Abstract
Testicular descent occurs in two consecutive stages: the transabdominal stage and the inguinoscrotal stage. Androgens play a crucial role in the second stage by influencing the development of the gubernaculum, a structure that pulls the testis into the scrotum. However, the mechanisms of androgen actions underlying many of the processes associated with gubernaculum development have not been fully elucidated. To identify the androgen-regulated genes, we conducted large-scale gene expression analyses on the gubernaculum harvested from luteinizing hormone/choriogonadotropin receptor knockout (Lhcgr KO) mice, an animal model of inguinoscrotal testis maldescent resulting from androgen deficiency. We found that the expression of secreted protein acidic and rich in cysteine (SPARC)-related modular calcium binding 1 (Smoc1) was the most severely suppressed at both the transcript and protein levels, while its expression was the most dramatically induced by testosterone administration in the gubernacula of Lhcgr KO mice. The upregulation of Smoc1 expression by testosterone was curtailed by the addition of an androgen receptor antagonist, flutamide. In addition, in vitro studies demonstrated that SMOC1 modestly but significantly promoted the proliferation of gubernacular cells. In the cultures of myogenic differentiation medium, both testosterone and SMOC1 enhanced the expression of myogenic regulatory factors such as paired box 7 (Pax7) and myogenic factor 5 (Myf5). After short-interfering RNA-mediated knocking down of Smoc1, the expression of Pax7 and Myf5 diminished, and testosterone alone did not recover, but additional SMOC1 did. These observations indicate that SMOC1 is pivotal in mediating androgen action to regulate gubernaculum development during inguinoscrotal testicular descent.
Keywords: androgen, androgen receptor, gubernaculum, Smoc1, testis descent
INTRODUCTION
Testicular descent progresses through two sequential stages: the transabdominal and inguinoscrotal stages. In the transabdominal stage, the testes move from the urogenital ridge to the internal inguinal ring. This phase is facilitated by the gubernaculum, a cylindrical, gelatinous structure attached caudally to the testis. The gubernaculum undergoes swelling and shortening that exert a traction to pull the testis downward during this initial descent phase. As the fetus enlarges, the gubernaculum holds the testis near the inguinal region.1,2 During embryogenesis, the growth and differentiation of the gubernaculum are regulated principally by insulin-like peptide 3 (INSL3). INSL3 is secreted primarily by Leydig cells within the testes. It binds to its specific receptor, relaxin family peptide receptor 2 (RXFP2), which is present in the gubernaculum. Mutations in either INSL3 or RXFP2 in humans and mice lead to transabdominal testis maldescent.3,4 In the transinguinal phase, the gubernaculum actively grows, continually differentiates to cremaster muscle, and ultimately facilitates the inguinoscrotal migration of the testes. The fusion of the peritoneum and cremaster muscle forms a sheath like processus, which provides the condition for the testes to move through the groin, causing them to descend into the peritoneal diverticulum in the subcutaneous part of the scrotum.1,2 It is known that androgens are involved in the regulation of the second phase of testicular descent and androgen deficiency in men and animals contributes to the second phase of testicular maldescent.5,6 However, the underlying mechanism of androgenic action is not fully understood.
Bilateral testis of luteinizing hormone/choriogonadotropin receptor (Lhcgr) knockout (Lhcgr KO) male mice located near the neck of the bladder at birth with utterly regression of the cranial suspensory ligament,7,8,9 indicating prenatal completion of the first phase of testicular descent (normally occurring between embryonic days 15.5 to 17.5 in mice4). Normal transabdominal testicular descent of Lhcgr KO males may attribute to a gonadotropin-independent secretion of INSL3 and testosterone by fetal Leydig cells.10,11 However, Lhcgr inactivation hampered the development of adult Leydig cells. As a result, postnatal serum testosterone dropped to almost nondetectable levels. The testes of Lhcgr KO mice were trapped in the inguinal region attached persistently with the gubernacular code. None of the testes of Lhcgr KO mice descended to the scrotum7,8,11 (normally occurring between postnatal day 14 and day 21 in mice4), indicating a defect specifically in the inguinoscrotal phase of testicular descent. Interestingly, this deficiency can be completely recovered through testosterone replacement therapy (TRT).9,12 Remedy of the maldescent by TRT requires androgen receptor (AR) activity in the gubernaculum.7,11 Therefore, Lhcgr KO mouse is a unique animal model for further understanding of the molecular mechanism by which androgens regulate inguinoscrotal testicular descent.
Although androgens can exert nongenomic effects, their primary functions are mediated through AR. AR belongs to the nuclear receptor gene family and acts as a ligand-dependent transcription factor.13 Liganded AR binds to specific DNA sequences at regulatory regions, known as androgen-responsive elements, to activate or inhibit the expression of specific genes in target cells and tissues.14 Given the crucial role of androgens and the gubernaculum in the second stage of testicular descent, identifying the genes regulated by androgens in gubernacular cells can provide valuable insights into the mechanism underlying testicular descent during the transinguinal phase. In the present study, we undertook large-scale gene expression analyses using gubernacular RNA extracted from wild-type, Lhcgr KO, and androgen-treated Lhcgr KO mice. Here, we provide evidence that secreted protein acidic and rich in cysteine (SPARC)-related modular calcium binding 1 (Smoc1) is one of the androgen responsive genes in the gubernaculum. It involves in the growth and differentiation of gubernacular cells and plays a role in regulation of inguinoscrotal testicular descent in response to androgens.
MATERIALS AND METHODS
Animals
The Lhcgr KO mouse model was created through targeted gene disruption, and genotyping was conducted via polymerase chain reaction (PCR) using tail genomic DNA, following previously established protocols.11 Adult heterozygous mice were bred to produce offspring with wild-type (+/+), heterozygous (+/−), and homozygous (−/−) genotypes. The mice were housed in an animal facility with consistent temperature and humidity levels, maintained under a 12 h/12 h cycle of light/darkness. Rodent chow and water were provided without restriction. Sacrifice of mice was carried out under ketamine anesthesia, with appropriate measures taken to minimize their discomfort. The Institutional Animal Care and Use Committee at the University of Louisville School of Medicine (Louisville, KY, USA) approved all experimental protocols involving mice (Approval No. 21933). All protocols involving mice were conducted in accordance with the Laboratory Animal Care and Use Guidelines of the National Institute of Health.
