1 expression was correlated with good-quality embryos, whereas increased PRM2 expression was correlated with the number of low-quality embryos in OT patients. These differences among the study groups could be indicative of deteriorating sperm features in teratozoospermia and oligoteratozoospermia. If the PRM1:PRM2 ratio is important in protecting genetic information, DNA becomes more susceptible to damage during transport to the oocyte. In most studies, the alteration of the PRM1:PRM2 ratio reflected differentially expressed PRM2 rather than PRM1,45,50 which might be explained by possibly less stringent regulatory and compensation mechanisms on the more recently evolved mammalian PRM2 protein than PRM1.53
Although TH2B expression was significantly correlated with tail abnormalities in our overall study group, this was not observed in the teratozoospermia and oligoteratozoospermia. Reduced PRM1:PRM2 protein levels are associated with increased histone retention, but the direct influence of TH2B on male infertility remains controversial.54 We observed no direct influence of TH2B expression or the TH2B:(PRM1+PRM2) ratio on preimplantation embryo development, though analyzing protein levels and protein modifications related to epigenetic regulation might be more valuable.17
Although we observed a correlation of embryo development with the alteration of sperm RNA quantity and the expression of protamine genes in our study group, there was none on implantation rates. The use of morphologically selected spermatozoa in ICSI rather than conventional IVF has been previously related to a similar association with ART outcome results.21,50 However, unlike our findings, normal embryological development was obtained with protamine-deficient spermatozoa in ART in these studies;21,50 in a mouse study with sperm protamine deficiency, embryo lethality was reported.55
In our study, genetic analysis was performed in processed sperm samples, so the results do not reflect transcript levels in whole samples, but a sperm fraction selected by morphology and motility. However, strict inclusion criteria were used in order not to mask the results by accompanying female infertility indications. The limitations of this study are the small sample size and having no data on the protein expression levels or the DNA fragmentation rates of the samples, which would be useful for comparing transcript and translation levels and allowing analysis of the influence of protein levels on embryo development. In this study, RNA quantity and gene expression levels were compared in the semen samples of the infertile couples who were enrolled in the ART program, so the results might differ from those of fertile couples with normozoospermic samples. Since normozoospermic patients may be diagnosed with unexplained infertility, spermatozoa which are morphologically normal according to the WHO criteria should not be taken as healthy and functionally capable cells. To understand the exact molecular mechanisms, the findings should be confirmed in fertile male individuals including data on protein levels. Since embryo transfer was performed routinely on D3, another limitation of this study is that we were unable to evaluate the further preimplantation embryo development.
We conclude that the RNA composition and quantity per spermatozoon are important factors that might influence early embryonic development and differ among male infertility patients. Sperm protamines and TH2B most probably do not function in early embryo development, but may be important for the proper transmission of genetic information to the oocyte. To understand the underlying biological mechanisms of preimplantation embryo development, the contribution of sperm transcripts to the zygote and developing embryo should be investigated further.
AUTHOR CONTRIBUTIONS
BOS, DSK, and NC performed the experiments. SBK contributed to the acquisition of patient data and the collection of the samples. BOS designed the experiments, analyzed the data, and drafted the manuscript. AK, GA, SBK, and NC contributed to the critical revision of the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
Contamination control of somatic cell markers. After the real-time PCR (qRT-PCR) method, the amplification products of the somatic cell markers were analyzed by agarose gel electrophoresis. No expression was detected in sperm samples (lanes 2, 4, and 6), whereas amplicon products were visual in the testicular tissue (lanes 1, 3, 5, and 7). The expected product sizes were confirmed for the studied genes and endogenous control gene in testicular tissue. Leukocyte cell marker (CD45), 439 bp; epithelial cell marker, E-cadherin (CHD1), 492 bp; germ cell marker, c-kit proto-oncogene (C-KIT), 436; endogenous control, beta actin (ACTB), 204 bp.
