Abstract
Primary ciliary dyskinesia (PCD) is a clinically rare, genetically and phenotypically heterogeneous condition characterized by chronic respiratory tract infections, male infertility, tympanitis, and laterality abnormalities. PCD is typically resulted from variants in genes encoding assembly or structural proteins that are indispensable for the movement of motile cilia. Here, we identified a novel nonsense mutation, c.466G>T, in cilia- and flagella-associated protein 300 (CFAP300) resulting in a stop codon (p.Glu156*) through whole-exome sequencing (WES). The proband had a PCD phenotype with laterality defects and immotile sperm flagella displaying a combined loss of the inner dynein arm (IDA) and outer dynein arm (ODA). Bioinformatic programs predicted that the mutation is deleterious. Successful pregnancy was achieved through intracytoplasmic sperm injection (ICSI). Our results expand the spectrum of CFAP300 variants in PCD and provide reproductive guidance for infertile couples suffering from PCD caused by them.
Keywords: CFAP300 variant, male infertility, primary ciliary dyskinesia, sperm flagella, whole-exome sequencing
INTRODUCTION
Infertility affects almost 16% of couples of reproductive age worldwide.1,2,3 Previous studies have shown that approximately half of these cases are the result of male infertility.4,5 Asthenozoospermia (ASZ), which is characterized by poor sperm motility owing to defective function of sperm flagellar, is the most prevalent phenotype of primary male infertility.6,7 Sperm flagella, which are a type of eukaryotic motile cilia, share a similar axonemal structure with motile cilia.8 Thus, ASZ caused by abnormal axonemal structure is usually related to primary ciliary dyskinesia (PCD; Online Mendelian Inheritance in Man [OMIM]: 244 400).9
PCD is a rare X-linked or autosomal recessive disease arising from mutations in genes encoding structural or assembly proteins that are indispensable for the movement of motile cilia and has a prevalence of 1 in 2200 to 40 000 individuals.10,11 Patients who suffer from PCD may exhibit chronic respiratory tract infections, male infertility, and tympanitis.12,13 Nearly half of PCD patients may exhibit laterality defects, such as Kartagener’s syndrome, which is characterized by bronchiectasis, chronic sinusitis, and situs inversus.14 Motile cilia of the fallopian tubes, brain ependyma, and respiratory epithelium have a 9+2 axonemal core structure, which is characterized by a central pair of microtubules and nine surrounding peripheral doublet microtubules. Among the 9+2 axonemal core structure, the outer dynein arm (ODA) and inner dynein arm (IDA)15,16 and the dynein arm motor subunits provide the motility involved in ciliary beating.17 Currently, the best diagnostic test for PCD is transmission electron microscopy (TEM) of the ciliary ultrastructure combined with genetic diagnosis.18 Up to present, over 40 genes responsible for PCD have been identified.19 However, PCD with a genetic basis accounts for less than 70% of cases worldwide.20
The cilia- and flagella-associated protein 300 (CFAP300; ENSG00000137691; also known as C11orf70) gene is among the genes reported to be involved in PCD pathogenesis. Its main transcript (NM 032930.3) contains 7 exons and encodes a protein composed of 267 amino acids.8,21 Previous studies have demonstrated that CFAP300 performs chaperone-related functions during preassembly and subsequent traffic into the motile ciliary axoneme of dynein arm motors.22,23,24,25,26 In humans, approximately nine pathogenic CFAP300 variants have been reported.27 However, most studies have focused on the ciliary phenotype of respiratory symptoms, rather than male infertility and the sperm flagellar phenotype.
Here, through whole-exome sequencing (WES) and Sanger sequencing, we identified a novel variant in CFAP300 in a Chinese patient with PCD and infertility. In vitro analyses to determine the pathogenicity of the CFAP300 variant were performed. These results expand the spectrum of CFAP300 variants in PCD and provide fertility support for infertile couples suffering from PCD owing to the mutation in CFAP300.
PATIENTS AND METHODS
Patients
An infertile male patient from a consanguineous lineage visited Jinling Hospital (Nanjing, China) for fertility treatment in July 2023. Routine semen examination was performed according to the 5th edition of the World Health Organization (WHO) guidelines.28 In addition, peripheral whole blood samples were obtained from the proband and his family members for genetic analyses. This study was approved by the Ethics Committee of Jinling Hospital (Approval No. 2018NZKY-005-02). Permission to publish the data involved in the paper has been obtained. Each individual signed the informed consent.