Complementary DNA (cDNA) microarray analysis
Twelve-day-old Lhcgr KO and wild-type (WT) male mice received a single intramuscular injection of either 5 mg testosterone propionate (T) or the vehicle control sesame oil (SO; MilliporeSigma, St. Louis, MO, USA). Although transinguinal testicular descent of Lhcgr KO mice, either young or old, can be induced by TRT,9,11,12 it is known that this phase of testicular descent in mice takes place normally around postnatal days 14–21.4 The rational of choosing 12-day-old mice for this experiment was that this timing could be a sensitive window to capture androgen-responsive genes that are critical for the onset of this phase of testicular descent. Each experimental group consisted of three animals (n = 3). Twenty-four hours postinjection, the mice were sacrificed, and gubernacular tissues were harvested for cDNA microarray and real-time reverse transcription polymerase chain reaction (RT-PCR) analyses.
Total RNA extracted from the tissues was labeled using Affymetrix IVT express kits and hybridized to the Affymetrix mouse genome MOE430 2.0 array chip (Affymetrix, Santa Clara, CA, USA), containing expression data for over 39 000 transcripts of mouse genes. The array data underwent normalization using the robust multichip average (RMA) algorithm, followed by background correction and adjustments. The resulting transformed signal intensities were analyzed utilizing Partek Genomic Suite (version 6.4, Partek Inc., Chesterfield, MO, USA). The genes exhibiting expression difference were identified through a two-way analysis of variance (ANOVA) comparison between Lhcgr KO and WT mice and the effects of testosterone treatment. Significant differentially expressed genes were determined as all genes that passed Benjamini-Hochberg’s false discovery rate (FDR) with an adjusted P < 0.05.
Gubernacular cell culture
The preparation and culture of mouse gubernacular cells were performed as previously reported.15,16 In short, gubernacula were obtained from 12-day-old WT mice and cut into approximately 1-mm3 pieces on ice. These tissue fragments were then placed in Dulbecco’s modified eagle medium (DMEM) containing 1 mg ml−1 type I collagenase and 0.13 mg ml−1 DNase I (MilliporeSigma) for 1 h at 37°C with continuous agitation. After filtration through a 100 μm cell strainer and centrifugation at 250g for 5 min using an Eppendorf 5417R microcentrifuge (Eppendorf, Enfield, CT, USA), the dispersed cells were seeded in DMEM supplemented with 10% fetal bovine serum (FBS; MilliporeSigma) and antibiotic-antimycotic solution (Invitrogen, Carlsbad, CA, USA) at 37°C with 5% CO2. The gubernacular tissues from three to five mice were combined for each experiment. These primarily cultured cells expressed CD44 molecule (CD44), CD105 antigen (CD105), and Rxfp2, suggesting that they were mainly gubernacular mesenchymal cells.
To test whether upregulation of Smoc1 by androgen was mediated by AR in the gubernaculum, the culture medium was replaced with fresh DMEM when the cells reached approximately 80% confluence. Subsequently, the cells were treated with 2 × 10−6 mol l−1 5α-dihydrotestosterone (DHT) or 2 × 10−6 mol l−1 flutamide (MilliporeSigma) or a combination of the both for 24 h. Cells treated with dimethyl sulfoxide (MilliporeSigma) served as a vehicle control. The expression levels of Smoc1 were determined using real-time RT-PCR, while SMOC1 protein levels were detected via western blot analysis.
Water-soluble tetrazolium-1 (WST-1) assay
Gubernacular cells were plated in a 96-well plate at a density of 5 × 104 cells per well in 100 μl of DMEM medium (MilliporeSigma). Then, the cells were treated with phosphate-buffered saline (PBS) containing or not containing 100 ng ml−1 recombinant mouse SMOC1 (R&D Systems, Minneapolis, MN, USA). After the designated incubation periods, the SMOC1 treatments were terminated by adding 10 μl of WST-1 mixture (Cayman Chemical, Ann Arbor, MI, USA) to each well and gently mixed. The plate was incubated for an additional 2 h. Absorbance was measured at a wavelength of 450 nm using a microplate reader (BMG Labtech, Cary, NC, USA).
Gubernacular cell differentiation assessment
To determine the effect of SMOC1 on the differentiation of gubernacular cells, the cells were cultured in DMEM (MilliporeSigma) medium containing 2% horse serum (MilliporeSigma) when they reached approximately 60% confluence. Subsequently, these cells were treated with PBS containing or not containing 100 ng ml−1 recombinant mouse SMOC1 (R&D Systems). At the end of the treatment, the cells were harvested and processed for real-time RT-PCR and western blot.
To knock down the Smoc1 expression, the cells were incubated with the mixed solution of 80 pmol l−1 Smoc1 short-interfering RNA (siRNA), 8 μl transfection reagent, and 1 ml siRNA transfection medium (Santa Cruz Biotech, Dallas, TX, USA) for 12 h at 37°C in a CO2 incubator. Then, 1 ml DMEM containing 4% horse serum (MilliporeSigma) was added for an additional 24 h. These cells were treated with 2 × 10−6 mol l−1 DHT or 100 ng ml−1 recombinant mouse SMOC1 in DMEM supplemented with 2% horse serum for 2 days. The protein levels of SMOC1, paired box 7 (PAX7), and myogenic factor 5 (MYF5) were determined by western blot. In parallel with the transfection of Smoc1 siRNA, a control siRNA (Santa Cruz Biotech) composed of scrambled RNA sequences was also transfected to serve as a specificity control for the knockdown of Smoc1.
Real-time RT-PCR
Total RNA was isolated from the samples using TRIzol reagent (Invitrogen). The quality and concentration of the extracted RNA were evaluated using a SpectroStar Nano Absorbance Microplate Reader (BMG Labtech). High-capacity cDNA reverse transcription kit (Applied Biosystems, Foster, CA, USA) was used to reverse transcribe 2 μg RNA into cDNA. The cDNA samples were then amplified with specific primer pairs for the target genes and 2× Universal SYBR Green Fast qPCR Mix (Abclonal, Woburn, MA, USA) using a StepOne Plus thermal cycler (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as a normalizer, and the fold change was determined using the 2−ΔΔCt method. Oligonucleotide primers were designed using sequences from GenBank through the Vector NTI 12.0 software (Invitrogen) and synthesized by Operon Technologies (Alameda, CA, USA). All primer sequences are provided in Supplementary Table 1.
Supplementary Table 1.
