Abstract
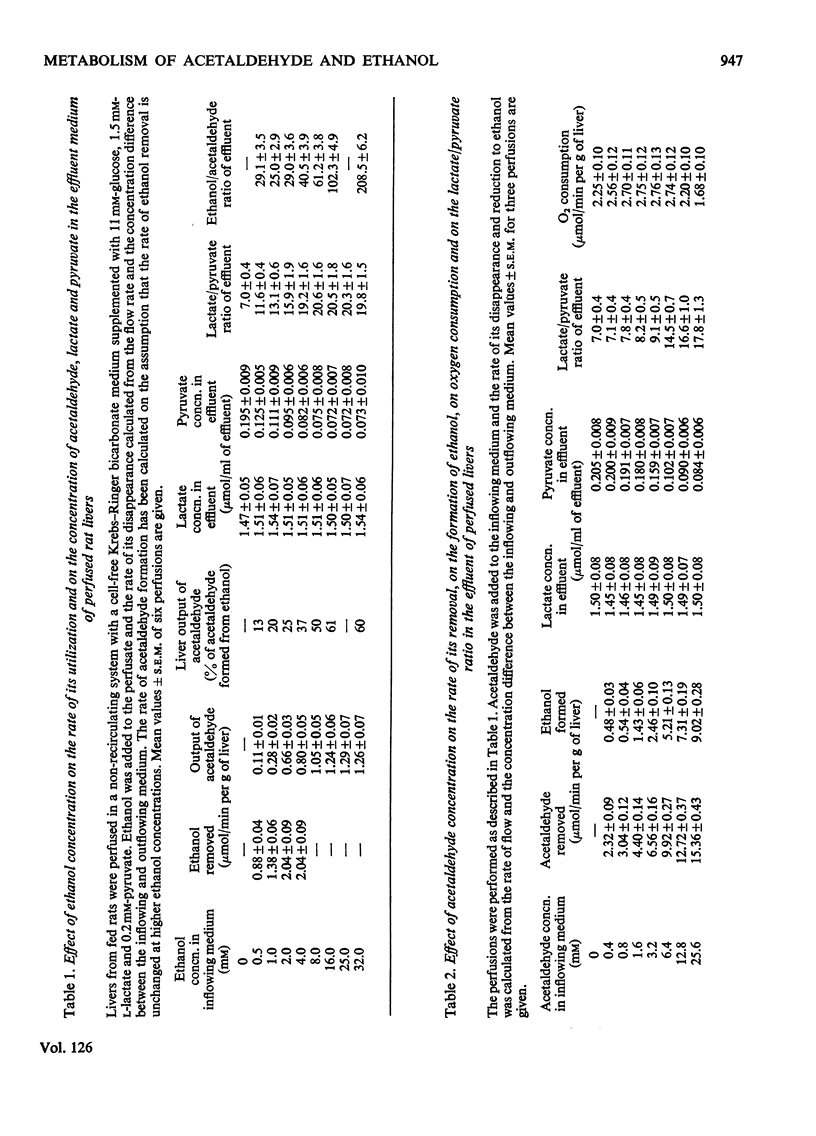
1. Removal of acetaldehyde and ethanol has been studied in perfused rat livers. 2. The maximum rate of ethanol oxidation was 2μmol/min per g of liver, which was less than the calculated capacity of the ethanol-oxidizing system. The lactate/pyruvate ratio of the medium increased with the rate of ethanol removal. At low ethanol concentrations most of the acetaldehyde formed was oxidized further, but at ethanol concentrations above 16mm about 60% of the acetaldehyde left the liver unmetabolized. 3. At lower concentrations the greater part of added acetaldehyde was oxidized, but above 5mm, 50–60% of that removed was recovered as ethanol. 4. When the reduction of acetaldehyde was blocked by pyrazole, removal was strongly diminished. There was no effect on the lactate/pyruvate ratio during oxidation of low concentrations of acetaldehyde, even in the presence of pyrazole, but at higher concentrations a gradual increase occurred. 5. The results indicate that during ethanol oxidation the ethanol/acetaldehyde pair is not in redox equilibrium with the lactate/pyruvate pair. Ethanol oxidation was abolished by addition of acetaldehyde. Under these conditions the lactate/pyruvate ratio was 1.5–1.8 times the ethanol/acetaldehyde ratio, indicating equilibration of the alcohol dehydrogenase and lactate dehydrogenase systems. 6. The results support the view that ultimately the rate of mitochondrial oxidation of NADH limits the removal of ethanol in the liver.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELES R. H., LEE H. A., Jr The dismutation of formaldehyde by liver alcohol dehydrogenase. J Biol Chem. 1960 May;235:1499–1503. [PubMed] [Google Scholar]
- Ammon H. P., Estler C. J., Heim F. Inactivation of coenzyme a by ethanol. I. Acetaldehyde as mediator of the inactivation of coenzyme A following the administration of ethanol in vivo. Biochem Pharmacol. 1969 Jan;18(1):29–33. doi: 10.1016/0006-2952(69)90005-7. [DOI] [PubMed] [Google Scholar]
- Dalziel K., Dickinson F. M. Aldehyde mutase. Nature. 1965 Apr 17;206(981):255–257. doi: 10.1038/206255a0. [DOI] [PubMed] [Google Scholar]
- Deitrich R. A. Tissue and subcellular distribution of mammalian aldehyde-oxydizing capacity. Biochem Pharmacol. 1966 Dec;15(12):1911–1922. doi: 10.1016/0006-2952(66)90220-6. [DOI] [PubMed] [Google Scholar]
- FROSANDER O. A., RAEIHAE N., SALASPURO M., MAEENPAEAE P. INFLUENCE OF ETHANOL ON THE LIVER METABOLISM OF FED AND STARVED RATS. Biochem J. 1965 Jan;94:259–265. doi: 10.1042/bj0940259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsander O. A., Hillbom M. E., Lindros K. O. Influence of thyroid function on the acetaldehyde level of blood and liver of intact rats during ethanol metabolism. Acta Pharmacol Toxicol (Copenh) 1969;27(6):410–416. doi: 10.1111/j.1600-0773.1969.tb00487.x. [DOI] [PubMed] [Google Scholar]
