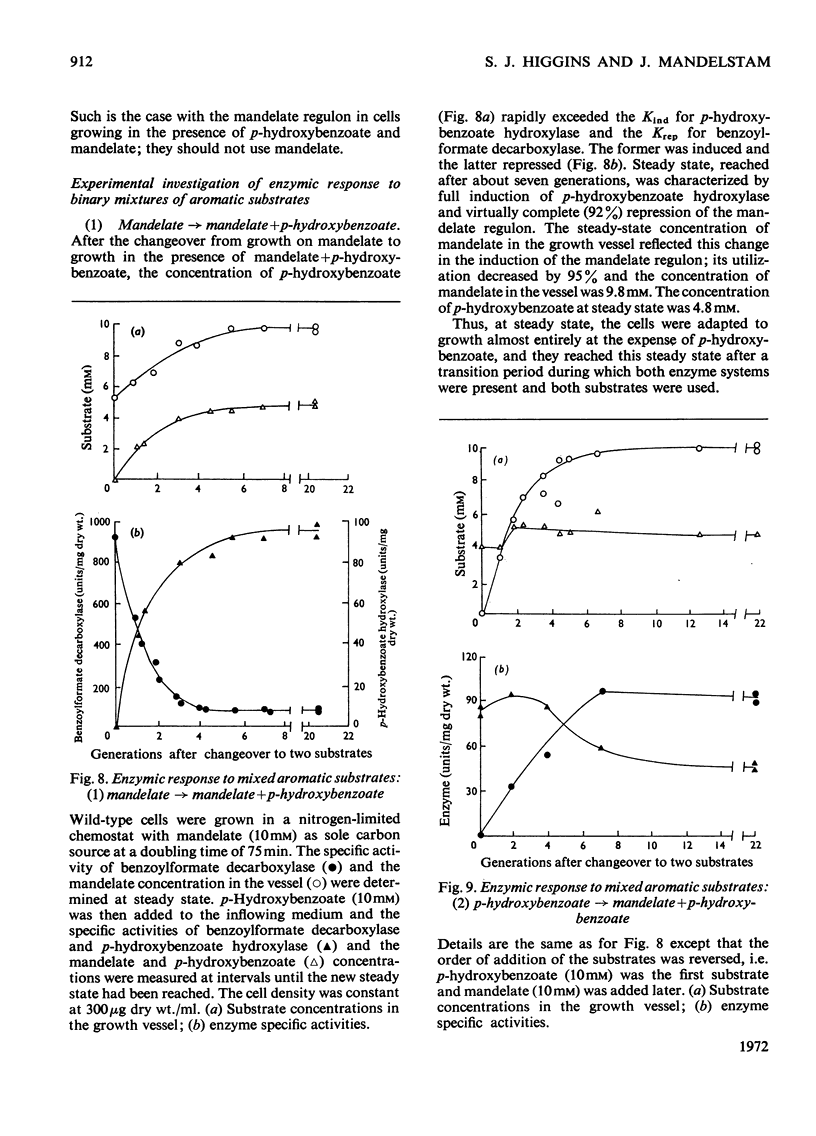
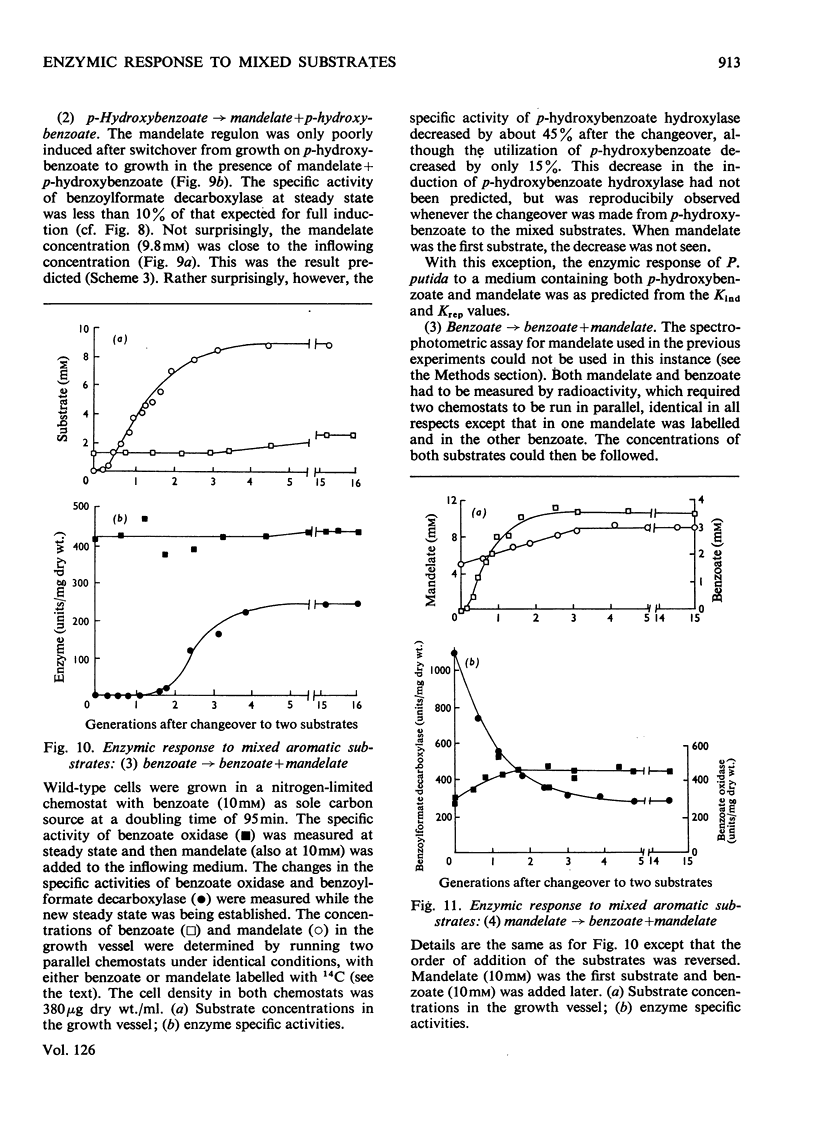
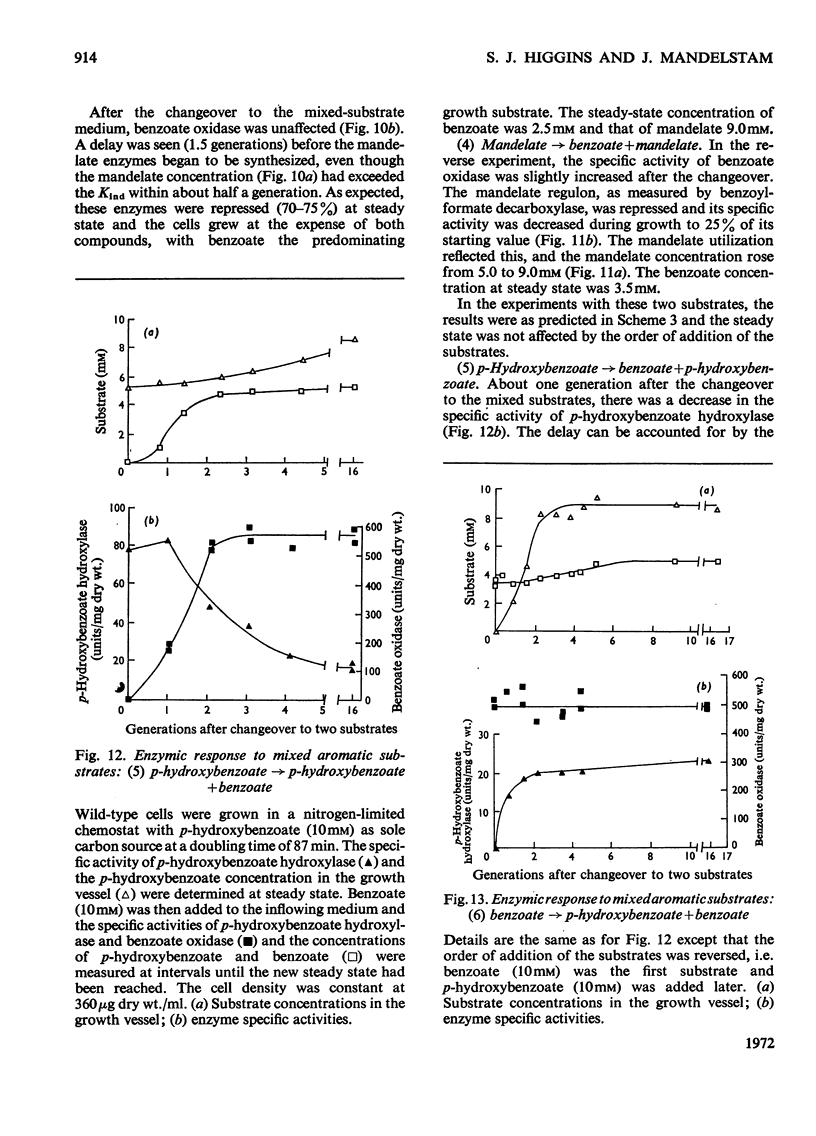
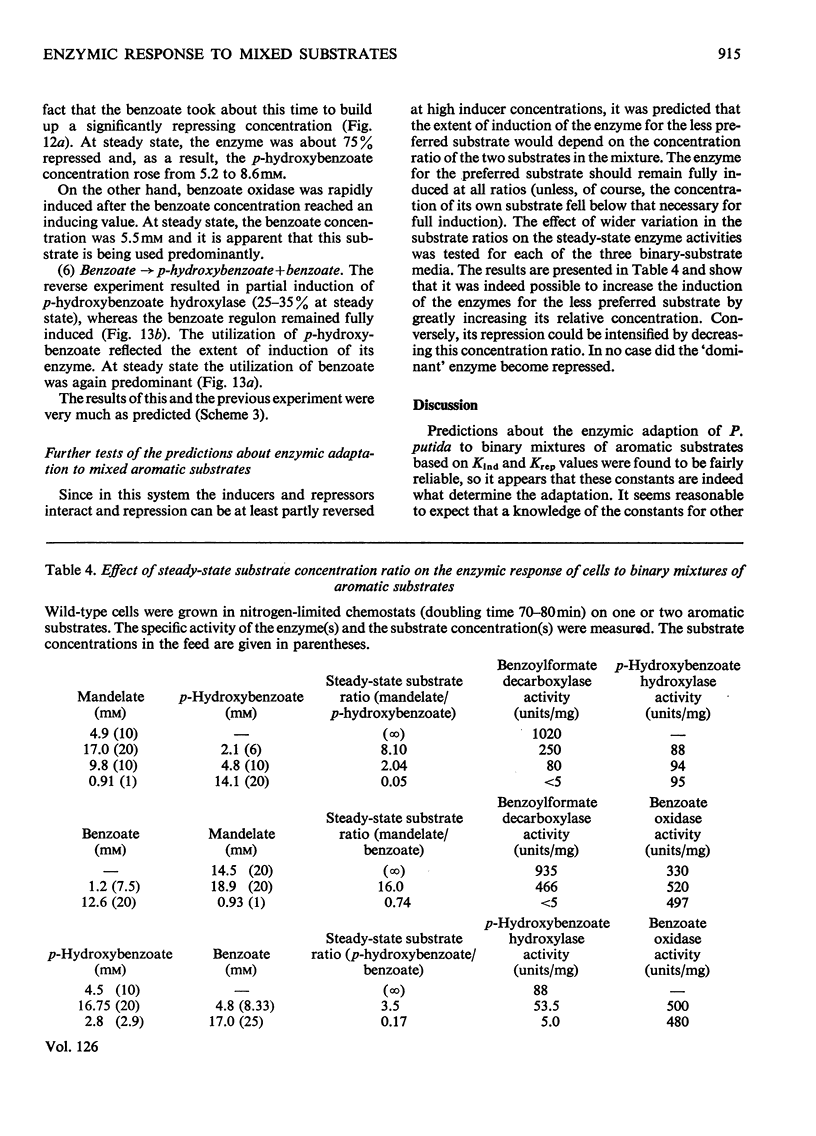
Abstract
1. Induction constants (Kind) and repression constants (Krep), which are a measure of the affinity of the inducers or repressors for the induction systems, were measured for mandelate, benzoate and p-hydroxybenzoate in Pseudomonas putida. 2. From these results, the enzymic response of the organism to media containing pairs of these substrates was predicted. Nitrogen-limited chemostats, operated at high growth rates, were used to investigate these predictions in cells grown first on one aromatic substrate with the second added later. 3. In general, the values of Kind and Krep predicted quite accurately the response to substrate mixtures. Thus, in the presence of mandelate and either benzoate or p-hydroxybenzoate, the enzymes of mandelate metabolism were repressed almost completely, and the bacteria were fully induced for the alternative substrate (benzoate or p-hydroxybenzoate), which was preferentially utilized for growth. When benzoate and p-hydroxybenzoate were the two substrates in the mixture, the enzymes for metabolism of the latter were strongly repressed and growth took place mainly on benzoate. 