List of polymerase chain reaction primers used for present study
| Genes | Forward (5’–3’) | Reverse (5’–3’) |
|---|---|---|
| Real-time PCR primers | ||
|
| ||
| Pa×3 | AACCCACTACCCAGACATTTAC | CCAGCTTGTTTCCTCCATCT |
| Pgr | CTACTCGCTGTGCCTTACCATG | CTGGCTTTGACTCCTCAGTCCT |
| Trhde | CAGAGTGTTCGACTGGATCGCA | CTGGCTGCATTGCCATACTTGTG |
| Prokr1 | ACTTGCGTACCGTCTCCCTCTA | GGATACTGACCACACCAGGAAG |
| Ntng1 | GACCTGAGGATCAGGCTGTTGA | ACACGAAGTGGCATGCAGGTTG |
| Pax7 | GAACCACATCCGTCACAAGATA | GGAGACACAACCATGGGAAA |
| Smoc1 | AATCCCGGAGTTGCTCTTATTC | GCTGGATCATCGCTCCTATTC |
| Rxfp2 | CTGTGGACCAAGCTGAGAATAG | AGTACTGACTGCCTGGAGAT |
| Slc26a7 | CAGGAGTGTGGATGTGTTCTT | CGGTGTCTAGGTCTCCATAGT |
| Myf5 | AGACAAGCTGGGCAGAATAC | CAGGCAGAGGAGAATCCATTATT |
| Enpp1 | ACCCTCAGTGGCAACTTGCGTT | TGCTTGAAGGCAGGTCCATAGC |
| MyoD1 | GGGTTCCCTGTTCTGTGTCGCTT | CGCTCCAACTGCTCTGATGGCAT |
| MyoG | AGTGAATGCAACTCCCACAG | ACGATGGACGTAAGGGAGTG |
| Des | GTGAAGATGGCCTTGGATGT | GTAGCCTCGCTGACAACCTC |
| Actn2 | GCCTCAAGCTCATGCTACTT | CTCCCTTGCTGGCTATGTAATC |
| Myh2 | GGCTTCAGGATTTGGTGGATAA | GGATCTTGCGGAACTTGGATAG |
| Tnnc1 | TGAAGGACGGTGACAAGAAC | GTGCAAGACCAGCATCTACT |
| CD44 | CAGTCACAGACCTACCCAATT | GTGTGTTCTATACTCGCCCTTC |
| CD105 | CTTGTCCCAGGAAGTCTACAA | GGAAGGCACCAAAGGTGATA |
| Sca1 | CAGTGTCGAAGTCTTGGTAGAG | TGGAACACGGCAGATCAAA |
| Gapdh | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
|
| ||
| Genotyping PCR primers | ||
|
| ||
| Neo | CGGAAGCCCGGCATTCTGCA | ATTCAGGGCACCGGACAGGTCG |
| Lhcgr | TGACCTGTTCCTGGGGCTGCTG | AAATGGCCTCAACGGGTGTGCA |
Pax3: paired box 3; MyoG: myogenin; Myod1: myogenic differentiation 1; Myh2: myosin heavy chain 2; Tnnc1: troponin C1; Sca1: spinocerebellar ataxia type 1; Actn2: actinin alpha 2; Des: desmin; Pgr: progesterone receptor; Trhde: thyrotropin-releasing hormone-degrading enzyme; Prokr1: prokineticin receptor 1; Ntng1: netrin G1; Pax7: paired box 7; Smoc1: SPARC-related modular calcium binding 1; SPARC: secreted protein acidic and rich in cysteine; Rxfp2: RELAXIN family peptide receptor 2; Slc26a7: solute carrier family 26 member 7; Myf5: myogenic factor 5; Enpp1: ectonucleotide pyrophosphatase/phosphodiesterase 1; Lhcgr: LUTEINIZING hormone/choriogonadotropin receptor; Gapdh: glyceraldehyde-3-phosphate dehydrogenase; PCR: polymerase chain reaction
Western blot analysis
Proteins in gubernacular tissues and cells were extracted using radioimmunoprecipitation lysis buffer containing complete proteinase inhibitor cocktails (Roche, Indianapolis, IN, USA). The protein concentrations were determined by a bicinchoninic acid protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). After boiling at 95°C, 20 μg protein per sample was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to the polyvinylidene fluoride membranes (MilliporeSigma). After blocking with nonfat milk (Bio-Rad Laboratories), the membranes were incubated overnight with primary antibodies as listed in Supplementary Table 2 at 4°C. The membranes were then washed with Tris-buffered saline containing 0.1% Tween-20. Followed by incubations with horseradish peroxidase-linked secondary antibodies and Clarity Western enhanced chemiluminescence substrate (Bio-Rad Laboratories), the immune complexes were visualized using the Li-Cor digital chemiluminescence detection system (Li-Cor Sciences, Lincoln, NE, USA). The molecular weight of proteins was confirmed by comparing them to a marker protein standard running in the same gel. The intensity of bands of appropriate size was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA) and normalized to the levels of β-actin.
Supplementary Table 2.
List of antibodies used for present study
| Antibody | Host | Dilution | Manufacturer |
|---|---|---|---|
| SMOC1 | Rabbit | 1:1000 | Abclonal |
| PAX7 | Rabbit | 1:2000 | Abclonal |
| MYF5 | Rabbit | 1:800 | R and D Systems |
| Ki67 | Rabbit | 1:800 | Abclonal |
| β-Actin | Mouse | 1:3000 | Santa Cruz Biotech |
Smoc1: SPARC-related modular calcium binding 1; SPARC: secreted protein acidic and rich in cysteine; Pax7: paired box 7; MYF5: Myf5: myogenic factor 5; Ki67: Kiel-67
Immunofluorescence
Gubernacular cells were seeded into two-chamber cell culture slides (MilliporeSigma) at a density of 6 × 104 cells per well in 0.5 ml of DMEM containing 10% FBS (MilliporeSigma). The cells were then treated with PBS containing or not containing 100 ng ml−1 recombinant mouse SMOC1 (R&D Systems) for 24 h. At the end of each treatment period, the cells were washed with PBS and subjected to immunofluorescence staining. First, the cells were fixed with 2% paraformaldehyde (MilliporeSigma) for 20 min. Subsequently, the cells were permeabilized with 0.5% Triton X-100 and blocked with 6% donkey serum for 30 min each. The cells were incubated with the primary antibody Kiel-67 (Ki67) at 4°C overnight and then exposed to a fluorescein isothiocyanate-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) for 2 h. Finally, all slides were covered with a 4’,6-diamidino-2-phenylindole-containing mounting medium (Santa Cruz Biotech). An Olympus fluorescence microscope (Olympus Corp., Center Valley, PA, USA) was used to capture fluorescent images.
