Abstract
Depression currently affects about 280 million people worldwide and its prevalence has been increasing dramatically, especially among the young and people of reproductive age, which consequently leads to an increase in antidepressant consumption. Antidepressants are associated with sexual dysfunction in both men and women; however, their role in male fertility has been scarcely studied. Fluoxetine and sertraline, two serotonin reuptake inhibitors (SSRIs), are among the most prescribed antidepressants worldwide. To determine their possible effects, human sperm cells were exposed to either sertraline or fluoxetine at concentrations previously found in blood and seminal fluid of patients undergoing treatment. Spermatozoa were incubated for up to 24 h at 37°C and 5% CO2, and important functional parameters such as sperm motility, viability, mitochondrial membrane potential, cellular reactive oxygen species (ROS) production, chromatin/DNA integrity, acrosome status, and tyrosine phosphorylation were assessed. At low levels, fluoxetine consistently decreased progressive motility throughout time while promoting fluctuations in ROS levels and sperm capacitation. Nevertheless, it did not affect viability, mitochondrial membrane potential, acrosome reaction nor chromatin/DNA integrity. Sertraline, on the other hand, had little to nonsignificant impact at low doses, but affected almost all tested parameters at supratherapeutic concentrations. Altogether, our results suggest that both antidepressants may impair sperm function, possibly through different mechanisms of action, but fluoxetine is the only exhibiting mild negative effects at doses found in vivo.
Keywords: fluoxetine, human sperm, male fertility, sertraline, sperm function, SSRIs
INTRODUCTION
Depression is currently the leading cause of disability worldwide, affecting about 280 million people. This disease usually results from an interaction between social, psychological, and biological factors and its effects are typically long lasting, affecting an individual’s daily life.1 Worryingly, its prevalence has been increasing, especially among the young and people of reproductive age. This increase, which boosted worldwide due to the coronavirus disease 2019 (COVID-19) pandemic, invariably leads to an increase in antidepressant (AD) consumption. Furthermore, while ADs are generally prescribed to treat long-term depression, they can also be used to treat disorders, such as anxiety, further increasing the number of people who take this type of medication over long periods of time.
Despite being divided into six major classes, serotonin reuptake inhibitors (SSRIs) are the most widely prescribed antidepressants worldwide. They are known to inhibit serotonin (5-HT) reuptake by blocking its transporter (5-HTT) and consequently increase extracellular serotonin levels.2,3 Even though ADs are widely known to cause some degree of sexual dysfunction in both men and women, their specific role in male fertility has been poorly studied and is mostly based on animal studies.2,4 In humans, SSRIs have been associated with harmful effects on sperm quality and increased oxidative stress, yet only a few functional parameters were assessed.2 In addition, since several serotonergic markers such as 5-HT receptors, 5-HTT and tryptophan hydroxylase and monoamine oxidase (responsible for 5-HT synthesis and degradation, respectively) were already found in human sperm, we can believe that serotonin may play a role in its physiology and, therefore, that SSRIs may impact sperm function.3
Considering the increasing number of people at reproductive age who (will) intend to father a child when taking antidepressants, as well as the fact that people facing infertility are at greater risk of experiencing depression symptoms,5,6,7,8,9,10,11 there is a great need for more controlled studies that focus on the effects of antidepressants, particularly SSRIs, on sperm functionality. Furthermore, spermatozoa can be directly exposed to antidepressants throughout their journey toward the oocyte, while within the male and female reproductive tracts,12,13 thus increasing their likelihood of being exposed to these drugs, consequently, being affected even after spermatogenesis. With that in mind, the present study aimed at determining the effects of two of the most consumed SSRIs, sertraline (SERT) and fluoxetine (FLU), on human sperm function at relevant in vivo concentrations, using a well-controlled environment (in vitro exposure) to avoid that any confounding factors may mask the results observed.
MATERIALS AND METHODS
Chemicals/reagents
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise. While FLU (#F132) was reconstituted in distilled water, SERT (#S6319) was dissolved in dimethyl sulfoxide (DMSO). Final stock concentrations of 1 mmol l−1 (FLU) and 2 mmol l−1 (SERT) were obtained.
Human samples
Human sperm samples were obtained from men undergoing routine semen analysis or assisted reproductive techniques (ART) at the Reproductive Medicine Unit of Coimbra Hospital and University Centre (CHUC; Coimbra, Portugal) and were used in accordance with the appropriate ethical and Internal Review Board guidelines. This study was approved by the Ethical Committee of CHUC in July 2021 (Approval No. OBS.SF.72-2021). Each patient was duly informed about the study and signed an informed consent, allowing the remainder of their sample, which would normally be discarded, to be used in the study. Semen samples were obtained by masturbation after 3–5 days of sexual abstinence. Only normozoospermic samples, i.e., the ones whose sperm concentration, motility, and morphology are considered normal according to the World Health Organization (WHO) criteria (≥15 × 106 sperm per ml, ≥32% of progressive motility or ≥40% of total motility [progressive + in situ], and ≥4% normal forms)14, were used (n = 10–20).