ACKNOWLEDGMENTS
The present work was supported by the Scientific Research Projects Coordination Unit of Istanbul University (No. 13930). The authors would like to thank Yesim Erkan, Gozde Koksal, Orhan Alak, and Gokce Deniz Kulekci (Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Türkiye) for their assistance in ART labwork and Selva Turkolmez (Department of Genetics, Aziz Sancar Institute of Experimental Medicine, Istanbul University) for her assistance in molecular genetics labwork during this project.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Ventelä S, Toppari J, Parvinen M. Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat:mechanisms of haploid gene product sharing. Mol Biol Cell. 2003;14:2768–80. doi: 10.1091/mbc.E02-10-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steger K. Transcriptional and translational regulation of gene expression in haploid spermatids. Anat Embryol. 1999;199:471–87. doi: 10.1007/s004290050245. [DOI] [PubMed] [Google Scholar]
- 3.Ostermeier GC, Dix DJ, Miller D, Khatri P, Krawetz SA. Spermatozoal RNA profiles of normal fertile men. Lancet. 2002;360:772–7. doi: 10.1016/S0140-6736(02)09899-9. [DOI] [PubMed] [Google Scholar]
- 4.Platts AE, Dix DJ, Chemes HE, Thompson KE, Goodrich R, et al. Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs. Hum Mol Genet. 2007;16:763–73. doi: 10.1093/hmg/ddm012. [DOI] [PubMed] [Google Scholar]
- 5.Avendaño C, Franchi A, Jones E, Oehninger S. Pregnancy-specific β-1-glycoprotein 1 and human leukocyte antigen-E mRNA in human sperm:differential expression in fertile and infertile men and evidence of a possible functional role during early development. Hum Reprod. 2009;24:270–7. doi: 10.1093/humrep/den381. [DOI] [PubMed] [Google Scholar]
- 6.Jodar M. Sperm and seminal plasma RNAs:what roles do they play beyond fertilization? Reproduction. 2019;158:R113–23. doi: 10.1530/REP-18-0639. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Shi J, Rassoulzadegan M, Tuorto F, Chen Q. Sperm RNA code programmes the metabolic health of offspring. Nat Rev Endocrinol. 2019;15:489–98. doi: 10.1038/s41574-019-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarozzi N, Nadalini M, Coticchio G, Zacà C, Lagalla C, et al. The paternal toolbox for embryo development and health. Mol Hum Reprod. 2021;27:gaab042. doi: 10.1093/molehr/gaab042. [DOI] [PubMed] [Google Scholar]
- 9.Hua M, Liu W, Chen Y, Zhang F, Xu B, et al. Identification of small non-coding RNAs as sperm quality biomarkers for in vitro fertilization. Cell Discov. 2019;5:20. doi: 10.1038/s41421-019-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balhorn R, Brewer L, Corzett M. DNA condensation by protamine and arginine-rich peptides:analysis of toroid stability using single DNA molecules. Mol Reprod Dev. 2000;56:230–4. doi: 10.1002/(SICI)1098-2795(200006)56:2+<230::AID-MRD3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Oliva R. Protamines and male infertility. Hum Reprod Update. 2006;12:417–35. doi: 10.1093/humupd/dml009. [DOI] [PubMed] [Google Scholar]
- 12.Tanphaichitr N, Sobhon P, Taluppeth N, Chalermisarachai P. Basic nuclear proteins in testicular cells and ejaculated spermatozoa in man. Exp Cell Res. 1978;117:347–56. doi: 10.1016/0014-4827(78)90148-9. [DOI] [PubMed] [Google Scholar]
- 13.Gatewood JM, Cook GR, Balhorn R, Bradbury EM, Schmid CW. Sequence-specific packaging of DNA in human sperm chromatin. Science. 1987;236:962–4. doi: 10.1126/science.3576213. [DOI] [PubMed] [Google Scholar]
