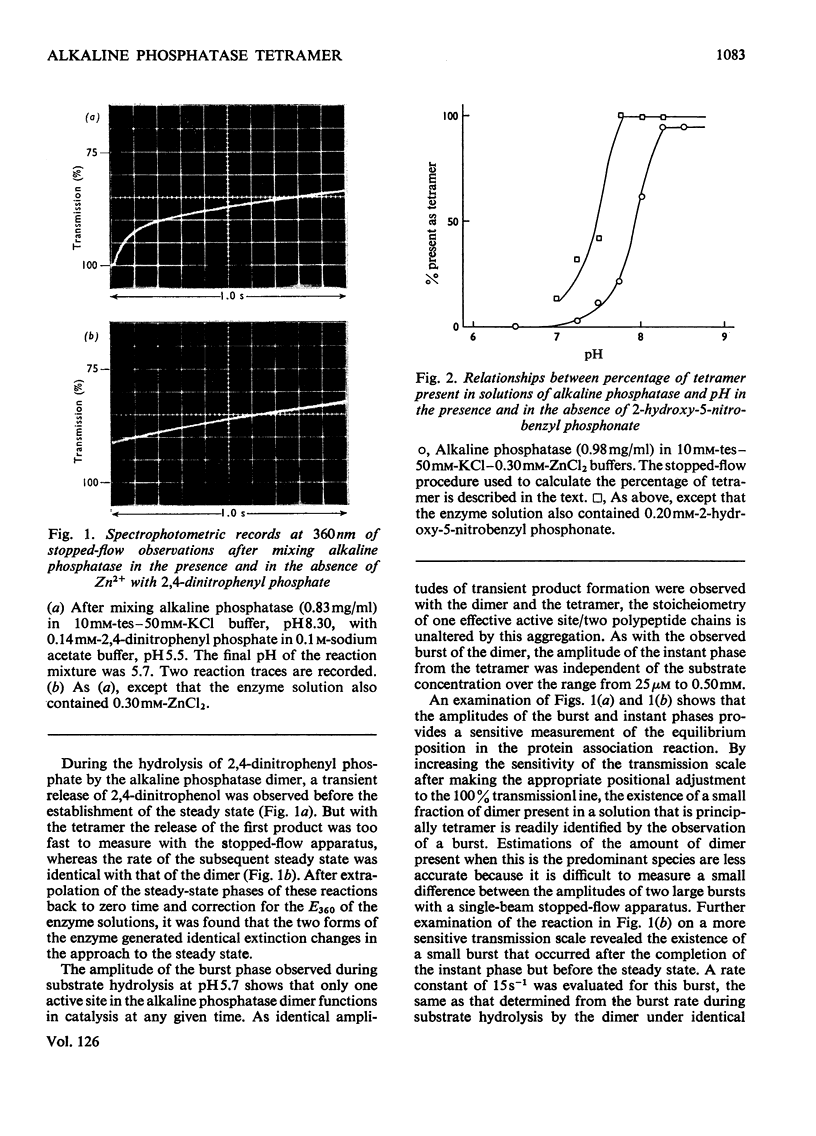
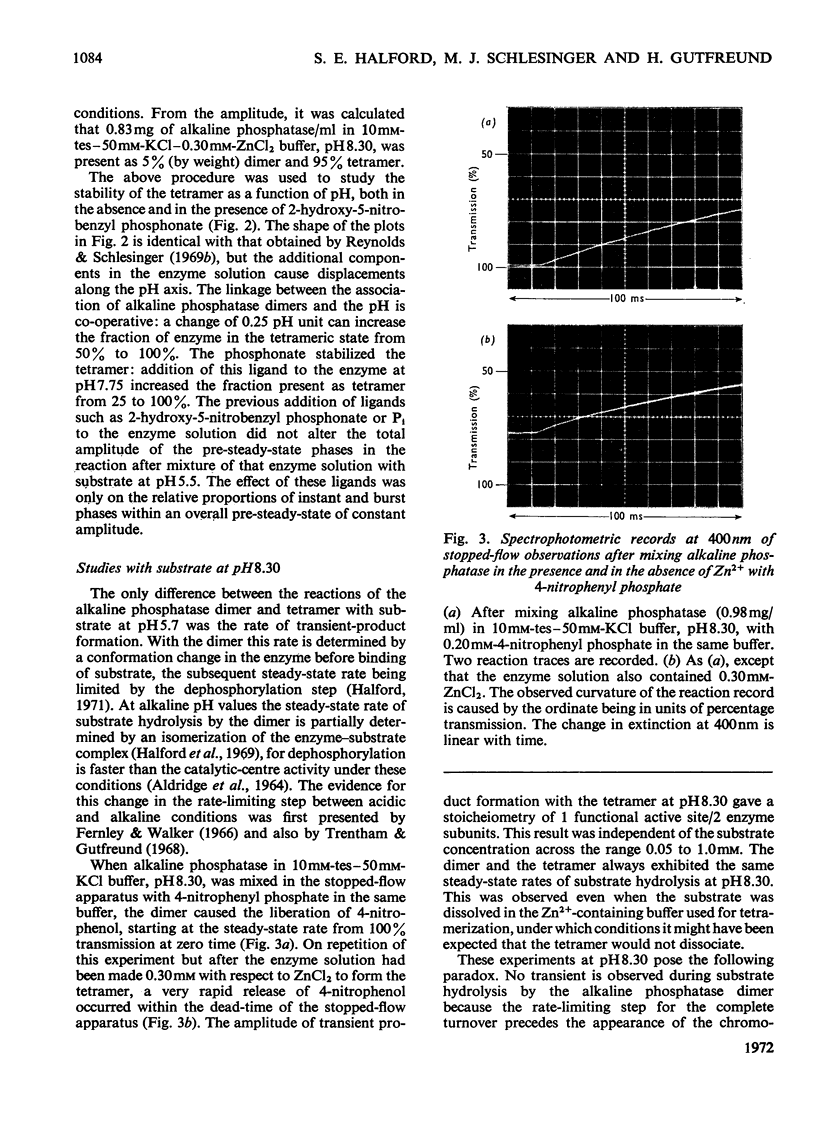
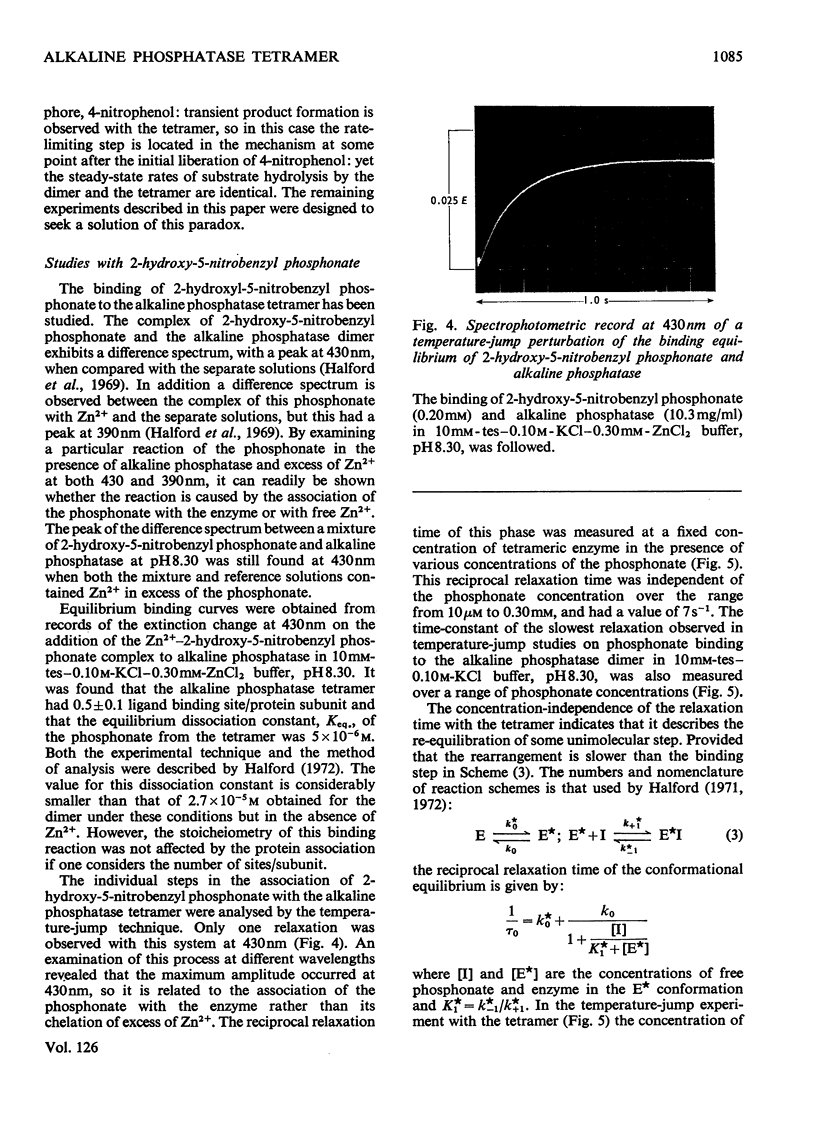
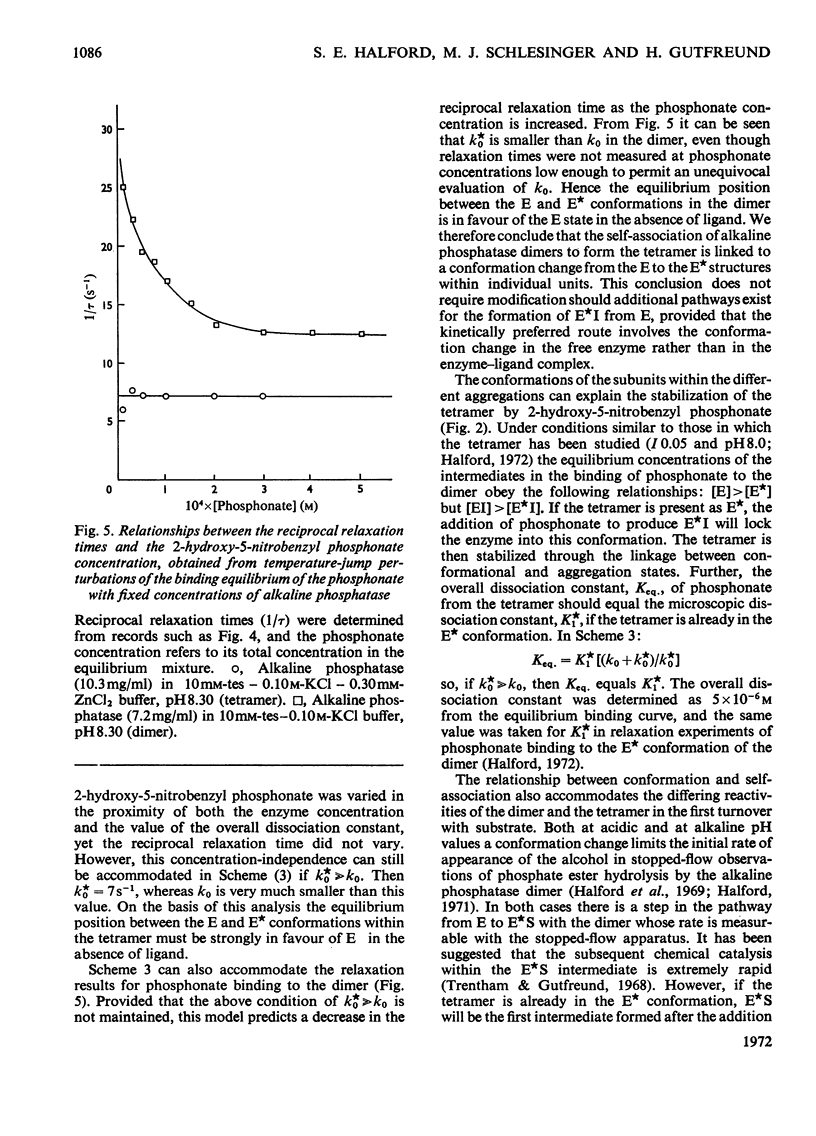
Abstract
1. The stability of the tetrameric form of Escherichia coli alkaline phosphatase was examined by analytical ultracentrifugation. 2. The stopped-flow technique was used to study the hydrolysis of nitrophenyl phosphates by the alkaline phosphatase tetramer at pH7.5 and 8.3. In both cases transient product formation was observed before the steady state was attained. Both transients consisted of the liberation of 1mol of nitrophenol/2mol of enzyme subunits within the dead-time of the apparatus. The steady-state rates were identical with those observed with the dimer under the same conditions. 3. The binding of 2-hydroxy-5-nitrobenzyl phosphonate to the alkaline phosphatase tetramer was studied by the temperature-jump technique. The self-association of two dimers to form the tetramer is linked to a conformation change within the dimer. This accounts for the differences between the transient phases in the reactions of the dimer and the tetramer with substrate. 