A total of 5 × 106 sperm cells were incubated with 100 nmol l−1–50 µmol l−1 FLU or SERT in a phosphate-buffered saline (PBS; Oxoid, Basingstoke Hampshire, UK)-supplemented medium at 37°C and 5% CO2. The PBS-supplemented medium contained 0.5 mmol l−1 MgCl2, 0.9 mmol l−1 CaCl2, 5.0 mmol l−1 D-glucose, 1.0 mmol l−1 Na-pyruvate, 10.0 mmol l−1 (v/v) Na-lactate, 25.0 mmol l−1 NaHCO3, 0.3% (w/v) bovine serum albumin (BSA) and 1.0% (v/v) Pen/Strep (7.2 ≤ pH ≤ 7.4; Gibco, Thermo Fisher Scientific, Winsford, UK). Effects were determined following immediate exposure (0 h), 3 h, and 24 h. In this study, the concentrations were chosen based on values found in the literature regarding FLU levels in the blood (387.94–1.62 μmol l−1) and seminal fluid (23.28–96.98 nmol l−1),12,15 as well as the 10 μmol l−1 concentration used in a previous study.13 For SERT, the available literature is scarcer, and therefore, the same concentrations were applied.13,16 The suprapharmacological 50 μmol l−1 dose was chosen as a massive positive control for both drugs. PBS-supplemented medium alone was used as a negative control for FLU, and for SERT, a solution containing 0.6% DMSO in the same medium was used. DMSO concentration was kept at 0.6% in all experiments to avoid any cell toxicity.17
Sperm motility
Sperm motility was evaluated following the WHO guidelines.14 After thoroughly mixing the sample, a wet preparation was prepared by placing a 10 μl drop on a slide. When the sample stopped drifting (within 60 s), the slide was examined by phase-contrast microscopy (200×–400× magnification; Nikon Eclipse E200; Nikon Instruments Inc., Melville, NY, USA) and at least 100 sperm cells were counted per condition and time point. Each spermatozoon was categorized according to its type of motility: progressive (when moving actively, either linearly or in a large circle), nonprogressive (when motile but without progression), or immotile (no movement at all). The results were presented as the percentage of progressive sperm and percentage of total motile (progressive + in situ) sperm, relative to the control (n = 13 for FLU; n = 11 for SERT).
Sperm viability and mitochondrial membrane potential (MMP)
Sperm viability and MMP were assessed simultaneously using the LIVE/DEAD sperm Viability kit (#L7011; Thermo Fisher Scientific) and the 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimi-dazolycarbocyanine iodide (JC-1) fluorescent probe (#T3168; Thermo Fisher Scientific), respectively, as described elsewhere.18 Briefly, sperm cells were incubated with 100 nmol l−1 SYBR14 and 240 nmol l−1 propidium iodide (PI), coupled with 2 μmol l−1 JC-1 for 20 min at 37°C and 5% CO2, in the dark. While SYBR14 is a cell membrane-permeant fluorescent dye that stains sperm nuclei green, PI only stains nuclei with compromised membrane integrity (red), overpowering the SYBR14 signal. On the other hand, JC-1 enters the mitochondria and, when MMP is high, accumulates in the matrix and forms aggregates that emit red fluorescence. In contrast, when the potential is low, it remains in a monomeric form and exhibits green fluorescence. For each condition and time point, 100 sperm cells were counted using a fluorescence microscope (630×–1000× magnification; DM4000B; Leica, Wetzlar, Germany). For viability, cells were classified according to the staining of the head (green or red) and MMP was evaluated considering the staining of the midpiece (green or reddish). The results were presented as the percentage of live (green) cells in relation to control for viability and as percentage of live sperm with high MMP relative to the respective control for MMP assessment (n = 13 for FLU; n = 11 for SERT). Representative images are found in Supplementary Figure 1 (56.5KB, tif) .
Cellular reactive oxygen species (ROS) production
To evaluate cellular ROS production in sperm after exposure to FLU or SERT, the dihydroethidium (DHE; #D1168; Thermo Fisher Scientific) fluorescent probe was used, as stated by Escada-Rebelo and Ramalho-Santos19 with minor alterations. This probe is commonly used to assess intracellular generation of superoxide (O2․–) in the cytosol. When oxidized by O2․–, DHE will intercalate with the cell’s DNA and exhibit a bright red fluorescence. Briefly, spermatozoa from each condition were firstly incubated with 2 μmol l−1 DHE and 100 nmol l−1 SYBR14 (to counterstain sperm nuclei green), for 15 min at 37°C, and then centrifuged for 5 min (300g; ScanSpeed 1730R; Labogene, Allerød, Denmark). After discarding the majority of the supernatant, the pellet was resuspended in the remaining volume and a 10 μl drop was placed on a glass slide under a coverslip. At least 100 spermatozoa were counted per condition and assay using a fluorescence microscope (630×–1000× magnification; DM4000B; Leica) and sperm were categorized according to the presence of red staining in the head, indicative of elevated cellular ROS production. The results were presented as the percentage of sperm with elevated ROS (n = 15 for FLU and SERT each).