- 14.Carrell DT, Hammoud SS. The human sperm epigenome and its potential role in embryonic development. Mol Hum Reprod. 2010;16:37–47. doi: 10.1093/molehr/gap090. [DOI] [PubMed] [Google Scholar]
- 15.Meyer RG, Ketchum CC, Meyer-Ficca ML. Heritable sperm chromatin epigenetics:a break to remember. Biol Reprod. 2017;97:784–97. doi: 10.1093/biolre/iox137. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton M, Russell S, Menezes K, Moskovtsev SI, Librach C. Assessing spermatozoal small ribonucleic acids and their relationship to blastocyst development in idiopathic infertile males. Sci Rep. 2022;12:20010. doi: 10.1038/s41598-022-24568-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, San Gabriel M, Zini A. Sperm nuclear histone to protamine ratio in fertile and infertile men:evidence of heterogeneous subpopulations of spermatozoa in the ejaculate. J Androl. 2006;27:414–20. doi: 10.2164/jandrol.05171. [DOI] [PubMed] [Google Scholar]
- 18.Steger K, Fink L, Failing K, Bohle RM, Kliesch S, et al. Decreased protamine-1 transcript levels in testes from infertile men. Mol Hum Reprod. 2003;9:331–6. doi: 10.1093/molehr/gag041. [DOI] [PubMed] [Google Scholar]
- 19.de Yebra L, Ballescá JL, Vanrell JA, Corzett M, Balhorn R, et al. Detection of P2 precursors in the sperm cells of infertile patients who have reduced protamine P2 levels. Fertil Steril. 1998;69:755–9. doi: 10.1016/s0015-0282(98)00012-0. [DOI] [PubMed] [Google Scholar]
- 20.Sakkas D, Moffatt O, Manicardi GC, Mariethoz E, Tarozzi N, et al. Nature of DNA damage in ejaculated human spermatozoa and the possible involvement of apoptosis. Biol Reprod. 2002;66:1061–7. doi: 10.1095/biolreprod66.4.1061. [DOI] [PubMed] [Google Scholar]
- 21.Nasr-Esfahani MH, Salehi MO, Razavi S, Mardani M, Bahramian H, et al. Effect of protamine-2 deficiency on ICSI outcome. Reprod Biomed Online. 2004;9:652–8. doi: 10.1016/s1472-6483(10)61776-2. [DOI] [PubMed] [Google Scholar]
- 22.Depa-Martynow M, Kempisty B, Jagodziński PP, Pawelczyk L, Jedrzejczak P. Impact of protamine transcripts and their proteins on the quality and fertilization ability of sperm and the development of preimplantation embryos. Reprod Biol. 2012;12:57–72. doi: 10.1016/s1642-431x(12)60077-1. [DOI] [PubMed] [Google Scholar]
- 23.Jodar M, Selvaraju S, Sendler E, Diamond MP, Krawetz SA, et al. The presence, role, and clinical use of spermatozoal RNAs. Hum Reprod Update. 2013;19:604–24. doi: 10.1093/humupd/dmt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernández-Silva G, Caballero-Campo P, Chirinos M. Sperm mRNAs as potential markers of male fertility. Reprod Biol. 2022;22:100636. doi: 10.1016/j.repbio.2022.100636. [DOI] [PubMed] [Google Scholar]
- 25.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment:proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 27.Ozsait-Selcuk B, Coban N, Sever-Kaya D, Bulgurcuoglu-Kuran S, Turkolmez S, et al. RNA isolation and detection of cellular RNA quantity of spermatozoa and embryos prior to gene expression analyses. J Istanb Fac Med. 2018;81:119–26. [Google Scholar]
- 28.Mao S, Goodrich RJ, Hauser R, Schrader SM, Chen Z, et al. Evaluation of the effectiveness of semen storage and sperm purification methods for spermatozoa transcript profiling. Syst Biol Reprod Med. 2013;59:287–95. doi: 10.3109/19396368.2013.817626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostermeier GC, Goodrich RJ, Moldenhauer JS, Diamond MP, Krawetz SA. A suite of novel human spermatozoal RNAs. J Androl. 2005;26:70–4. [PubMed] [Google Scholar]