TEM
Semen samples from healthy individuals with normal sperm parameters, hereafter referred to as controls. The proband were processed as follows: the samples were centrifuged at 800g for 10 min (Eppendorf, Hamburg, Germany) after being washed three times with 1× phosphate-buffered saline (PBS; C0221A; Beyotime, Shanghai, China) and fixed with 2.5% glutaraldehyde (pH 6.9) at 4°C for 2 h. Following the fixation process, the spermatozoa were postfixed for 2 h at 4°C with 1% osmium tetroxide, dyed for 2 h with 2% uranium acetate, and then dried using a graded series (50%, 70%, 90%, and 100%) of ethanol and 100% acetone. Afterward, the fixed spermatozoa were embedded in the epoxy resin EPON 812 (E8000; Head, Beijing, China) and then cut into 100-nm-thick ultrathin slices. Finally, the sections were stained with lead citrate and examined with a transmission electron microscope (Talos L120C G2; Thermo Fisher Scientific Waltham, MA, USA).
Immunofluorescence staining
We collected sperm from the proband and a normal control. Briefly,29 after being washed, the spermatozoa samples were fixed on slides with 4% paraformaldehyde. Then, the semen smears were permeabilized with 0.5% Triton X-100 and blocked with 1% bovine serum albumin (BSA; ST023-1000g; Beyotime). For immunofluorescence staining, semen smears were first incubated with first antibody anti-dynein axonemal light chain 1 (DNAL1; 1:100, A8267; ABclonal, Wuhan, China), fluorescein isothiocyanate (FITC)-conjugated anti-tubulin (1:100, ab64503; Abcam, Boston, MA, USA), and anti-dynein axonemal heavy chain 2 (DNAH2; 1:100, 18841-1-AP; Proteintech, Chicago, IL, USA) for 2.5 h at 37°C. The slides were subsequently incubated with secondary antibodies (A0468; Beyotime) for 1.5 h at 37°C. Finally, 4’,6-diamidino-2-phenylindole (DAPI) was used to stain the slides for 5 min. Images were acquired on a fluorescence microscope (IX73; Olympus Corporation, Tokyo, Japan).
WES and bioinformatic analysis
Peripheral blood samples were collected from the proband’s family members. Genomic DNA was extracted with a TIANamp Blood DNA Kit (TIANGEN, Beijing, China) according to the manufacturer’s protocols. WES was performed to screen and identify variants across the whole exome, and new rare CFAP300 variants were identified through comparison with the reference sequence Genome Reference Consortium Human Build 37 (GRCh37). The DNA of the proband was analyzed through WES using the Illumina HiSeq ×10 platform (Suzhou Basecare Medical Corporation Limited, Soochow, China).
The screening criteria for candidate genes were as follows: (1) a variant frequency less than 1% in the public databases (gnomAD, 1000 Genomes Project, and Exome Aggregation Consortium [ExAC]); (2) a homozygous variant that was predicted to be deleterious by Sorting Intolerant From Tolerant (SIFT), MutationTaster, and PolyPhen-2; and (3) a function related to cilia or flagella.
Sanger sequencing
The variant of CFAP300 was confirmed by Sanger sequencing using the appropriate primers (forward primer: 5´-GAATGTCAGTTCCTAATG-3´; reverse primer: 5´-ATAACCACTGAATGATGT-3´). Polymerase chain reaction (PCR) amplification was performed with Ex Taq DNA Polymerase (TAKARA, Kyoto, Japan), and bidirectional sequencing was subsequently performed by Sangon Biotech (Shanghai, China).
Ovarian stimulation and intracytoplasmic sperm injection (ICSI) procedures
In brief, the proband’s partner underwent a prolonged follicular phase protocol.30 Semen samples from the proband were obtained simultaneously through masturbation, and viable spermatozoa were selected for ICSI through hypo-osmotic swelling test. Individual viable sperm were injected into metaphase II oocytes. Then, the fertilized oocytes were cultured separately. Finally, two blastocyst stage embryos were transcervically transferred.