Chromatin/DNA integrity
Chromatin/DNA integrity was assessed using a modified version of the Diff-Quik® rapid staining kit (Dade Behring Inc., Newark, NJ, USA), which is typically used to evaluate sperm morphology but can be applied to measure chromatin integrity in a straightforward and reproducible manner as described elsewhere.20 Briefly, a smear was prepared using 10 μl of the sample and allowed to air dry. Slides were then sequentially dipped in methanol, eosin, and thiazine solutions (10–20 s in each), rinsed in water, and left to air dry. Finally, the definitive preparations were assembled by gluing a coverslip to the slide using the Eukitt® Quick-Hardening mounting medium for microscopy. Slides were examined under a bright-field microscope (1000× magnification; Nikon Eclipse E200; Nikon Instruments Inc.) and at least 100 spermatozoa were counted per condition and assay. Based on the premise that thiazines are DNA-binding dyes and that a decrease in sperm chromatin compaction or increased DNA breaks can increase the number of dye-binding sites, sperm cells classified with normal/condensed chromatin present lightly stained nuclei, whereas sperm with decondensed chromatin/damaged DNA are darkly stained. The results were presented as the percentage of light-colored sperm, representative of chromatin/DNA integrity (n = 20 for both FLU and SERT).
Capacitation and acrosomal status
Protein phosphorylation, one of the physiological and functional changes involved in sperm capacitation, was evaluated through the detection of phosphotyrosine (PY) residues by immunocytochemistry using a rabbit anti-human phosphotyrosine polyclonal antibody, as described elsewhere.18,21 First, spermatozoa were fixed (2% [v/v] formaldehyde in PBS; 40 min), permeabilized (1% [v/v] Triton X-100 in PBS; 20 min), and blocked (0.1% [w/v] BSA and 100 mmol l−1 glycine in PBS; at least 30 min). Next, the cells were incubated with a rabbit anti-human phosphotyrosine polyclonal antibody (1:10; #61-5800; Thermo Fisher Scientific) overnight at 37°C and posteriorly washed with 0.1% Triton X-100 in PBS for 30 min. Spermatozoa were then incubated with an anti-rabbit secondary antibody (Texas Red®-X Goat Anti-Rabbit IgG; 1:200; Molecular Probes, Eugene, OR, USA) for 1 h at 37°C followed by a new 15-min wash with 0.1% Triton X-100 in PBS. Finally, the cells were mounted on glass slides with Vectashield® Mounting Media containing the DNA-binding dye 4’,6-diamino-2-phenyl-indole (DAPI; #H1200; Vector Labs, Newark, CA, USA) that counterstains the DNA blue. Sperm were considered capacitated when they exhibited red fluorescent staining, regardless of their homogeneous or spotted appearance.
To evaluate acrosome integrity, the Pisum sativum agglutinin conjugated with fluorescein isothiocyanate (PSA-FITC),18,21 a marker that binds to the acrosomal content of human spermatozoa, was used. Sperm acrosome was considered intact when the entire acrosomal region exhibited a homogeneous fluorescent green staining and considered reacted when there was no fluorescent signal in this region, when the signal presented heterogeneous staining, or when only a band was observed in the equatorial zone of the sperm head. After the sperm cells were fixed, permeabilized, and blocked as described in the capacitation protocol, they were incubated with PSA-FITC (1:200) for 1 h at 37°C, washed with 0.1% (v/v) Triton X-100 in PBS for 15 min, and finally mounted with Vectashield® Mounting Media with DAPI (Vector Labs) as described above.
For each of the assays, at least 100 spermatozoa were counted using a fluorescence microscope (630×–1000× magnification; DM4000B; Leica). The results were presented as the percentage of capacitated sperm and percentage of intact acrosomes (n = 10 for both FLU and SERT). Representative images are found in Supplementary Figures 2 (47KB, tif) and 3 (73.8KB, tif) .
Statistical analyses
Statistical analysis was carried out using the IBM® SPSS® software version 26.0 for Windows (SPSS Inc., Chicago, IL, USA). Sample sizes for sperm experiments were estimated by power analysis using a power value of at least 80% to detect a 10% difference between groups, a significance below 0.05 (P < 0.05) and 95% confidence intervals as criteria. The results were expressed as median and interquartile range, and all variables were checked for normal distribution using the Shapiro–Wilk test. Within each incubation time, comparisons between the control and each concentration were carried out either by the independent t-test for normal variables or the Mann–Whitney U test for nonnormal variables. P < 0.05 was considered statistically significant.