4. Addition of Pi to the alkaline phosphatase tetramer caused it to dissociate into dimers. The tetramer is unable to bind this ligand. It is suggested that the tetramer undergoes a compulsory dissociation before the completion of its first turnover with substrate. 5. On the basis of these findings a mechanism is proposed for the involvement of the alkaline phosphatase tetramer in the physiology of E. coli.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frieden C. Treatment of enzyme kinetic data. II. The multisite case: comparison of allosteric models and a possible new mechanism. J Biol Chem. 1967 Sep 25;242(18):4045–4052. [PubMed] [Google Scholar]
- Halford S. E., Bennett N. G., Trentham D. R., Gutfeund H. A substate-induced conformation change in the reaction of alkaline phosphatase from Escherichia coli. Biochem J. 1969 Sep;114(2):243–251. doi: 10.1042/bj1140243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford S. E. Escherichia coli alkaline phosphatase. An analysis of transient kinetics. Biochem J. 1971 Nov;125(1):319–327. doi: 10.1042/bj1250319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford S. E. Escherichia coli alkaline phosphatase. Relaxation spectra of ligand binding. Biochem J. 1972 Feb;126(3):727–738. doi: 10.1042/bj1260727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K. R. Conformationally distinct subunits in protein oligomers with dihedral symmetry. J Mol Biol. 1968 Nov 28;38(1):133–136. doi: 10.1016/0022-2836(68)90134-4. [DOI] [PubMed] [Google Scholar]
- Kellett G. L., Gutfreund H. Reactions of haemoglobin dimers after ligand dissociation. Nature. 1970 Aug 29;227(5261):921–926. doi: 10.1038/227921a0. [DOI] [PubMed] [Google Scholar]
- Lazdunski M., Petitclerc C., Chappelet D., Lazdunski C. Flip-flop mechanisms in enzymology. A model: the alkaline phosphatase of Escherichia coli. Eur J Biochem. 1971 May 11;20(1):124–139. doi: 10.1111/j.1432-1033.1971.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Schlesinger M. J. Alterations in the structure and function of Escherichia coli alkaline phosphatase due to Zn2+ binding. Biochemistry. 1969 Feb;8(2):588–593. doi: 10.1021/bi00830a019. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Schlesinger M. J. Formation and properties of a tetrameric form of Escherichia coli alkaline phosphatase. Biochemistry. 1969 Nov;8(11):4278–4282. doi: 10.1021/bi00839a008. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Olsen R. A new, simple, rapid procedure for purification of Escherichia coli alkaline phosphatase. Anal Biochem. 1970 Jul;36(1):86–90. doi: 10.1016/0003-2697(70)90334-9. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Reynolds J. A., Schlesinger S. Formation and localization of the alkaline phosphatase of Escherichia coli. Ann N Y Acad Sci. 1969 Oct 14;166(2):368–379. doi: 10.1111/j.1749-6632.1969.tb46408.x. [DOI] [PubMed] [Google Scholar]
- TORRIANI A., ROTHMAN F. Mutants of Escherichia coli constitutive for alkaline phosphatase. J Bacteriol. 1961 May;81:835–836. doi: 10.1128/jb.81.5.835-836.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R., Gutfreund H. The kinetics of the reaction of nitrophenyl phosphates with alkaline phosphatase from Escherichia coli. Biochem J. 1968 Jan;106(2):455–460. doi: 10.1042/bj1060455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYMAN J., Jr LINKED FUNCTIONS AND RECIPROCAL EFFECTS IN HEMOGLOBIN: A SECOND LOOK. Adv Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]