Immunohistochemical staining
Briefly, formalin-fixed sections were heated by microwave for 13 min, treated with 0.3% H2O2 to deactivate endogenous peroxidase activity, and then blocked with 5% normal goat serum (MilliporeSigma). Next, the slices were exposed to primary antibodies as listed in Supplementary Table 2 at 4°C overnight. After washing with PBS, the slices were treated with biotinylated secondary antibody (diluted at 1:100) for 1 h and incubated with an avidin-biotin peroxidase complex (Vector Lab, Burlingame, CA, USA) for another 1 h. Immunoreactivity was visualized by incubating slices with substrate 3’3-diaminobenzidine (Vector Lab). Finally, the slides were counterstained with hematoxylin (MilliporeSigma) and visualized using an Olympus IX71 optical microscope (Olympus Corp.).
Computational prediction of AR-binding sites and calculation of protein sequence similarity
The JASPAR program (https://genome.ucsc.edu/cgi-bin/, last accessed on October 10, 2023) was employed to predict the binding sites of AR in the mouse Smoc1 gene promoter sequence. The analysis was conducted within a region spanning 3000 base pairs upstream from the first known transcribed nucleotide of the Smoc1 gene. SMOC1 protein sequence similarity was calculated by CLUSTALW multiple alignment program (https://www.genome.jp/toolsbin/clustalw, last accessed on October 10, 2023).
Statistical analyses
The data were presented as means ± standard error of the mean (s.e.m.). Statistical significance was assessed using Student’s t-test and one-way ANOVA. The analyses were conducted using SPSS version 13.0 (SPSS lnc., Armonk, NY, USA), with a significance level set at P < 0.05.
RESULTS
The expression of Smoc1 mRNA and corresponding protein is regulated by androgen in gubernacular tissues
To unravel the intricate mechanisms of androgen actions, we investigated how androgens influence the gene expression patterns in the gubernaculum of WT and Lhcgr KO mice. Twelve-day-old male mice were administered a single dose of either 5 mg T or SO alone. After 24 h, the mice were sacrificed to collect gubernacular tissues. The results of cDNA array analysis and verified with real-time RT-PCR showed that compared to WT mice, Lhcgr KO mice had 6 genes (solute carrier family 26 member 7 [Slc26a7], thyrotropin-releasing hormone-degrading enzyme [Trhde], netrin G1 [Ntng1], prokineticin receptor 1 [Prokr1], ectonucleotide pyrophosphatase/phosphodiesterase 1 [Enpp1], and progesterone receptor [Pgr]) significantly upregulated and 2 genes (Smoc1 and Rxfp2) remarkably downregulated. The expression of these genes in the gubernaculum of Lhcgr KO mice treated with testosterone was completely reversed (Supplementary Figure 1 (154KB, tif) ). Of these differentially regulated mRNAs, we found that Smoc1 mRNA was the most severely suppressed in the gubernaculum of Lhcgr KO mice, and the most dramatically induced by testosterone treatment as validated by real-time RT-PCR analysis (Figure 1a).
Figure 1.

Expression of Smoc1 and corresponding protein and SMOC1 immunohistochemical staining in the gubernacular tissues from Lhcgr KO (KO) and wild-type (WT) mice. The expression of Smoc1 was measured by real-time RT-PCR. The relative mRNA levels of Smoc1 were calculated using the comparative 2-ΔΔCt method with Gapdh as an internal control. Western blot assays were conducted to assessed SMOC1 protein expression. The relative protein levels of SMOC1 were determined using intensity-based quantification with β-actin as a reference control. (a) Consistent with microarray data, real-time RT-PCR shows that Smoc1 mRNA levels are decreased in KO mice. Treatment with testosterone propionate (T) increases Smoc1 mRNA levels in KO mice compared to KO mice given sesame oil. (b) Western blot assays reveal that T-treatment significantly increases SMOC1 protein levels in the gubernacular tissues. (c) Representative immunostaining pictures of the gubernacular cremaster muscle for WT, KO, and treatment with T (T/KO). Immunostaining indicates the presence of SMOC1 in the cremaster muscle. The immunostaining intensity of SMOC1 is diminished in KO mice compared to WT, while T-treatment increases SMOC1 in KO mice. Scale bars = 50 μm; n = 3; *P < 0.05, KO compared to WT. Smoc1: secreted protein acidic and rich in cysteine-related modular calcium binding 1; Lhcgr KO: luteinizing hormone/choriogonadotropin receptor knockout; WT: wild-type; RT-PCR: reverse transcription-polymerase chain reaction; Gapdh: glyceraldehyde-3-phosphate dehydrogenase.
Smoc1 mRNA encodes a secreted acidic cysteine rich glycoprotein. SMOC1 proteins of mice and rats exhibited a high degree of similarity, with homologies exceeding 98%. Similarly, there was a considerable overlap in protein sequences between mice and humans, with homologies surpassing 95% (Supplementary Figure 2 (53.6KB, tif) ). Immunohistochemical staining and western blot analysis demonstrated that gubernacular SMOC1 protein levels are drastically reduced in Lhcgr KO mice compared to that of WT mice, while the protein levels were noticeably elevated by testosterone treatment (Figure 1b and 1c).
AR-dependent regulation of Smoc1 expression in gubernacular cells
To interrogate whether androgen directly regulates Smoc1 expression, we performed primary cultures of gubernacular cells of neonatal mice and treated them with a potent androgen, DHT (2 × 10−6 mol l−1). Real-time RT-PCR and western blot results showed that DHT increased both Smoc1 mRNA and corresponding protein levels in a time-dependent manner (Figure 2a and 2b). The upregulatory effects of DHT were completely inhibited by cotreatment with an androgen antagonist flutamide. Flutamide treatment alone did not affect the expression of Smoc1 (Figure 2c and 2d). These results suggested that upregulation of Smoc1 expression by DHT is an AR-mediated action. Interestingly, computational analysis of mouse Smoc1 gene promoter region (approximately 3 kb upstream from the transcription start site) by JASPAR program predicted two putative AR-binding sequences (Supplementary Figure 3 (57.6KB, tif) ). Together, these in vitro data further confirm the initial large-scale gene expression analyses, indicating that Smoc1 is an androgen-responsive gene in the gubernaculum.