RESULTS
Motility and viability
Motility is key for spermatozoa to naturally fertilize an oocyte in vivo. A significant decrease in progressive motility was found with increasing concentrations of FLU (all P < 0.05; Figure 1a); yet, this decrease was not observed in total motility (progressive + in situ), at least at the lower concentrations (P > 0.05; Figure 1b), suggesting that this antidepressant does not immobilize spermatozoa but renders them nonprogressive. At high doses (10 μmol l−1 and 50 μmol l−1), however, the effect was massive and clearly detected by the significant decrease in total motility, particularly at 50 μmol l−1 (P < 0.01 at 0 h, P < 0.01 at 3 h, and P < 0.001 at 24 h; Figure 1b). For SERT, the results showed a less noticeable effect on progressive motility. In this case, a decrease was observed at all time points when compared to the DMSO control, but only significantly at the higher concentrations (P < 0.05 for 10 μmol l−1 and P < 0.001 for 50 μmol l−1; Figure 1d). Total motility was only affected at 50 μmol l−1, our positive control (P < 0.01 at 0 h and P < 0.001 upon 3 h and 24 h; Figure 1e), and its decrease was concomitant with the decrease in viability (P < 0.01 at 0 h and P < 0.001 upon 3 h and 24 h; Figure 1f).
Figure 1.
Effects of fluoxetine (FLU) on sperm (a) progressive motility, (b) total motility and (c) viability. Sertraline (SERT) effects on sperm (d) progressive and (e) total motility, and (f) viability. Sperm cells were either exposed to FLU or SERT for up to 24 h at 37°C and 5% CO2 (FLU: n = 13; SERT: n = 11). Results are expressed as median and interquartile range in relation to control. *P < 0.05, **P < 0.01, and ***P < 0.001 symbolize significant differences when compared to the control group.
Interestingly, both compounds only caused a decrease in sperm viability at 50 μmol l−1, suggesting that they are not cytotoxic at lower levels (Figure 1c and 1f).
MMP
MMP, often used as an indicator of mitochondrial function, was found reduced by FLU but only following 24 h of exposure at 10 μmol l−1 (P < 0.05) and 50 μmol l−1 concentrations (P = 0.001; Figure 2a). For this higher concentration, a trend could also be observed after 3 h of incubation, suggesting that the mitochondrial function may have started to become impaired at this time point. In contrast, SERT led to a decrease in MMP at all time points but, again, mainly at the highest dose (50 μmol l−1) tested (P < 0.01 at 0 h and P < 0.001 at 3 h and 24 h; Figure 2b).
Figure 2.
(a) Fluoxetine (FLU) and (b) sertraline (SERT) effects on high MMP. Sperm cells were either exposed to FLU or SERT for up to 24 h at 37°C and 5% CO2 (FLU: n = 13; SERT: n = 11). Results are expressed as median and interquartile range in relation to control. *P < 0.05, **P < 0.01, and ***P < 0.001 symbolize significant differences when compared to the control group; #0.05<P<0.07 symbolizes a statistical trend when compared to the control group. MMP: mitochondrial membrane potential.
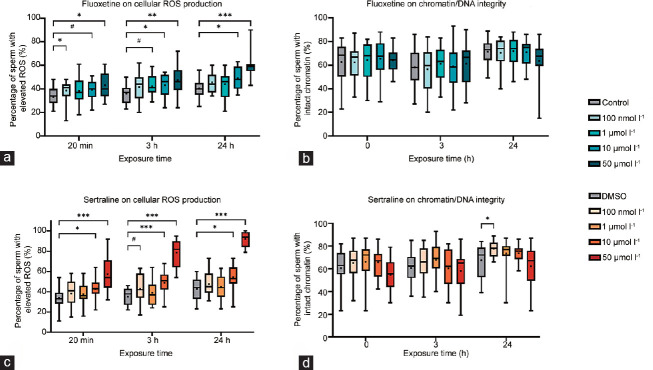
Cellular ROS production and chromatin integrity
Spermatozoa are particularly susceptible to ROS damage which, among other effects, can lead to a decrease in motility and an increase in DNA damage. To ascertain if our previous results could be attributed to an increase in cellular ROS production and if chromatin/DNA integrity could possibly be affected, we decided to evaluate both parameters.
Our results show that FLU only led to a consistent and significant increase in cellular ROS at the 10 μmol l−1 (P < 0.05 at 3 h and 24 h) and 50 μmol l−1 (P < 0.05 at 0 h, P < 0.01 at 3 h, and P < 0.001 at 24 h) concentrations. This AD also led to an increase in this parameter at the lowest concentration (100 nmol l−1; P < 0.05) immediately after exposure and potentially at 1 μmol l−1 after a 3-h exposure (Figure 3a), but those differences were further lost and may have little biological relevance. SERT showed similar results, affecting ROS production mainly at 10 μmol l−1 (P < 0.05 at 0 h, P < 0.001 at 3 h, and P < 0.05 at 24 h) and 50 μmol l−1 at all time points (all P < 0.001; Figure 3c).