RESULTS
Clinical identification of PCD patient
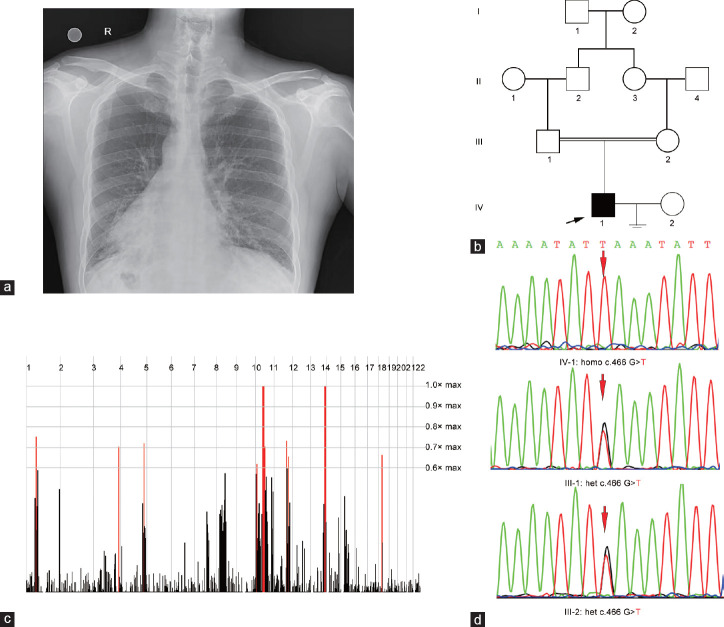
At the age of 33 years, the proband presented typical symptoms and signs of PCD, including chronic bronchiectasis, dextrocardia, and male infertility with completely immotile spermatozoa (0) and low sperm concentration (6.0%–21.9%). Chest radiography of the proband revealed increased lung markings and dextrocardia (Figure 1a). His parents were consanguineous, and his wife did not have PCD-related clinical features (Figure 1b). Thus, these results demonstrated that the proband suffers from PCD combined with male infertility.
Figure 1.
The clinic and genetic data of the proband. (a) Chest radiography of the proband revealed increased lung markings and dextrocardia. R: right. (b) Pedigree of the family affected with PCD and infertility. Squares: male family members; circles: female members; arrow: proband. (c) Homozygosity mapping of the family. Homozygous regions with the strongest signal are indicated in red. (d) Sanger sequencing confirmation of CFAP300 mutations in the proband and his parents. Homo: homozygous; het: heterozygous; PCD: primary ciliary dyskinesia; CFAP300: cilia- and flagella-associated protein 300.
Identification of the CFAP300 variant
WES of the proband was subsequently conducted, and a recessive inheritance model was used for homozygosity mapping through a homozygosity mapper due to his parental consanguinity (Figure 1c). Homozygous regions >5.0 Mb were considered candidate regions. Next, the PCD-related gene list combined with the screening data from the public database was used to filter candidate genes. We identified a novel homozygous mutation in CFAP300 (c.466G>T) resulting in a stop codon (p.Glu156*) that has not been recorded in gnomAD or other public databases, such as the ExAC, 1000G, and ClinVar databases (Table 1). Sanger sequencing confirmed the homozygous mutation in the proband. Moreover, the proband’s parents were heterozygous mutation carriers, which is consistent with the autosomal recessive mode of inheritance (Figure 1d). Thus, we inferred that the novel mutation in CFAP300 is the cause of PCD combined with male infertility in this patient.31
Table 1.