Figure 2.

Effect of DHT on Smoc1 expression in the primary cultures of gubernacular cells isolated from neonatal WT mice. The expression of Smoc1 was measured by real-time RT-PCR. The relative mRNA contents of Smoc1 were calculated using the comparative 2-ΔΔCt method with Gapdh as an endogenous control. Western blot assays were conducted to assessed SMOC1 protein expression. The relative protein abundances of SMOC1 were determined using intensity-based quantification with β-actin as a reference control. Time-dependent upregulation of (a) Smoc1 mRNA and (b) corresponding protein levels by DHT (D). Flutamide (F) blocked the DHT induced upregulation of (c) Smoc1 mRNA and (d) SMOC1 protein levels. n = 5; **P < 0.01 compared to control (C). D/F: DHT and flutamide; DHT: 5α-dihydrotestosterone; WT: wild-type; Smoc1: secreted protein acidic and rich in cysteine-related modular calcium binding 1; RT-PCR: reverse transcription-polymerase chain reaction; Gapdh: glyceraldehyde-3-phosphate dehydrogenase.
Effect of SMOC1 on the proliferation of gubernacular cells
To explore the biological relevance of SMOC1 in gubernacular development, we first evaluated cell proliferative effect of SMOC1. Gubernacular cells isolated from neonatal WT mice were cultured in DMEM medium with 10% FBS and exposed to 100 ng ml−1 recombinant mouse SMOC1. Data obtained from WST-1 assays showed that exposure to SMOC1 for 24 h modestly but significantly changed the cell viability in cultures when compared to the control group (P < 0.01; Figure 3a). However, prolongation of culture time to 48 h, there was no significant difference between SMOC1 and the control groups. Immunofluorescent staining revealed an increase in Ki67-positive nuclei in the cells treated with SMOC1 for 24 h compared to the control group (Figure 3b).
Figure 3.
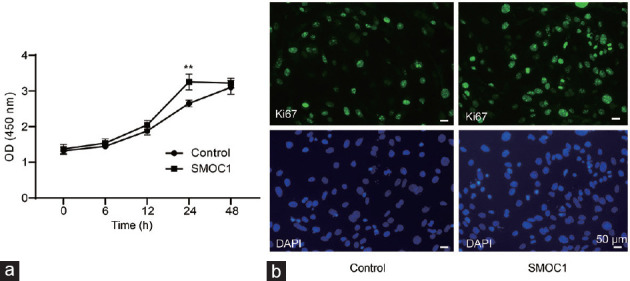
SMOC1 moderately stimulates cell proliferation in the primary cultures of gubernacular cells isolated from neonatal WT mice as determined by (a) WST-1 assays and (b) immunofluorescent staining of a cell proliferation marker Ki67. Scale bars = 50 μm; n = 5; **P < 0.01 compared to control. SMOC1: secreted protein acidic and rich in cysteine-related modular calcium binding 1; WT: wild-type; OD: optical density; DAPI: 4’,6-diamidino-2-phenylindole; WST-1: water-soluble tetrazolium-1; Ki67: Kiel-67.
Effect of SMOC1 on myogenic differentiation gene expression in gubernacular cells
To determine whether SMOC1 affects the myogenic differentiation of gubernacular cells, we cultured gubernacular cells in differentiation medium containing 2% horse serum, treated cells with recombinant SMOC1 protein, and screened for several myogenesis-related gene expressions through real-time RT-PCR. Noticeably, the expression of Pax7 and Myf5 mRNA was markedly increased, while the others, including paired box 3 (Pax3), myogenin (MyoG), myogenic differentiation 1 (Myod1), myosin heavy chain 2 (Myh2), CD44, CD105, troponin C1 (Tnnc1), spinocerebellar ataxia type 1 (Sca1), actinin alpha 2 (Actn2), and desmin (Des), were not changed (Figure 4). Moreover, real-time RT-PCR (Figure 5a and 5b) and western blot (Figure 5c and 5d) results showed that SMOC1 elevated Myf5 and Pax7 mRNA and corresponding protein levels in a time-dependent manner.
Figure 4.

Effect of SMOC1 on the expression of myogenic differentiation-related genes in the gubernacular cells isolated from the gubernacula of neonatal WT mice. The cells were cultured in myogenic differentiation medium and treated without (control) and with SMOC1 for 5 days. The expression of myogenic differentiation-related genes was measured by real-time RT-PCR. The relative mRNA levels are calculated using the comparative 2-ΔΔCt method with Gapdh as an internal control. n = 6; **P < 0.01 compared to control. SMOC1: secreted protein acidic and rich in cysteine-related modular calcium binding 1; WT: wild-type; Gapdh: glyceraldehyde-3-phosphate dehydrogenase; RT-PCR: reverse transcription-polymerase chain reaction; Pax3: paired box 3; Pax7: paired box 7; Myf5: myogenic factor 5; MyoG: myogenin; Myod1: myogenic differentiation 1; Myh2: myosin heavy chain 2; Tnnc1: troponin C1; Sca1: spinocerebellar ataxia type 1; Actn2: actinin alpha 2; Des: desmin.
Figure 5.

Time-dependent upregulation of Myf5 and Pax7 expression by SMOC1 in the gubernacular cells isolated from the gubernacula of neonatal WT mice. The cells were cultured in myogenic differentiation medium and treated without (control; C) and with SMOC1 (S). The mRNA levels of (a) Myf5 and (b) Pax7 were measured by real-time RT-PCR. The protein levels of (c) MYF5 and (d) PAX7 were detected by western blot. The relative mRNA contents were calculated using the comparative 2-ΔΔCt method with Gapdh as an internal control. The relative protein abundances were determined using intensity-based quantification with β-actin as a reference control. n = 6; *P < 0.05, **P < 0.01 compared to control. Myf5: myogenic factor 5; Pax7: paired box 7; SMOC1: secreted protein acidic and rich in cysteine-related modular calcium binding 1; WT: wild-type; RT-PCR: reverse transcription-polymerase chain reaction; Gapdh: glyceraldehyde-3-phosphate dehydrogenase.