Figure 3.
Effects of fluoxetine (FLU) on (a) cellular ROS production and (b) chromatin/DNA integrity. Sertraline (SERT) effects on (c) cellular ROS production and (d) chromatin/DNA integrity. Sperm cells were either exposed to FLU or SERT for up to 24 h at 37°C and 5% CO2 (ROS: n = 15, for FLU and SERT; chromatin/DNA: n = 20, for FLU and SERT). Results are expressed as median and interquartile range. *P < 0.05, **P < 0.01, and ***P < 0.001 symbolize significant differences when compared to the control group; #0.05<P<0.07 symbolizes a statistical trend when compared to the control group. ROS: reactive oxygen species.
Regarding chromatin/DNA integrity, no relevant differences were observed upon exposure to FLU (all P > 0.05; Figure 3b) and SERT (all P > 0.05; Figure 3d) in this study.
Capacitation and acrosomal status
To be able to fertilize an oocyte, sperm need to undergo a maturation process known as capacitation, which comprises several physiological and functional changes. One of those changes is protein phosphorylation.
A 3-h incubation with 1 μmol l−1 FLU resulted in a significant decrease in the percentage of capacitated sperm when compared to the control (P < 0.05), a statistical significance that was further lost following 24 h of exposure (P > 0.05; Figure 4a). No other concentrations caused significant differences. In sharp contrast, SERT caused an increase in the percentage of capacitated spermatozoa (Figure 4c) after 3 h and 24 h of exposure to 10 μmol l−1 (P < 0.05 for both time points), as well as after 3 h of exposure to the positive massive control (50 μmol l−1; P < 0.05).
Figure 4.
The effects of fluoxetine (FLU) on (a) sperm capacitation and (b) acrosome status. Sertraline (SERT) effects on (c) sperm capacitation and (d) acrosome status. Sperm cells were either exposed to FLU or SERT for up to 24 h at 37°C and 5% CO2 (FLU and SERT: n = 10). Results are expressed as median and interquartile range. *P < 0.05, **P < 0.01, and ***P < 0.001 symbolize significant differences when compared to the control group.
Sperm acrosome integrity, essential for the timely release of acrosomal contents and, consequently, penetration of the oocyte zona pellucida, was found affected by SERT. This antidepressant significantly decreased acrosome-intact sperm upon contact at 1 μmol l−1 and 50 μmol l−1, the latter dose inducing a permanent effect (1 μmol l−1: P < 0.05 at 0 h; 50 μmol l−1: P < 0.05 at 0 h, P < 0.01 at 3 h, and P < 0.001 at 24 h; Figure 4d). In the opposite direction, all tested FLU concentrations promoted no differences at all time points (all P > 0.05; Figure 4b).
DISCUSSION
SSRIs have been previously described to affect human sperm quality by decreasing sperm concentration, motility and normal morphology, and increasing DNA fragmentation.2,22,23,24,25,26 Strikingly, these studies did not address more functional parameters that reflect sperm fertilizing ability. Herein, we observed a significant and immediate decrease in progressive motility after exposure to both antidepressants, with FLU being the only showing a significant and consistent deleterious effect at levels found in vivo. Indeed, FLU was able to promote a shift from progressive to in situ motility at low doses, suggesting that this AD may eventually hamper fertility or cause some sort of subfertility phenotype in vivo. On the other hand, SERT failed to promote the same effect at low doses, but at high concentrations, both behaved similarly, leading to a significant decrease in total motility (progressive + in situ).
According to the literature, SSRIs can also interfere with mitochondrial function and trigger apoptosis in different cell types,27,28 but no previous studies were performed on human sperm, as we acknowledged. We show that viability was not affected by antidepressants at low concentrations, suggesting that they may not be cytotoxic at these levels. However, at the highest, massive concentration, both promoted significant cell loss, in particular SERT, with a more obvious effect already observed after immediate exposure. Notwithstanding, similar results were obtained for the MMP with the 50 μmol l−1 concentration, promoting a decrease in the percentage of cells with highly active mitochondria. However, the 10 μmol l−1 concentration of both drugs was found to decrease MMP after 24 h of exposure without affecting viability, suggesting an impairment of mitochondrial function only after longer exposures and before cell death.
Sperm capacitation and acrosome reaction are crucial processes for sperm to be able to naturally fertilize the oocyte. So far, as we acknowledged, no studies evaluating acrosome and capacitation status have been performed in humans using SSRIs. In our study, we found that 1 μmol l−1 FLU led to a decrease in the percentage of capacitated sperm after 3 h of incubation, a significant difference that disappeared after 24 h of incubation, thus suggesting a delay and not a complete impairment of this process. SERT, on the other hand, actually led to an increase in the percentage of tyrosine-phosphorylated sperm but only at high concentrations (10 μmol l−1 and 50 μmol l−1). In addition, at these concentrations, SERT also led to a significant and consistent decrease in the percentage of sperm with intact acrosomes. Despite the fact that SERT only affects these parameters at suprapharmacological and not biologically relevant concentrations, these outcomes strengthen the idea that FLU and SERT may be acting through different mechanisms of action as they affect different parameters differently.