Detailed description of the bi-allelic mutations in cilia- and flagella-associated protein 300 identified in the proband
| Gene | Position | RefSeq ID | AA alteration | Mutation type | Status | 1000 Ga | gnomADa | ExACa | ClinVara | MutationTasterb | CADDb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CFAP300 | chr11:101946634 | NM_032930.3 | c.466G>T p.Glu156* | Nonsense | Homo | NA | NA | NA | NA | D | D |
aFrequency of corresponding mutations in 1000G, gnomAD, ExAC and ClinVar; bMutation assessment by MutationTaster and CADD. CFAP300: cilia- and flagella-associated protein 300; D: disease causing; homo: homozygous; NA: not available; AA: amino acid; gnomAD: The Genome Aggregation Database; ExAC: The Exome Aggregation Consortium; CADD: Combined Annotation Dependent Depletion
CFAP300 with a stop-gain mutation results in a lack of dynein arms in sperm flagella
Several CFAP300 variants have been identified in PCD patients (Figure 2a). The homozygous nonsense variant in this proband (c.466G>T) is located in exon 5 of the CFAP300 gene, and the Glu156 residue (Figure 2b) is located within the DUF4498 domain of the CFAP300 protein. To evaluate the pathogenicity of this mutation, multiple sequence alignment was performed, which indicated that the mutant nucleotide site in this patient is highly conserved among mammalian species (Figure 2c). MutationTaster predicted that this mutation is a deleterious mutation, and it has a CADD score of 41, indicating that the variant is among the top 0.1% of deleterious variants in the genome. SWISS-MODEL was used to predict the effect of the novel mutation on the CFAP300 protein structure (Figure 2d). The molecular weight of the normal CFAP300 protein is 30 kDa. The novel loss-of-function mutation, c.466G>T, in CFAP300 could result in a stop codon (p.Glu156*), which may truncate the protein or cause nonsense-mediated decay of the transcript. According to the in silico analysis, the identified stop-gain mutation in CFAP300 might be pathogenic.
Figure 2.
The analysis of the mutation in CFAP300. (a) The genomic structure of CFAP300 and the CFAP300 protein shows the locations of the identified disease variants in our and previous research.8,19,21,27 The only known conserved domain is a ‘‘domain of unknown function’’ (DUF4498). (b) The CFAP300 protein variant. (c) Alignment of multiple CFAP300 nucleotide sequences across species. (d) Structural changes in the CFAP300 protein based on SWISS-MODEL. CFAP300: cilia- and flagella-associated protein 300; AA: amino acid.
To confirm the functional effect of the identified stop-gain variant in CFAP300, we investigated ultrastructural defects in the proband’s spermatozoa using TEM. Consistent with previous reports on cilia, the sperm flagella lacked the IDA and ODA but exhibited a normal microtubule arrangement (Figure 3a). Furthermore, immunofluorescence staining of the ODA marker DNAL1 and the IDA marker DNAH2 confirmed that the proband’s sperm flagella lacked both arms (Figure 3b and 3c). Both the ODA and IDA, although absent from the sperm flagella, were found in the apical part of the cytoplasm, close to the base of the axoneme. Taken together, these data suggest that the novel CFAP300 variant affects the dynein arm assembly of sperm flagella.
Figure 3.
Morphological ultrastructure defects in CFAP300 mutant spermatozoa. (a) The main finding in TEM of sperm flagella cross sections in the proband was the loss (red asterisks) of both the outer (blue arrowheads) and inner (green arrowheads) dynein arms. (b) Immunostaining of the inner dynein arm marker DNAH2. (c) Immunostaining of the outer dynein arm marker DNAL1. Scar bars = 5 µm. CFAP300: cilia- and flagella-associated protein 300; DNAL1: dynein axonemal light chain 1; DNAH2: dynein axonemal heavy chain 2; TEM: transmission electron microscopy; DAPI: 4’,6-diamidino-2-phenylindole.
ICSI outcome
After the proband’s partner was screened for the CFAP300 variant, the couple selected ICSI as an infertility treatment. Thirty-six hours after human chorionic gonadotropin (hCG) injection, 19 oocytes were acquired. In one stimulated cycle, 16 metaphase II oocytes were fertilized. Through standard embryo culture, six blastocysts were formed and two were implanted (Table 2). After 2 weeks, the embryos were transferred into the partner, and a bigeminal pregnancy was confirmed via two hCG tests of peripheral blood. Finally, clinical pregnancy was confirmed by ultrasound according to the presence of two gestational sacs and heartbeats, 35 days after the transfer of embryos. As of the writing, the partner of the proband is still pregnant.
Table 2.