- HOLZER H., LYNEN F., SCHULTZ G. Bestimmung des Quotienten DPNH/DPN in lebenden Hefezellen durch Analyse stationärer-Alkohol- und Acetaldehyd-Konzentrationen. Biochem Z. 1956;328(4):252–263. [PubMed] [Google Scholar]
- Hassinen I. E., Ylikahri R. H., Kähönen M. T. Effect of ethanol, thyrxine and fructose on the intracellular redox state of a perfused liver as studied by surface fluorometry. Ann Med Exp Biol Fenn. 1970;48(3):176–183. [PubMed] [Google Scholar]
- Hedlung S. G., Kiessling K. H. The physiological mechanism involved in hangover. 1. The oxidation of some lower aliphatic fusel alcohols and aldehydes in rat liver and their effect on the mitochondrial oxidation of various substrates. Acta Pharmacol Toxicol (Copenh) 1969;27(5):381–396. doi: 10.1111/j.1600-0773.1969.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Israel Y., Khanna J. M., Lin R. Effect of 2,4-dinitrophenol on the rate of ethanol elimination in the rat in vivo. Biochem J. 1970 Nov;120(2):447–448. doi: 10.1042/bj1200447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer R. J., Deitrich R. A. Isolation and characterization of human liver aldehyde dehydrogenase. J Biol Chem. 1968 Dec 25;243(24):6402–6408. [PubMed] [Google Scholar]
- Krebs H. A., Freedland R. A., Hems R., Stubbs M. Inhibition of hepatic gluconeogenesis by ethanol. Biochem J. 1969 Mar;112(1):117–124. doi: 10.1042/bj1120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDQUIST F., FUGMANN U., KLANING E., RASMUSSEN H. The metabolism of acetaldehyde in mammalian tissues: reactions in rat-liver suspensions under anaerobic conditions. Biochem J. 1959 Jul;72:409–419. doi: 10.1042/bj0720409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDQUIST F., FUGMANN U., RASMUSSEN H., SVENDSEN I. The metabolism of acetaldehyde in mammalian tissues. Reactions in rat-liver suspensions under aerobic conditions. Biochem J. 1962 Aug;84:281–286. doi: 10.1042/bj0840281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindros K. O., Aro H. Ethanol-induced changes in levels of metabolites related to the redox state and ketogenesis in rat liver. Ann Med Exp Biol Fenn. 1969;47(1):39–42. [PubMed] [Google Scholar]
- Rawat A. K. Effects of ethanol infusion on the redox state and metabolite levels in rat liver in vivo. Eur J Biochem. 1968 Dec 5;6(4):585–592. doi: 10.1111/j.1432-1033.1968.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R., Albersheim P., Mcclearn G. Aldehyde dehydrogenase and ethanol preference in mice. J Biol Chem. 1970 Jun 10;245(11):2876–2882. [PubMed] [Google Scholar]
- Videla L., Israel Y. Factors that modify the metabolism of ethanol in rat liver and adaptive changes produced by its chronic administration. Biochem J. 1970 Jun;118(2):275–281. doi: 10.1042/bj1180275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]