ROS production at physiological levels is necessary for the regulation of several events; however, oxidative stress resulting from an imbalance between ROS production and scavenging by antioxidants has been associated with male infertility. Indeed, several studies have correlated excessive ROS production with the loss of sperm motility and viability, mitochondrial dysfunction, DNA damage, impairment of capacitation, and acrosome reaction.19,29 Several SSRIs, including FLU, have already been associated with an increase in oxidative stress and oxidative damage, at least in rats.2,30 However, although FLU led to an increase in cellular ROS production at low levels (100 nmol l−1 at 0 h, and a trend of 1 μmol l−1 at 3 h), those differences were not consistent throughout time and thus relevant in this scenario, which implies that they are not responsible for the motility decrease herein reported at all time points. Furthermore, SERT consistently affected cellular ROS production but only at high doses, as observed for many of the parameters addressed, pinpointing a possible underlying role of high ROS generation in sperm dysfunction at these specific concentrations. Interestingly, neither FLU nor SERT had a significant impact on chromatin/DNA integrity throughout time. Although it is well-established the association between ROS production and macromolecule damage, we hypothesize that the higher ROS levels produced may not be enough to induce lower chromatin/DNA integrity in this study. To our knowledge, no studies have addressed the effects of these ADs on human sperm DNA damage so far. Conversely, paroxetine, an AD also belonging to the SSRI category, was associated with a significant increase in DNA fragmentation in 35 healthy male volunteers, without affecting other semen parameters.26
In summary, the results from this study suggest that FLU is more harmful than SERT when it comes to decreasing sperm motility at low concentrations, and thus more therapeutic concentrations. Since spermatozoa need to be progressively motile to naturally fertilize the oocyte in vivo, we hypothesize that, although the decrease in motility was not dramatic along the 24-h timeframe, FLU exposure may lead to fertility impairment or subfertility on the long run. Both compounds can also affect other relevant functional parameters at high doses. Our results also suggest that both compounds may act through different mechanisms of action, which is especially interesting since they belong to the same category of antidepressants. For instance, while FLU did not have any impact on the acrosome status, SERT caused a decrease in the number of intact acrosomes at the highest dose, suggesting that it may promote a premature acrosome reaction. Since only capacitated sperm with an intact acrosome can fertilize an oocyte, a premature release of acrosomal contents may likely affect fertilization and compromise an individual’s fertility. However, this effect was consistently observed only after exposure to the SERT’s positive control.
As this study carried out in vitro exposures for up to 24 h, we cannot evaluate the effects of continuous exposure during spermatogenesis, which will possibly be affected since antidepressants are usually consumed over a long period of time. However, it allows us to evaluate the effects of possible exposure to different concentrations of antidepressants present in the seminal fluid and the female reproductive tract, based on values reported in previous studies.12,15 The massive concentrations used herein were added to validate our system approach. Moreover, although it depends on the antidepressant concentrations taken by the individuals and compound pharmacokinetics, we cannot discard the hypothesis that these may build up in reproductive fluids and reach higher levels than the ones used herein. Indeed, to our knowledge, no studies have reported the levels of SERT and FLU in biological fluids throughout intake. Furthermore, one should keep in mind that human spermatozoa can be kept for up to one week in the female reproductive tract in vivo and thus, even lower concentrations than the ones used here can further induce deleterious effects as time passes by, particularly in what concerns FLU. Nevertheless, even though more comprehensive studies are certainly warranted, especially in vivo, the present controlled in vitro study allows us to pinpoint specific alterations in sperm function that can only be attributed to these ADs. In fact, since it is shown that sperm motility can be compromised in this 1-day scenario, we can hypothesize that individuals who take these specific medications, especially over long periods of time, may potentially have their fertility compromised. This is the first in vitro study to use biologically relevant AD concentrations, based on previously quantified values both in blood and seminal fluid; all others used higher doses with little biological relevance.31
AUTHOR CONTRIBUTIONS
RAS and RT established the concept and designed the research study. RAS acquired the data. RAS, APS, TAS, JRS, and RST contributed to the analysis and interpretation of the results. RAS wrote the original draft, and RST reviewed and edited it. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Representative images of live spermatozoa with high (a and c) and low mitochondrial membrane potential (d). (b) represents a dead sperm cell with no staining in the midpiece. The assay was performed with the JC1 dye coupled with the live/dead kit (×1000).
Representative images of fully (a), partially (b), and noncapacitated (c) spermatozoa detected by tyrosine phosphorylation, a hallmark of capacitation. Nuclei are counterstained with DAPI (×1000). DAPI: DNA-binding dye 4’,6-diamino-2-phenyl-indole.