Clinical features of the partner undergoing intracytoplasmic sperm injection treatment
| Parameter | Value |
|---|---|
| Female age (year) | 33 |
| Length of primary infertility history (year) | 6 |
| BMI (kg m−2) | 20 |
| Basal hormones | |
| FSH (IU l−1) | 7.6 |
| LH (IU l−1) | 5.5 |
| E2 (pmol l−1) | 108 |
| Prog (nmol l−1) | 6.12 |
| Cycle 1 | |
| Protocol | Long |
| E2 level on the trigger day (pmol l−1) | 7976 |
| Number of follicles ≥14 mm on the trigger day (n) | 23 |
| Number of follicles ≥18 mm on the trigger day (n) | 5 |
| Number of oocytes retrieved (n) | 19 |
| ICSI progress | |
| MII (n) | 16 |
| Oocytes injected (n) | 16 |
| 2PN (n) | 9 |
| 8-cell formation (n) | 6 |
| Blastocyst formation (n) | 6 |
ICSI: intracytoplasmic sperm injection; BMI: body mass index; FSH: follicle-stimulating hormone; LH: luteinizing hormone; E2: estradiol; Prog: progesterone; MII: metaphase II; PN: pronucleus
DISCUSSION
In this study, we identified a novel germline mutation in CFAP300 through WES in a Chinese patient with parental consanguinity suffering from PCD and male infertility. A lack of dynein arms was the main defect observed in the immotile cilia of the PCD patient, as described in previous studies.8,19,21,32 A series of gene mutations, including those in dynein axonemal assembly factor 2 (DNAAF2), dynein axonemal assembly factor 4 (DNAAF4), dynein axonemal assembly factor 6 (DNAAF6), and Leucine-rich repeat containing protein 6 (LRRC6), have been reported to be associated with a lack of dynein arms in PCD patients.32 CFAP300 is a known PCD disease-causing gene involved in dynein arm preassembly in cilia, and previous studies have reported that patients with CFAP300 mutations exhibit a PCD phenotype characterized by immotile cilia with dual deficiency of the IDA and ODA and laterality defects beginning in early life.8,19,21,32,33,34 Previous studies have reported approximately 8 mutations in CFAP300 in PCD patients.8,19,27 In patients with compound heterozygous nonsense mutations at c.361C>T (p.Arg121*) and c.154C>T (p.Gln52*) and homozygous missense mutations at c.776A>G (p.His259Arg), ODA loss was found in 67%–95% of axonemal cross sections, and IDA loss was found in 75%–95% of axonemal cross sections.8 Dynein axonemal intermediate chain 2 (DNAI2), DNALI1, and dynein axonemal intermediate chain 1 (DNAI1) were reported to be absent in cilia in a patient with a homozygous nonsense mutation in CFAP300 (c.361C>T, p.Arg121*), and only ODA abnormalities were observed in cilia via TEM.32 According to TEM, the in-frame deletion c.98_106del (p.Arg33_Arg35del) found in six Greek-Cypriot patients was linked to IDA and ODA loss in the majority of cilia cross-sections, and the sperm had an incredibly rigid ciliary beat pattern.33 Variable ciliary beating was also reported for patients with the c.98_106del and c.198_200delinsCC frameshift variants.27,33 In this patient, the novel loss-of-function mutation (c.466G>T), which is located before a known pathogenic mutation site (c.776A>G)8 in CFAP300, is predicted to produce a truncated protein or undergo nonsense-mediated decay of the transcript. Loss of function of CFAP300 is a known pathogenic mechanism of PCD.8,21 Through TEM and immunofluorescence staining, we confirmed the lack of ODA and IDA in the sperm flagella of the proband. Combined with the prediction of the CFAP300 mutant protein structure and in silico analysis, we speculated that the identified stop-gain mutation in CFAP300 is pathogenic.
Male infertility in PCD patients is rarely diagnosed until adulthood, and most studies have focused on the ciliary phenotype and respiratory symptoms, ignoring male infertility and the sperm flagellar phenotype in patients with PCD caused by CFAP300 variants.8,19,27 To date, only a few PCD-related genes, such as dynein regulatory complex subunit 5 (DRC5), dynein regulatory complex subunit 1 (DRC1), radial spoke head component 4A (RSPH4A), and LRRC6,35,36,37,38,39 have been demonstrated to be associated with human male infertility. In our study, the proband with the CFAP300 variant exhibited male infertility, and his spermatozoa were completely immotile and lacked the IDA and ODA, which is consistent with defects in airway cilia.27 Thus, CFAP300 in spermatozoa could perform chaperone-related functions during the preassembly of dynein arm motors, as in cilia.22 In this study, the nonsense mutation of CFAP300 might result in a truncated protein without protein domains that are likely critical for its interaction with DNAL1 and DNAH2, which are essential for the formation of IDA and ODA complexes and functional cilia and flagella motors. In airway cilia, correct polarization of basal bodies is a prerequisite for ciliary motility.40 CFAP298, another factor involved in dynein arm preassembly, has been reported to play a more direct role in basal body polarization.41 Further studies are needed to explore the specific role of CFAP300 in the preassembly of and subsequent trafficking in sperm flagella. Our study identified a novel mutation in a PCD-related infertility gene and revealed the possibility of undiagnosed infertility in PCD patients.