Upregulation of Pax7 and Myf5 by androgen is mediated by SMOC1 in gubernacular cells
To determine whether SMOC1 mediated androgen to upregulate the Pax7 and Myf5 expression, we first demonstrated that DHT (2 × 10−6 mol l−1) treatment elevated PAX7 and MYF5 protein levels in gubernacular cells in a time-dependent manner (Figure 6a and 6b). The effects of DHT on PAX7 and MYF5 protein expression were abolished when the endogenous SMOC1 in gubernacular cells was depleted by Smoc1 siRNA (Supplementary Figure 4 (47.6KB, tif) ). Additional exogenous SMOC1 but not DHT significantly reversed the above changes (Figure 6c and 6d).
Figure 6.

Time-dependent upregulation of (a) MYF5 and (b) PAX7 protein expression by DHT, and the effects of Smoc1 siRNA on DHT and SMOC1 induced upregulation of (c) MYF5 and (d) PAX7 protein expression in the gubernacular cells. The cells were isolated from the gubernacula of neonatal WT mice and cultured in myogenic differentiation medium. (a and b) Cells were treated without (C) and with DHT (D). (c and d) Cells were treated with scramble control siRNA (I), Smoc1 siRNA (II), Smoc1 siRNA/DHT (III), Smoc1 siRNA/SMOC1 (IV). MYF5 and PAX7 protein expression were measured by western blot assays. The band intensity of each target protein normalized to β-actin in each sample was used to determine the relative protein levels. n = 4; *P < 0.05, **P < 0.01 compared to control. MYF5: myogenic factor 5; PAX7: paired box 7; siRNA: short-interfering RNA; DHT: 5α-dihydrotestosterone; WT: wild-type; Smoc1: secreted protein acidic and rich in cysteine-related modular calcium binding 1.
DISCUSSION
After the testes descend through the abdomen, the gubernaculum fixes the testes near the groin. During the inguinal scrotal stage, androgens stimulate the growth and differentiation of the gubernaculum, and the traction generated by this growth promotes the movement of the testicles through the groin. The indispensable requirement of androgens in the process of testicular descent from the inguinal to the scrotum was overwhelmingly demonstrated in numerous past studies.5,6,17 Most of the patients with transinguinal testis maldescent are associated with decreased production or action of androgens.5 Lhcgr inactivation in mice led to a defective inguinoscrotal testicular descent. Exogenous androgen manipulations completely corrected the defect and androgen actions are directly targeted to gubernacular cells, where the cells express abundant AR.7,11,18,19 Kaftanovskaya et al.18 reported that the eversion and growth of the gubernaculum at the groin scrotal stage require AR signals in gubernacular cells. Specific AR ablation in the gubernacular ligament resulted in aberrant outgrowth of the gubernaculum and maldifferentiation of gubernacular cells to cremaster muscle.18 Together, these studies clearly establish the signaling mechanism by which AR mediated androgens in gubernacular cells to modulate gubernacular development. However, the genes in response to androgens that are important in gubernacular cell outgrowth and myogenic differentiation remain to be completely defined.
In this study, using high throughput cDNA microarrays with androgen-stimulated gubernacula from Lhcgr KO mice confirms that Rxfp2, which is well known to be crucial in modulating gubernaculum development and differentiation during inguinoscrotal testicular descent, is mediated by androgen. Previous studies have shown that a direct action of androgens on gubernaculum is to sustain the expression of Rxfp2, which is then activated by its cognate ligand INSL3, thereby influencing gubernacular development.16 The connection of the remaining differentially expressed genes with testicular descent, such as Smoc1, Slc26a7, Trhde, Ntng1, Prokr1, Pgr, and Enpp1, remains uncertain. Extensive investigations are necessary to thoroughly analyze each of these genes. It is potentially offering valuable insights into the molecular etiology of inguinoscrotal testis maldescent.
Of these differentially expressed genes, Smoc1 mRNA was the most profoundly depressed in the gubernaculum of Lhcgr KO mice and robustly responded to androgen treatment. Smoc1 encodes a secreted modular protein that is evolutionarily conserved across the species and found in organisms from worms to humans. It is a member of the basement membrane protein-40 (BM-40, also referred to as SPARC/osteonectin) family widely distributed in the basement membrane and extracellular matrix of many tissues.20,21,22 Pazin and Albrecht23 reported the expression of Smoc1 in fetal gonad/mesonephros complexes, suggesting that SMOC1 plays a role in gonad and reproductive tract development. The present study reveals that gubernacular cells and cremaster muscle express Smoc1. Both in vivo and in vitro experiments show that Smoc1 mRNA and corresponding proteins levels are suppressed by androgen depletion and induced by androgen replenishment. It is consistent that Smoc1 mRNA abundance or SMOC1 protein levels in the prostate tissue and sperm are positively correlated with androgen levels.24,25,26 It is further demonstrated by the in vitro study that androgens regulate Smoc1 expression. The study showed that cotreatment of a specific AR inhibitor flutamide significantly blunted the upregulation of Smoc1 by DHT in gubernacular cells. Moreover, our computational decipherment identified several putative AR-binding sites in the promoter region of Smoc1 gene, indicating that androgen may have a direct regulatory effect on Smoc1.
Matricellular protein SMOC1 is highly expressed during embryogenesis and in the tissues undergoing extensive remodeling. SMOC1 has been shown to influence a wide variety of cellular functions including growth factor signaling, cell attachment, proliferation, differentiation, and angiogenesis.27,28 However, the role of SMOC1 in the gubernaculum development has not been investigated. We first set up primary gubernacular cell cultures to test if SMOC1 affected gubernacular cell proliferation. Next, we examined whether SMOC1 involved myogenic differentiation of gubernacular cells. Our results show that SMOC1 significantly promotes gubernacular cell proliferation. When gubernacular cells cultured in myogenic differentiation medium, SMOC1 is found to pronouncedly enhance Pax7 and Myf5 expression. PAX7 and MYF5 are well-established primary regulators of striated muscle commitment and differentiation in both muscle and nonmuscle cells,29,30 suggesting that SMOC1 plays a role as a promyogenic factor in the gubernaculum. Finally, either AR blocking or Smoc1 silencing attenuated Pax7 and Myf5 expression. The phenomenon cannot be reversed by the addition of androgen but can be reversed by SMOC1. Our data further provide the regulatory cascade of androgen, Smoc1, and Pax7/Myf5 in gubernacular cells. Together, SMOC1 may act as a progrowth and promyogenic differentiation factor participating in the complex processes of growth and differentiation of the gubernaculum orchestrated by androgen during the inguinoscrotal testicular descent.