Representative images of acrosome-reacted (a and b)and acrosome-intact (c) spermatozoa using the PSA-FITC staining. Nuclei are counterstained with DAPI (×1000). PSA-FITC: Pisum sativum agglutinin conjugated with fluorescein isothiocyanate; DAPI: DNA-binding dye 4’,6-diamino-2-phenyl-indole.
ACKNOWLEDGMENTS
The authors would like to acknowledge all members of the Reproductive Biology and Stem Cells Group for all their constructive ideas and fruitful discussions. We would also like to give special thanks to Maria Inês Alfaiate (CNC-UC) for her valuable insights and her willingness to help when needed. Finally, the authors would also like to thank John S Jones (CNC-UC) for proofreading the final manuscript.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.World Health Organization. Depression. 2021. [[Last accessed on 2023 Mar 29]]. Available from: https://www.who.int/news-room/fact-sheets/detail/depression .
- 2.Beeder LA, Samplaski MK. Effect of antidepressant medications on semen parameters and male fertility. Int J Urol. 2020;27:39–46. doi: 10.1111/iju.14111. [DOI] [PubMed] [Google Scholar]
- 3.Jimeńez-Trejo F, Tapia-Rodríguez M, Cerboń M, Kuhn DM, Manjarrez-Gutiérrez G, et al. Evidence of 5-HT components in human sperm:implications for protein tyrosine phosphorylation and the physiology of motility. Reproduction. 2012;144:677–85. doi: 10.1530/REP-12-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins A, Nash M, Lynch AM. Antidepressant-associated sexual dysfunction:impact effects, and treatment. Drug Healthc Patient Saf. 2010;2:141–50. doi: 10.2147/DHPS.S7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galhardo A, Moura-Ramos M, Cunha M, Pinto-Gouveia J. The infertility trap:how defeat and entrapment affect depressive symptoms. Hum Reprod. 2016;31:419–26. doi: 10.1093/humrep/dev311. [DOI] [PubMed] [Google Scholar]
- 6.Galhardo A, Alves J, Moura-Ramos M, Cunha M. Infertility-related stress and depressive symptoms –the role of experiential avoidance:a cross-sectional study. J Reprod Infant Psychol. 2019;38:139–50. doi: 10.1080/02646838.2019.1612046. [DOI] [PubMed] [Google Scholar]
- 7.Moura-Ramos M, Gameiro S, Canavarro MC, Soares I, Almeida-Santos T. Does infertility history affect the emotional adjustment of couples undergoing assisted reproduction?The mediating role of the importance of parenthood. Br J Health Psychol. 2016;21:302–17. doi: 10.1111/bjhp.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simionescu G, Doroftei B, Maftei R, Obreja BE, Anton E, et al. The complex relationship between infertility and psychological distress (review) Exp Ther Med. 2021;21:1–6. doi: 10.3892/etm.2021.9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavousi SA, Behjati M, Milajerdi A, Mohammadi AH. Psychological assessment in infertility:a systematic review and meta-analysis. Front Psychol. 2022;13:961722. doi: 10.3389/fpsyg.2022.961722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendrick V, Gitlin M, Altshuler L, Korenman S. Antidepressant medications, mood and male fertility. Psychoneuroendocrinology. 2000;25:37–51. doi: 10.1016/s0306-4530(99)00038-4. [DOI] [PubMed] [Google Scholar]
- 11.Valoriani V, Lotti F, Lari D, Miccinesi G, Vaiani S, et al. Differences in psychophysical well-being and signs of depression in couples undergoing their first consultation for assisted reproduction technology (ART):an Italian pilot study. Eur J Obstet Gynecol Reprod Biol. 2016;197:179–85. doi: 10.1016/j.ejogrb.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Mazzilli R, Curto M, De Bernardini D, Olana S, Capi M, et al. Psychotropic drugs levels in seminal fluid:a new therapeutic drug monitoring analysis? Front Endocrinol (Lausanne) 2021;12:620936. doi: 10.3389/fendo.2021.620936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahban R, Rehfeld A, Schiffer C, Brenker C, Palme DL, et al. The antidepressant sertraline inhibits CatSper Ca2+ channels in human sperm. Hum Reprod. 2021;36:2638–48. doi: 10.1093/humrep/deab190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 15.Unterecker S, Riederer P, Proft F, Maloney J, Deckert J, et al. Effects of gender and age on serum concentrations of antidepressants under naturalistic conditions. J Neural Transm. 2013;120:1237–46. doi: 10.1007/s00702-012-0952-2. [DOI] [PubMed] [Google Scholar]
- 16.Reis M, Åberg-Wistedt A, Ågren H, Höglund P, Äkerblad AC, et al. Serum disposition of sertraline, N-desmethylsertraline and paroxetine:a pharmacokinetic evaluation of repeated drug concentration measurements during 6 months of treatment for major depression. Hum Psychopharmacol. 2004;19:283–91. doi: 10.1002/hup.599. [DOI] [PubMed] [Google Scholar]