The usual method of treating male asthenozoospermia is ICSI.42 ICSI with ejaculated spermatozoa (EJ-ICSI) and ICSI utilizing spermatozoa extracted from the testis (TESE-ICSI) are effective without negatively impacting fetal development. Several successful cases of ICSI use to overcome PCD-related infertility have been reported, and the proband’s partner has achieved a successful pregnancy after ICSI.43,44 In this study, the couple chose EJ-ICSI, and a successful pregnancy was achieved. Thus, ICSI is a useful method for treating male infertility resulting from CFAP300 mutations and may provide guidance for treating PCD-related male infertility in the future.
In our study, a novel mutation in CFAP300 was identified as a cause of PCD combined with ODA and IDA loss, situs inversus, and male infertility. Our research would have important clinical implications for improving our understanding of the genetic basis of this disease and counseling affected families. Therefore, genetic diagnosis is an acceptable method that can be used to assist in the diagnosis of PCD in men with infertility, allowing timely and effective treatment to be provided.
AUTHOR CONTRIBUTIONS
ZZ, QQ, BY, JZM, and LC designed the research. ZZ, BY, QQ, and JZM performed the research. WHW, JD, YMF, and ZCZ contributed new reagents and analytic tools. ZZ, QQ JZM, and BY analyzed data. ZZ, QQ, and LC wrote the paper. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We are grateful to all the patients and families participating in this study. This work was supported by the China Postdoctoral Science Foundation Grant (2023M734294), Jiangsu Provincial Medical Key Discipline Cultivation Unit (JSDW202215), and the National Natural Science Foundation of China (No. 82001618).
REFERENCES
- 1.Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, et al. Male infertility. Lancet. 2021;397:319–33. doi: 10.1016/S0140-6736(20)32667-2. [DOI] [PubMed] [Google Scholar]
- 2.Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol. 2018;15:369–84. doi: 10.1038/s41585-018-0003-3. [DOI] [PubMed] [Google Scholar]
- 3.Krausz C, Riera-Escamilla A, Moreno-Mendoza D, Holleman K, Cioppi F, et al. Genetic dissection of spermatogenic arrest through exome analysis:clinical implications for the management of azoospermic men. Genet Med. 2020;22:1956–66. doi: 10.1038/s41436-020-0907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colpi GM, Francavilla S, Haidl G, Link K, Behre HM, et al. European Academy of Andrology guideline management of oligo-astheno-teratozoospermia. Andrology. 2018;6:513–24. doi: 10.1111/andr.12502. [DOI] [PubMed] [Google Scholar]
- 5.Krausz C. Male infertility:pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab. 2011;25:271–85. doi: 10.1016/j.beem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Punab M, Poolamets O, Paju P, Vihljajev V, Pomm K, et al. Causes of male infertility:a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum Reprod. 2017;32:18–31. doi: 10.1093/humrep/dew284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephen EH, Chandra A. Declining estimates of infertility in the United States:1982-2002. Fertil Steril. 2006;86:516–23. doi: 10.1016/j.fertnstert.2006.02.129. [DOI] [PubMed] [Google Scholar]
- 8.Fassad MR, Shoemark A, Le Borgne P, Koll F, Patel M, et al. C11orf70 mutations disrupting the intraflagellar transport-dependent assembly of multiple axonemal dyneins cause primary ciliary dyskinesia. Am J Hum Genet. 2018;102:956–72. doi: 10.1016/j.ajhg.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutton C, Escoffier J, Martinez G, Arnoult C, Ray PF. Teratozoospermia:spotlight on the main genetic actors in the human. Hum Reprod Update. 2015;21:455–85. doi: 10.1093/humupd/dmv020. [DOI] [PubMed] [Google Scholar]
- 10.Kuehni CE, Frischer T, Strippoli MP, Maurer E, Bush A, et al. Factors influencing age at diagnosis of primary ciliary dyskinesia in European children. Eur Respir J. 2010;36:1248–58. doi: 10.1183/09031936.00001010. [DOI] [PubMed] [Google Scholar]