It is more common for patients with cryptorchidism to have disruption in the inguinoscrotal stage.18,31 Androgen deficiency or malfunction has been identified as one of the factors contributing to this blemish.5,32 The current study provides the evidence that SMOC1 acts as an androgen regulated factor. Its deficiency is secondary to the defective androgen action and is associated with inguinoscrotal undescended testis. On the other hand, congenital mutation of SMOC1 gene may have cryptorchidism as one of the manifestations. Indeed, there are several reports that cryptorchidism in addition to the malformation of eyes and limbs,33,34,35 referred to as ophthalmo-acromelic syndrome or Waardenburg anophthalmia syndrome, have been found in boys with homozygous SMOC1 mutations.34,35,36 It is tempting to suggest that SMOC1 is likely one of the genetic factors associated with cryptorchidism in humans. Testicular descent is a very complex process involving genetic, hormonal, anatomical, and environmental factors.37 The dysfunction of one or more of these factors can directly or indirectly affect testicular descent and cause cryptorchidism. Therefore, a better understanding of the molecular basis of testicular descent is necessary to lead to diagnostic and therapeutic advancements.
In conclusion, our study highlights the roles of SMOC1 in modulating gubernacular cell proliferation and differentiation and reveals SMOC1 as an important downstream molecule of androgenic action on gubernacular development process during the transinguinal migration of the testis.
AUTHOR CONTRIBUTIONS
ZML, HLW, YS, ZYZ, and LYL conceived and designed the experiments. ZYZ, YS, XL, and LYL conducted the experiments and analyzed the data. HLW, ZYZ, YS, and ZML wrote and revised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
(a) Differential expression of androgen-responsive genes in LhcgrKO (KO) and wild-type (WT) mouse gubernaculum. (b) Heatmap for androgen-responsive genes in KO mice. Eight specific genes were differentially expressed in LhcgrKO mice compared with WT and following treatment with testosterone (T). LhcgrKO: luteinizing hormone/choriogonadotropin receptor knockout.
SMOC1 is highly conserved across multiple vertebrate animal species. SMOC1 amino acid sequences of mouse and rat share almost 99% identical and mouse and human SMOC1 amino acid sequences are 96% identical. Protein sequence similarity was calculated by ClustalW multiple alignment program. SMOC1: secreted protein acidic and rich in cysteine-related modular calcium binding 1.
JASPER program computationally predicts high probability of AR binding to an ARE in the promoter region of mouse Smoc1 gene. AR: androgen receptor; ARE: androgen response element.
Western blot assays indicated that mouse Smoc1siRNA significantly suppressed SMOC1 protein by about 70% in gubernacular cells. n = 3; **P < 0.01 compared to control. Smoc1: secreted protein acidic and rich in cysteine-related modular calcium binding 1; siRNA: short-interfering RNA; Cont siRNA: control siRNA.
ACKNOWLEDGMENTS
This work was supported in part by the Department of OB/GYN research funds (University of Louisville, Louisville, KY, USA) and Jilin Province Health Technology Capability Enhancement funds (No. 2022JC055). The authors are grateful to Ms. Robin J. Lawrence’s (University of Louisville) helpful advice in the preparation and editing of this manuscript.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Hutson JM, Balic A, Nation T, Southwell B. Cryptorchidism. Semin Pediatr Surg. 2010;19:215–24. doi: 10.1053/j.sempedsurg.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Hutson JM, Hasthorpe S, Heyns CF. Anatomical and functional aspects of testicular descent and cryptorchidism. Endocr Rev. 1997;18:259–80. doi: 10.1210/edrv.18.2.0298. [DOI] [PubMed] [Google Scholar]
- 3.Nef S, Parada LF. Cryptorchidism in mice mutant for Insl3. Nat Genet. 1999;22:295–9. doi: 10.1038/10364. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, et al. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol Endocrinol. 1999;13:681–91. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]
- 5.Rodprasert W, Virtanen HE, Makela JA, Toppari J. Hypogonadism and cryptorchidism. Front Endocrinol. 2020;10:906. doi: 10.3389/fendo.2019.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarila G, Hutson JM, Vikraman J. Testicular descent:a review of a complex, multistaged process to identify potential hidden causes of UDT. J Pediatr Surg. 2022;57:479–87. doi: 10.1016/j.jpedsurg.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Lei ZM, Mishra S, Zou W, Xu B, Foltz M, et al. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol. 2001;15:184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- 8.Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172–83. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- 9.Lei ZM, Mishra S, Ponnuru P, Li X, Yang ZW, et al. Testicular phenotype in luteinizing hormone receptor knockout animals and the effect of testosterone replacement therapy. Biol Reprod. 2004;71:1605–13. doi: 10.1095/biolreprod.104.031161. [DOI] [PubMed] [Google Scholar]
- 10.Zhang FP, Pakarainen T, Poutanen M, Toppari J, Huhtaniemi I. The low gonadotropin-independent constitutive production of testicular testosterone is sufficient to maintain spermatogenesis. Proc Natl Acad Sci U S A. 2003;100:13692–7. doi: 10.1073/pnas.2232815100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan FP, Lin DX, Rao CV, Lei ZM. Cryptorchidism in LhrKO animals and the effect of testosterone-replacement therapy. Hum Reprod. 2006;21:936–42. doi: 10.1093/humrep/dei433. [DOI] [PubMed] [Google Scholar]
- 12.Pakarainen T, Zhang FP, Makela S, Poutanen M, Huhtaniemi I. Testosterone replacement therapy induces spermatogenesis and partially restores fertility in luteinizing hormone receptor knockout mice. Endocrinology. 2005;146:596–606. doi: 10.1210/en.2004-0913. [DOI] [PubMed] [Google Scholar]
- 13.Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, et al. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988;240:327–30. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- 14.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, et al. The nuclear receptor superfamily:the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boockfor FR, Fullbright G, Bullesbach EE, Schwabe C. Relaxin-like factor (RLF) serum concentrations and gubernaculum RLF receptor display in relation to pre- and neonatal development of rats. Reproduction. 2001;122:899–906. doi: 10.1530/rep.0.1220899. [DOI] [PubMed] [Google Scholar]