- 17.Bisconti M, Grosjean P, Arcolia V, Simon JF, Hennebert E. Influence of two widely used solvents, ethanol and dimethyl sulfoxide, on human sperm parameters. Int J Mol Sci. 2023;24:505. doi: 10.3390/ijms24010505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baptista M, Publicover SJ, Ramalho-Santos J. In vitro effects of cationic compounds on functional human sperm parameters. Fertil Steril. 2013;99:705–12. doi: 10.1016/j.fertnstert.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Escada-Rebelo S, Ramalho-Santos J. Oxidative and nitrosative stress detection in human sperm using fluorescent probes. In: Pellicciari C, Biggiogera M, Malatesta M, editors. Histochemistry of Single Molecules:Methods and Protocols. Totowa: Humana Press; 2023. pp. 45–52. [DOI] [PubMed] [Google Scholar]
- 20.Sousa AP, Tavares RS, Velez de la Calle JF, Figueiredo H, Almeida V, et al. Dual use of Diff-Quik-like stains for the simultaneous evaluation of human sperm morphology and chromatin status. Hum Reprod. 2009;24:28–36. doi: 10.1093/humrep/den365. [DOI] [PubMed] [Google Scholar]
- 21.Mota PC, Tavares RS, Cordeiro M, Pereira SP, Publicover SJ, et al. Acute effects of TCDD administration:special emphasis on testicular and sperm mitochondrial function. Asian Pacific J Reprod. 2012;1:269–76. [Google Scholar]
- 22.Elnazer HY, Baldwin DS. Treatment with citalopram, but not with agomelatine, adversely affects sperm parameters:a case report and translational review. Acta Neuropsychiatr. 2014;26:125–9. doi: 10.1017/neu.2013.60. [DOI] [PubMed] [Google Scholar]
- 23.Tanrikut C, Schlegel PN. Antidepressant-associated changes in semen parameters. Urology. 2007;69:185.e5–7. doi: 10.1016/j.urology.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 24.Akasheh G, Sirati L, Reza A, Kamran N, Sepehrmanesh Z. Comparison of the effect of sertraline with behavioral therapy on semen parameters in men with primary premature ejaculation. Urology. 2014;83:800–4. doi: 10.1016/j.urology.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Koyuncu H, Serefoglu EC, Yencilek E, Atalay H, Akbas NB, et al. Escitalopram treatment for premature ejaculation has a negative effect on semen parameters. Int J Impot Res. 2011;23:257–61. doi: 10.1038/ijir.2011.35. [DOI] [PubMed] [Google Scholar]
- 26.Tanrikut C, Feldman AS, Altemus M, Paduch DA, Schlegel PN. Adverse effect of paroxetine on sperm. Fertil Steril. 2010;94:1021–26. doi: 10.1016/j.fertnstert.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 27.de Oliveira MR. Fluoxetine and the mitochondria:a review of the toxicological aspects. Toxicol Lett. 2016;258:185–91. doi: 10.1016/j.toxlet.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Couch L, Higuchi M, Fang JL, Guo L. Mitochondrial dysfunction induced by sertraline, an antidepressant agent. Toxicol Sci. 2012;127:582–91. doi: 10.1093/toxsci/kfs100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majzoub A, Agarwal A. Systematic review of antioxidant types and doses in male infertility:benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J Urol. 2018;16:113–24. doi: 10.1016/j.aju.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakr HF, Abbas AM, Elsamanoudy AZ, Ghoneim FM. Effect of fluoxetine and resveratrol on testicular functions and oxidative stress in a rat model of chronic mild stress-induced depression. J Physiol Pharmacol. 2015;66:515–27. [PubMed] [Google Scholar]
- 31.Kumar VS, Sharma VL, Tiwari P, Singh D, Maikhuri JP, et al. The spermicidal and antitrichomonas activities of SSRI antidepressants. Bioorg Med Chem Lett. 2006;16:2509–12. doi: 10.1016/j.bmcl.2006.01.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative images of live spermatozoa with high (a and c) and low mitochondrial membrane potential (d). (b) represents a dead sperm cell with no staining in the midpiece. The assay was performed with the JC1 dye coupled with the live/dead kit (×1000).
Representative images of fully (a), partially (b), and noncapacitated (c) spermatozoa detected by tyrosine phosphorylation, a hallmark of capacitation. Nuclei are counterstained with DAPI (×1000). DAPI: DNA-binding dye 4’,6-diamino-2-phenyl-indole.
Representative images of acrosome-reacted (a and b)and acrosome-intact (c) spermatozoa using the PSA-FITC staining. Nuclei are counterstained with DAPI (×1000). PSA-FITC: Pisum sativum agglutinin conjugated with fluorescein isothiocyanate; DAPI: DNA-binding dye 4’,6-diamino-2-phenyl-indole.