- 11.Lucas JS, Burgess A, Mitchison HM, Moya E, Williamson M, et al. Diagnosis and management of primary ciliary dyskinesia. Arch Dis Child. 2014;99:850–6. doi: 10.1136/archdischild-2013-304831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frija-Masson J, Bassinet L, Honoré I, Dufeu N, Housset B, et al. Clinical characteristics, functional respiratory decline and follow-up in adult patients with primary ciliary dyskinesia. Thorax. 2017;72:154–60. doi: 10.1136/thoraxjnl-2015-207891. [DOI] [PubMed] [Google Scholar]
- 13.Davis SD, Ferkol TW, Rosenfeld M, Lee HS, Dell SD, et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med. 2015;191:316–24. doi: 10.1164/rccm.201409-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang D, Liu BC, Luo J, Huang TX, Liu CT. Kartagener syndrome. QJM. 2019;112:297–8. doi: 10.1093/qjmed/hcy242. [DOI] [PubMed] [Google Scholar]
- 15.Satir P, Christensen ST. Structure and function of mammalian cilia. Histochem Cell Biol. 2008;129:687–93. doi: 10.1007/s00418-008-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milla CE. The evolving spectrum of ciliopathies and respiratory disease. Curr Opin Pediatr. 2016;28:339–47. doi: 10.1097/MOP.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pennekamp P, Menchen T, Dworniczak B, Hamada H. Situs inversus and ciliary abnormalities:20 years later, what is the connection? Cilia. 2015;4:1. doi: 10.1186/s13630-014-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro AJ, Davis SD, Polineni D, Manion M, Rosenfeld M, et al. Diagnosis of primary ciliary dyskinesia. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2018;197:e24–39. doi: 10.1164/rccm.201805-0819ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zietkiewicz E, Bukowy-Bieryllo Z, Rabiasz A, Daca-Roszak P, Wojda A, et al. CFAP300:mutations in slavic patients with primary ciliary dyskinesia and a role in ciliary dynein arms trafficking. Am J Respir Cell Mol Biol. 2019;61:440–9. doi: 10.1165/rcmb.2018-0260OC. [DOI] [PubMed] [Google Scholar]
- 20.Mirra V, Werner C, Santamaria F. Primary ciliary dyskinesia:an update on clinical aspects, genetics, diagnosis, and future treatment strategies. Front Pediatr. 2017;5:135. doi: 10.3389/fped.2017.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoben IM, Hjeij R, Olbrich H, Dougherty GW, Nothe-Menchen T, et al. Mutations in C11orf70 cause primary ciliary dyskinesia with randomization of left/right body asymmetry due to defects of outer and inner dynein arms. Am J Hum Genet. 2018;102:973–84. doi: 10.1016/j.ajhg.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horani A, Ustione A, Huang T, Firth AL, Pan J, et al. Establishment of the early cilia preassembly protein complex during motile ciliogenesis. Proc Natl Acad Sci U S A. 2018;115:E1221–8. doi: 10.1073/pnas.1715915115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchison HM, Schmidts M, Loges NT, Freshour J, Dritsoula A, et al. Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat Genet. 2012;44:381–9. doi: 10.1038/ng.1106. S1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olcese C, Patel MP, Shoemark A, Kiviluoto S, Legendre M, et al. X-linked primary ciliary dyskinesia due to mutations in the cytoplasmic axonemal dynein assembly factor PIH1D3. Nat Commun. 2017;8:14279. doi: 10.1038/ncomms14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowkes ME, Mitchell DR. The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. Mol Biol Cell. 1998;9:2337–47. doi: 10.1091/mbc.9.9.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King SM. Axonemal dynein arms. Cold Spring Harb Perspect Biol. 2016;8:a028100. doi: 10.1101/cshperspect.a028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultz R, Elenius V, Fassad MR, Freke G, Rogers A, et al. CFAP300 mutation causing primary ciliary dyskinesia in Finland. Front Genet. 2022;13:985227. doi: 10.3389/fgene.2022.985227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 29.Shen Y, Zhang F, Li F, Jiang X, Yang Y, et al. Loss-of-function mutations in QRICH2 cause male infertility with multiple morphological abnormalities of the sperm flagella. Nat Commun. 2019;10:433. doi: 10.1038/s41467-018-08182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lainas TG, Sfontouris IA, Zorzovilis IZ, Petsas GK, Lainas GT, et al. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF:a prospective randomised controlled trial (RCT) Hum Reprod. 2010;25:683–9. doi: 10.1093/humrep/dep436. [DOI] [PubMed] [Google Scholar]
