Abstract
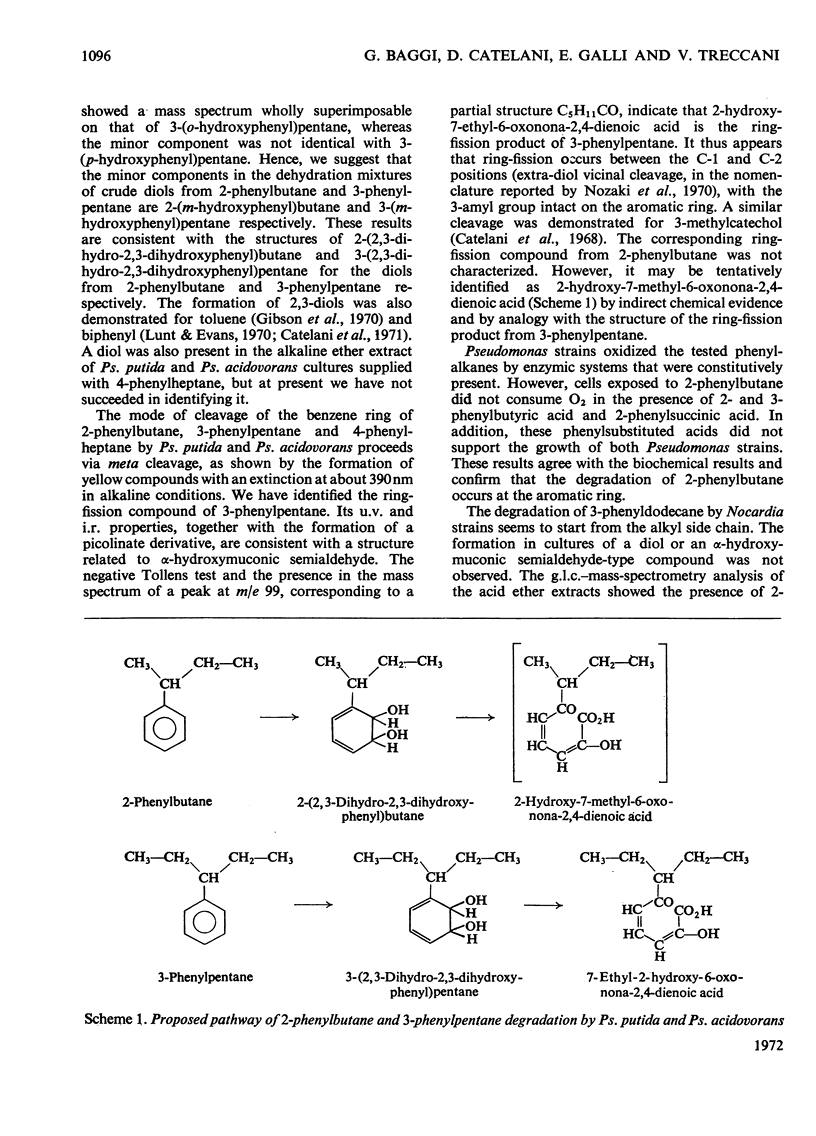
1. Two Pseudomonas strains capable of utilizing 2-phenylbutane, 3-phenylpentane and 4-phenylheptane as the sole carbon and energy source were isolated. 2. Two Nocardia strains capable of utilizing only 3-phenyldodecane as the sole carbon and energy source were isolated. 3. All the isolated strains were unable to grow on the corresponding phenylalkane-p-sulphonates. 4. From liquid cultures of Pseudomonas strains utilizing 2-phenylbutane, 2-(2,3-dihydro-2,3-dihydroxyphenyl)butane was isolated and identified. Evidence for a meta cleavage of the benzene ring was also obtained. 5. From liquid cultures of Pseudomonas strains utilizing 3-phenylpentane, 3-(2,3-dihydro-2,3-dihydroxyphenyl)pentane and 2-hydroxy-7-ethyl-6-oxonona-2,4-dienoic acid were isolated and identified. 6. Evidence for the formation of both a diol and a meta-cleavage compound was obtained from liquid cultures of both Pseudomonas strains utilizing 4-phenylheptane. 7. Liquid cultures of both Nocardia strains utilizing 3-phenyldodecane never formed a diol or a semialdehyde-related compound. 2-Phenylbutyric acid, 3-phenylvaleric acid and 4-phenylhexanoic acid were shown to be present in these cultures.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYLAND E., WILTSHIRE G. H. Metabolism of naphthalene by liver slices. Biochem J. 1953 Feb;53(3):424–426. doi: 10.1042/bj0530424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANONICA L., BONATI A., GAUDENZI L., MOTTA G. Anticolesterolemici di sintesi. II. Acidi fenilvalerianici e fenildimetilacrilici. Farmaco Sci. 1959;14(2):112–121. [PubMed] [Google Scholar]
- Catelani D., Fiecchi A., Galli E. Formation of 2-hydroxy-6-oxo-2, trans-4, trans-heptad-ienoic acid from 3-methylcatechol by a Pseudomonas. Experientia. 1968 Feb 15;24(2):113–113. doi: 10.1007/BF02146927. [DOI] [PubMed] [Google Scholar]
- Catelani D., Mosselmans G., Nienhaus J., Sorlini C., Treccani V. Microbial degradation of aromatic hydrocarbons used as reactor coolants. Experientia. 1970 Aug 15;26(8):922–923. doi: 10.1007/BF02114264. [DOI] [PubMed] [Google Scholar]
- Catelani D., Sorlini C., Treccani V. The metabolism of biphenyl by Pseudomonas putida. Experientia. 1971 Oct 15;27(10):1173–1174. doi: 10.1007/BF02286908. [DOI] [PubMed] [Google Scholar]
- EL-BAGOURY S., FLETCHER S., MORRISON R. B. Effect of chloramphenicol in maintaining the viability of Escherichia coli. Nature. 1956 Dec 29;178(4548):1467–1467. doi: 10.1038/1781467a0. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Cardini G. E., Maseles F. C., Kallio R. E. Incorporation of oxygen-18 into benzene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1631–1635. doi: 10.1021/bi00809a024. [DOI] [PubMed] [Google Scholar]
- Nozaki M., Kotani S., Ono K., Seno S. Metapyrocatechase. 3. Substrate specificity and mode of ring fission. Biochim Biophys Acta. 1970 Nov 11;220(2):213–223. doi: 10.1016/0005-2744(70)90007-0. [DOI] [PubMed] [Google Scholar]
- RAYMOND R. L., DAVIS J. B. n-Alkane utilization and lipid formation by a Nocardia. Appl Microbiol. 1960 Nov;8:329–334. doi: 10.1128/am.8.6.329-334.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- WEBLEY D. M., DUFF R. B., FARMER V. C. Beta-oxidation of fatty acids by Nocardia opaca. J Gen Microbiol. 1955 Oct;13(2):361–369. doi: 10.1099/00221287-13-2-361. [DOI] [PubMed] [Google Scholar]
- WEBLEY D. M. The morphology of Nocardia opaca Waksman & Henrici (proactinomyces opacus Jensen) when grown on hydrocarbons, vegetable oils, fatty acids and related substances. J Gen Microbiol. 1954 Dec;11(3):420–425. doi: 10.1099/00221287-11-3-420. [DOI] [PubMed] [Google Scholar]