4. The enzymic response to mixed substrates did not result in the metabolism of the better growth substrate, but in the substrate requiring the synthesis of fewer enzymes. Thus benzoate is used in preference to mandelate although the latter supports a faster growth rate. It is nevertheless considered that, with our present knowledge of the natural habitat of the organism, it is impossible to decide whether protein economy or growth rate was the factor determining the evolution of this control system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baich A., Johnson M. Evolutionary advantage of control of a biosynthetic pathway. Nature. 1968 May 4;218(5140):464–465. doi: 10.1038/218464a0. [DOI] [PubMed] [Google Scholar]
- Blackmore M. A., Quayle J. R. Choice between autotrophy and heterotrophy in Pseudomonas oxalaticus. Growth in mixed substrates. Biochem J. 1968 May;107(5):705–713. doi: 10.1042/bj1070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. 3. Isolation and properties of constitutive mutants. J Bacteriol. 1966 Mar;91(3):1161–1167. doi: 10.1128/jb.91.3.1161-1167.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. II. Isolation and properties of blocked mutants. J Bacteriol. 1966 Mar;91(3):1155–1160. doi: 10.1128/jb.91.3.1155-1160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Mandelstam J. Evidence for induced synthesis of an active transport factor for mandelate in Pseudomonas putida. Biochem J. 1972 Feb;126(4):917–922. doi: 10.1042/bj1260917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K. Regulation of synthesis of early enzymes of p-hydroxybenzoate pathway in Pseudomonas putida. J Biol Chem. 1970 Oct 25;245(20):5304–5308. [PubMed] [Google Scholar]
- MAAS W. K. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. II. DOMINANCE OF REPRESSIBILITY IN DIPLOIDS. J Mol Biol. 1964 Mar;8:365–370. doi: 10.1016/s0022-2836(64)80200-x. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J., JACOBY G. A. INDUCTION AND MULTI-SENSITIVE END-PRODUCT REPRESSION IN THE ENZYMIC PATHWAY DEGRADING MANDELATE IN PSEUDOMONAS FLUORESCENS. Biochem J. 1965 Mar;94:569–577. doi: 10.1042/bj0940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. The repression of constitutive beta-galactosidase in Escherichia coli by glucose and other carbon sources. Biochem J. 1962 Mar;82:489–493. doi: 10.1042/bj0820489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTON L. N., STANIER R. Y. MECHANISM OF BETA-KETOADIPATE FORMATION BY BACTERIA. Nature. 1964 Dec 26;204:1279–1283. doi: 10.1038/2041279a0. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- Roepke R. R., Libby R. L., Small M. H. Mutation or Variation of Escherichia coli with Respect to Growth Requirements. J Bacteriol. 1944 Oct;48(4):401–412. doi: 10.1128/jb.48.4.401-412.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLEEPER B. P., STANIER R. Y. The bacterial oxidation of aromatic compounds; adaptive patterns with respect to polyphenolic compounds. J Bacteriol. 1950 Jan;59(1):117–127. doi: 10.1128/jb.59.1.117-127.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Stevenson I. L., Mandelstam J. Induction and multi-sensitive end-product repression in two converging pathways degrading aromatic substances in Pseudomonas fluorescens. Biochem J. 1965 Aug;96(2):354–362. doi: 10.1042/bj0960354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamenhof S., Eichhorn H. H. Study of microbial evolution through loss of biosynthetic functions: establishment of "defective" mutants. Nature. 1967 Nov 4;216(5114):456–458. doi: 10.1038/216456a0. [DOI] [PubMed] [Google Scholar]