- 31.Richards S, Aziz N, Bale S, Bick D, Das S, et al. Standards and guidelines for the interpretation of sequence variants:a joint consensus recommendation of the American College of Medical Genetics and genomics and the association for molecular pathology. Genet Med. 2015;17:405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aprea I, Raidt J, Hoben IM, Loges NT, Nothe-Menchen T, et al. Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility. PLoS Genet. 2021;17:e1009306. doi: 10.1371/journal.pgen.1009306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yiallouros PK, Kouis P, Kyriacou K, Evriviadou A, Anagnostopoulou P, et al. Implementation of multigene panel NGS diagnosis in the national primary ciliary dyskinesia cohort of Cyprus:an island with a high disease prevalence. Hum Mutat. 2021;42:e62–77. doi: 10.1002/humu.24196. [DOI] [PubMed] [Google Scholar]
- 34.Bolkier Y, Barel O, Marek-Yagel D, Atias-Varon D, Kagan M, et al. Whole-exome sequencing reveals a monogenic cause in 56% of individuals with laterality disorders and associated congenital heart defects. J Med Genet. 2022;59:691–6. doi: 10.1136/jmedgenet-2021-107775. [DOI] [PubMed] [Google Scholar]
- 35.Pereira R, Carvalho V, Dias C, Barbosa T, Oliveira J, et al. Characterization of a DRC1 null variant associated with primary ciliary dyskinesia and female infertility. J Assist Reprod Genet. 2023;40:765–78. doi: 10.1007/s10815-023-02755-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou S, Wu H, Zhang J, He X, Liu S, et al. Bi-allelic variants in human TCTE1/DRC5 cause asthenospermia and male infertility. Eur J Hum Genet. 2022;30:721–9. doi: 10.1038/s41431-022-01095-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Wang R, Yang D, Lu C, Xu Y, et al. Novel RSPH4A variants associated with primary ciliary dyskinesia-related infertility in three Chinese families. Front Genet. 2022;13:922287. doi: 10.3389/fgene.2022.922287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Li Y, Wang Y, Meng L, Tan C, et al. Identification of novel biallelic LRRC6 variants in male Chinese patients with primary ciliary dyskinesia and infertility. J Assist Reprod Genet. 2023;40:41–51. doi: 10.1007/s10815-022-02681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Jiang C, Zhang X, Liu M, Sun Y, et al. The effect of a novel LRRC6 mutation on the flagellar ultrastructure in a primary ciliary dyskinesia patient. J Assist Reprod Genet. 2021;38:689–96. doi: 10.1007/s10815-020-02036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- 41.Jaffe KM, Grimes DT, Schottenfeld-Roames J, Werner ME, Ku TS, et al. c21orf59/kurly controls both cilia motility and polarization. Cell Rep. 2016;14:1841–9. doi: 10.1016/j.celrep.2016.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLachlan RI, Ishikawa T, Osianlis T, Robinson P, Merriner DJ, et al. Normal live birth after testicular sperm extraction and intracytoplasmic sperm injection in variant primary ciliary dyskinesia with completely immotile sperm and structurally abnormal sperm tails. Fertil Steril. 2012;97:313–8. doi: 10.1016/j.fertnstert.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Tu C, Nie H, Meng L, Li D, et al. Novel DNAAF6 variants identified by whole-exome sequencing cause male infertility and primary ciliary dyskinesia. J Assist Reprod Genet. 2020;37:811–20. doi: 10.1007/s10815-020-01735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozkavukcu S, Celik-Ozenci C, Konuk E, Atabekoglu C. Live birth after laser assisted viability assessment (LAVA) to detect pentoxifylline resistant ejaculated immotile spermatozoa during ICSI in a couple with male Kartagener's syndrome. Reprod Biol Endocrinol. 2018;16:10. doi: 10.1186/s12958-018-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]