- 30.Luo CH, Tang YG, Hong SH, Tang Y, Zhang Y, et al. Selection and evaluation of optimal reference genes for quantitative reverse transcription-polymerase chain reaction analyses of gene expression in human spermatozoa. Reprod Dev Med. 2020;4:212–8. [Google Scholar]
- 31.Pérez-Rico A, Crespo F, Sanmartín ML, De Santiago A, Vega-Pla JL. Determining ACTB, ATP5B and RPL32 as optimal reference genes for quantitative RT-PCR studies of cryopreserved stallion semen. Anim Reprod Sci. 2014;149:204–11. doi: 10.1016/j.anireprosci.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 33.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3:a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 34.Cappallo-Obermann H, Schulze W, Jastrow H, Baukloh V, Spiess AN. Highly purified spermatozoal RNA obtained by a novel method indicates an unusual 28S/18S rRNA ratio and suggests impaired ribosome assembly. Mol Hum Reprod. 2011;17:669–78. doi: 10.1093/molehr/gar037. [DOI] [PubMed] [Google Scholar]
- 35.Miller D, Briggs D, Snowden H, Hamlington J, Rollinson S, et al. A complex population of RNAs exists in human ejaculate spermatozoa:implications for understanding molecular aspects of spermiogenesis. Gene. 1999;237:385–92. doi: 10.1016/s0378-1119(99)00324-8. [DOI] [PubMed] [Google Scholar]
- 36.Aoki VW, Liu L, Carrell DT. A novel mechanism of protamine expression deregulation highlighted by abnormal protamine transcript retention in infertile human males with sperm protamine deficiency. Mol Hum Reprod. 2006;12:41–50. doi: 10.1093/molehr/gah258. [DOI] [PubMed] [Google Scholar]
- 37.Carrell DT, Emery BR, Hammoud S. Altered protamine expression and diminished spermatogenesis:what is the link? Hum Reprod Update. 2007;13:313–27. doi: 10.1093/humupd/dml057. [DOI] [PubMed] [Google Scholar]
- 38.Sassone-Corsi P. Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science. 2002;296:2176–8. doi: 10.1126/science.1070963. [DOI] [PubMed] [Google Scholar]
- 39.Grunewald S, Paasch U, Glander HJ, Anderegg U. Mature human spermatozoa do not transcribe novel RNA. Andrologia. 2005;37:69–71. doi: 10.1111/j.1439-0272.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 40.Gur Y, Breitbart H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006;20:411–6. doi: 10.1101/gad.367606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou W, Stanger SJ, Anderson AL, Bernstein IR, De Iuliis GN, et al. Mechanisms of tethering and cargo transfer during epididymosome-sperm interactions. BMC Biol. 2019;17:35. doi: 10.1186/s12915-019-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodrich R, Johnson G, Krawetz SA. The preparation of human spermatozoal RNA for clinical analysis. Arch Androl. 2007;53:161–7. doi: 10.1080/01485010701216526. [DOI] [PubMed] [Google Scholar]
- 43.Lalancette C, Platts AE, Johnson GD, Emery BR, Carrell DT, et al. Identification of human sperm transcripts as candidate markers of male fertility. J Mol Med. 2009;87:735–48. doi: 10.1007/s00109-009-0485-9. [DOI] [PubMed] [Google Scholar]
- 44.Chemes EH, Rawe YV. Sperm pathology:a step beyond descriptive morphology. Origin, characterization and fertility potential of abnormal sperm phenotypes in infertile men. Hum Reprod Update. 2003;9:405–28. doi: 10.1093/humupd/dmg034. [DOI] [PubMed] [Google Scholar]