- 16.Yuan FP, Li X, Lin J, Schwabe C, Bullesbach EE, et al. The role of RXFP2 in mediating androgen-induced inguinoscrotal testis descent in LH receptor knockout mice. Reproduction. 2010;139:759–69. doi: 10.1530/REP-09-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutson JM, Southwell BR, Li R, Lie G, Ismail K, et al. The regulation of testicular descent and the effects of cryptorchidism. Endocr Rev. 2013;34:725–52. doi: 10.1210/er.2012-1089. [DOI] [PubMed] [Google Scholar]
- 18.Kaftanovskaya EM, Huang Z, Barbara AM, De Gendt K, Verhoeven G, et al. Cryptorchidism in mice with an androgen receptor ablation in gubernaculum testis. Mol Endocrinol. 2012;26:598–607. doi: 10.1210/me.2011-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan JT, Robbins AK, Mateson AB, Sawamoto K, Tomatsu S, et al. Regional variation in androgen receptor expression and biomechanical properties may contribute to cryptorchidism susceptibility in the LE/orl rat. Front Endocrinol (Lausanne) 2018;9:738. doi: 10.3389/fendo.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vannahme C, Smyth N, Miosge N, Gosling S, Frie C, et al. Characterization of SMOC-1, a novel modular calcium-binding protein in basement membranes. J Biol Chem. 2002;277:37977–86. doi: 10.1074/jbc.M203830200. [DOI] [PubMed] [Google Scholar]
- 21.Abouzeid H, Boisset G, Favez T, Youssef M, Marzouk I, et al. Mutations in the SPARC-related modular calcium-binding protein 1 gene, SMOC1, cause Waardenburg anophthalmia syndrome. Am J Hum Genet. 2011;88:92–8. doi: 10.1016/j.ajhg.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradshaw AD. Diverse biological functions of the SPARC family of proteins. Int J Biochem Cell Biol. 2012;44:480–8. doi: 10.1016/j.biocel.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pazin DE, Albrecht KH. Developmental expression of Smoc1 and Smoc2 suggests potential roles in fetal gonad and reproductive tract differentiation. Dev Dyn. 2009;238:2877–90. doi: 10.1002/dvdy.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaeffer EM, Marchionni L, Huang Z, Simons B, Blackman A, et al. Androgen-induced programs for prostate epithelial growth and invasion arise in embryogenesis and are reactivated in cancer. Oncogene. 2008;27:7180–91. doi: 10.1038/onc.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Love HD, Booton SE, Boone BE, Breyer JP, Koyama T, et al. Androgen regulated genes in human prostate xenografts in mice:relation to BPH and prostate cancer. PLoS One. 2009;4:e8384. doi: 10.1371/journal.pone.0008384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grande G, Barrachina F, Soler-Ventura A, Jodar M, Mancini F, et al. The role of testosterone in spermatogenesis:lessons from proteome profiling of human spermatozoa in testosterone deficiency. Front Endocrinol. 2022;13:852661. doi: 10.3389/fendo.2022.852661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bornstein P, Sage EH. Matricellular proteins:extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–16. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 28.Gao Q, Mok HP, Zhuang J. Secreted modular calcium-binding proteins in pathophysiological processes and embryonic development. Chin Med J (Engl) 2019;132:2476–84. doi: 10.1097/CM9.0000000000000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–13. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKinnell IW, Ishibashi J, Le Grand F, Punch VG, Addicks GC, et al. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol. 2008;10:77–84. doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elder JS. Ultrasonography is unnecessary in evaluating boys with a nonpalpable testis. Pediatrics. 2002;110:748–51. doi: 10.1542/peds.110.4.748. [DOI] [PubMed] [Google Scholar]
- 32.Elamo HP, Virtanen HE, Toppari J. Genetics of cryptorchidism and testicular regression. Best Pract Res Cl En. 2022;36:101619. doi: 10.1016/j.beem.2022.101619. [DOI] [PubMed] [Google Scholar]
- 33.Okada I, Hamanoue H, Terada K, Tohma T, Megarbane A, et al. SMOC1 is essential for ocular and limb development in humans and mice. Am J Hum Genet. 2011;88:30–41. doi: 10.1016/j.ajhg.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rainger J, van Beusekom E, Ramsay JK, McKie L, Al-Gazali L, et al. Loss of the BMP antagonist, SMOC-1, causes ophthalmo-acromelic (Waardenburg anophthalmia) syndrome in humans and mice. PLoS Genet. 2011;7:e1002114. doi: 10.1371/journal.pgen.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamshidi J, Abdollahi S, Ghaedi H, Alehabib E, Tafakhori A, et al. A novel mutation in SMOC1 and variable phenotypic expression in two patients with Waardenburg anophthalmia syndrome. Eur J Med Genet. 2017;60:578–82. doi: 10.1016/j.ejmg.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Oehl-Jaschkowitz B, Vanakker OM, De Paepe A, Menten B, Martin T, et al. Deletions in 14q24.1q24.3 are associated with congenital heart defects, brachydactyly, and mild intellectual disability. Am J Med Genet A. 2014;164A:620–6. doi: 10.1002/ajmg.a.36321. [DOI] [PubMed] [Google Scholar]
- 37.Bay K, Main KM, Toppari J, Skakkebaek NE. Testicular descent:INSL3, testosterone, genes and the intrauterine milieu. Nat Rev Urol. 2011;8:187–96. doi: 10.1038/nrurol.2011.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Differential expression of androgen-responsive genes in LhcgrKO (KO) and wild-type (WT) mouse gubernaculum. (b) Heatmap for androgen-responsive genes in KO mice. Eight specific genes were differentially expressed in LhcgrKO mice compared with WT and following treatment with testosterone (T). LhcgrKO: luteinizing hormone/choriogonadotropin receptor knockout.
SMOC1 is highly conserved across multiple vertebrate animal species. SMOC1 amino acid sequences of mouse and rat share almost 99% identical and mouse and human SMOC1 amino acid sequences are 96% identical. Protein sequence similarity was calculated by ClustalW multiple alignment program. SMOC1: secreted protein acidic and rich in cysteine-related modular calcium binding 1.
JASPER program computationally predicts high probability of AR binding to an ARE in the promoter region of mouse Smoc1 gene. AR: androgen receptor; ARE: androgen response element.
Western blot assays indicated that mouse Smoc1siRNA significantly suppressed SMOC1 protein by about 70% in gubernacular cells. n = 3; **P < 0.01 compared to control. Smoc1: secreted protein acidic and rich in cysteine-related modular calcium binding 1; siRNA: short-interfering RNA; Cont siRNA: control siRNA.