- 45.Aoki VW, Liu LH, Carrell DT. Identification and evaluation of a novel sperm protamine abnormality in a population of infertile males. Hum Reprod. 2005;20:1298–306. doi: 10.1093/humrep/deh798. [DOI] [PubMed] [Google Scholar]
- 46.Simon L, Castillo J, Oliva R, Lewis SE. Relationships between human sperm protamines, DNA damage and assisted reproduction outcomes. Reprod Biomed Online. 2011;23:724–34. doi: 10.1016/j.rbmo.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Savadi-Shiraz E, Edalatkhah H, Talebi S, Heidari-Vala H, Zandemami M, et al. Quantification of sperm specific mRNA transcripts (PRM1, PRM2, and TNP2) in teratozoospermia and normozoospermia:New correlations between mRNA content and morphology of sperm. Mol Reprod Dev. 2015;82:26–35. doi: 10.1002/mrd.22440. [DOI] [PubMed] [Google Scholar]
- 48.Lambard S, Galeraud-Denis I, Martin G, Levy R, Chocat A, et al. Analysis and significance of mRNA in human ejaculated sperm from normozoospermic donors:relationship to sperm motility and capacitation. Mol Hum Reprod. 2004;10:535–41. doi: 10.1093/molehr/gah064. [DOI] [PubMed] [Google Scholar]
- 49.Ganguly I, Gaur GK, Kumar S, Mandal DK, Kumar M, et al. Differential expression of protamine 1 and 2 genes in mature spermatozoa of normal and motility impaired semen producing crossbred Frieswal (HF×Sahiwal) bulls. Res Vet Sci. 2013;94:256–62. doi: 10.1016/j.rvsc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Carrell DT, Liu L. Altered protamine 2 expression is uncommon in donors of known fertility, but common among men with poor fertilizing capacity, and may reflect other abnormalities of spermiogenesis. J Androl. 2001;22:604–10. [PubMed] [Google Scholar]
- 51.Schneider S, Balbach M, Jikeli JF, Fietz D, Nettersheim D, et al. Re-visiting the Protamine-2 locus:deletion, but not haploinsufficiency, renders male mice infertile. Sci Rep. 2016;6:36764. doi: 10.1038/srep36764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balhorn R, Reed S, Tanphaichitr N. Aberrant protamine 1/protamine 2 ratios in sperm of infertile human males. Experientia. 1988;44:52–5. doi: 10.1007/BF01960243. [DOI] [PubMed] [Google Scholar]
- 53.Lewis JD, Song Y, de Jong ME, Bagha SM, Ausio J. A walk though vertebrate and invertebrate protamines. Chromosoma. 2003;111:473–82. doi: 10.1007/s00412-002-0226-0. [DOI] [PubMed] [Google Scholar]
- 54.Hammoud S, Liu L, Carrell DT. Protamine ratio and the level of histone retention in sperm selected from a density gradient preparation. Andrologia. 2009;41:88–94. doi: 10.1111/j.1439-0272.2008.00890.x. [DOI] [PubMed] [Google Scholar]
- 55.Cho C, Jung-Ha H, Willis WD, Goulding EH, Stein P, et al. Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biol Reprod. 2003;69:211–7. doi: 10.1095/biolreprod.102.015115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contamination control of somatic cell markers. After the real-time PCR (qRT-PCR) method, the amplification products of the somatic cell markers were analyzed by agarose gel electrophoresis. No expression was detected in sperm samples (lanes 2, 4, and 6), whereas amplicon products were visual in the testicular tissue (lanes 1, 3, 5, and 7). The expected product sizes were confirmed for the studied genes and endogenous control gene in testicular tissue. Leukocyte cell marker (CD45), 439 bp; epithelial cell marker, E-cadherin (CHD1), 492 bp; germ cell marker, c-kit proto-oncogene (C-KIT), 436; endogenous control, beta actin (ACTB), 204 bp.


