Abstract
1. Arapaima gigas bile salts were hydrolysed by alkali or cleaved with dioxan–trichloroacetic acid to give cholic acid, arapaimic acid, arapaimol-A and arapaimol-B. 2. I.r., n.m.r. and mass spectroscopy and [α]D measurements indicated that arapaimic acid and arapaimol-A and -B are respectively 2α,3α,7α,12α-tetrahydroxy-5β,25∈-cholestan-26-oic acid, 5β,25R-cholestane-2β,3α,7α,12α,26-pentol and 5β-cholestane-2β,3α,7α,12α,26,27-hexol. 3. Partial synthesis of 2β,3α,7α,12α-tetrahydroxy-5α- and -5β-cholan-24-oic acid and their spectral examination fully confirmed these conclusions. 4. A. gigas bile salts show primitive features in that they comprise alcohol sulphates and a C27 acid; they are also specialized in showing 2β-hydroxylation.
Full text
PDF
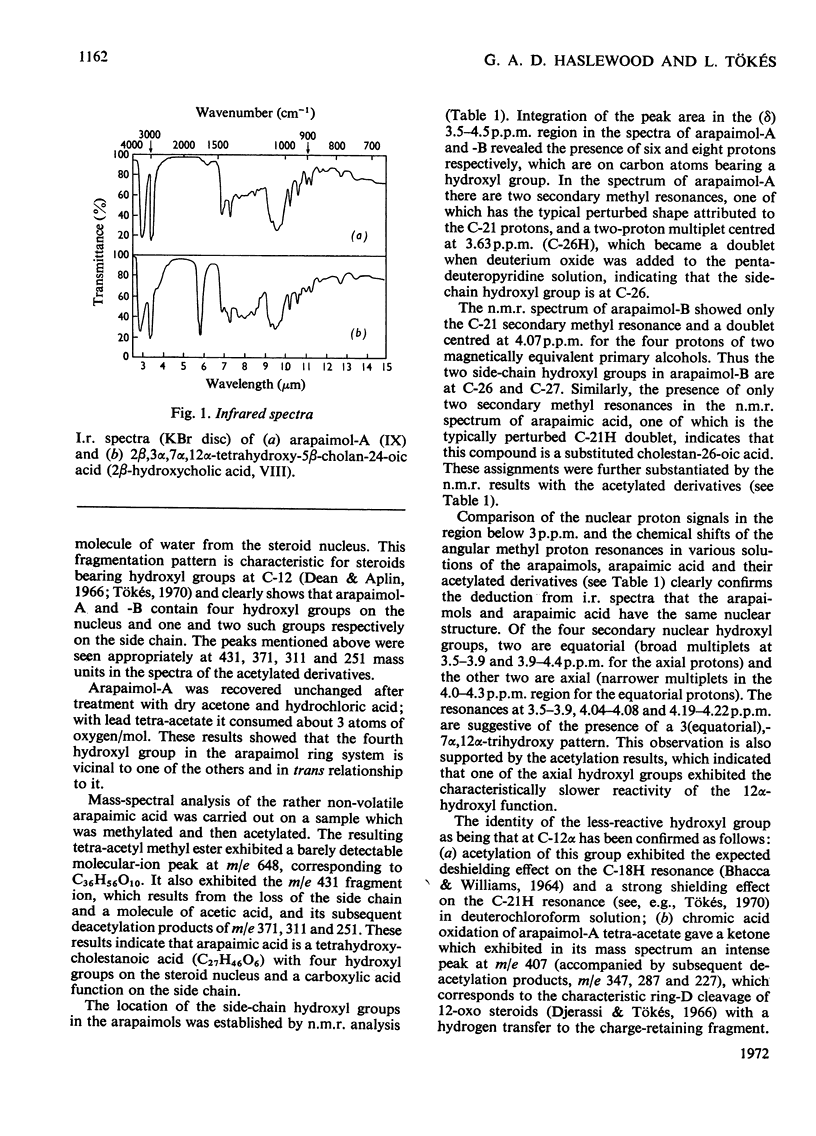

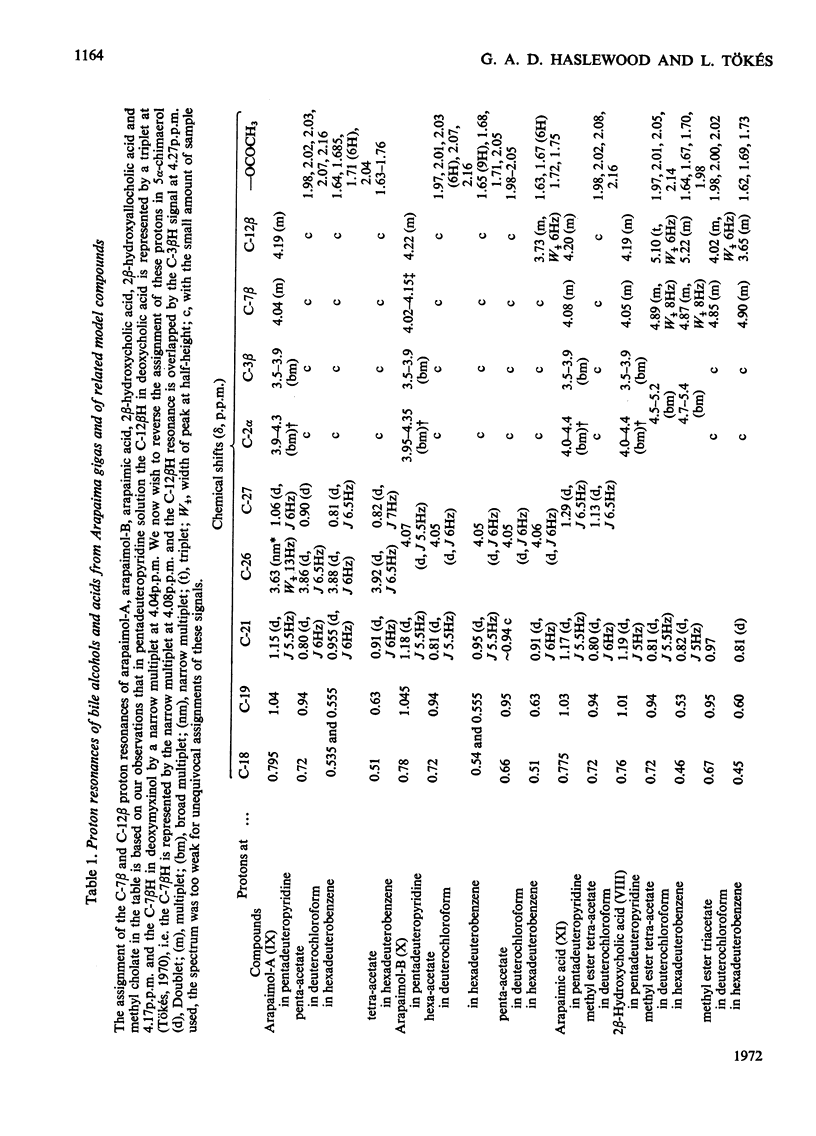
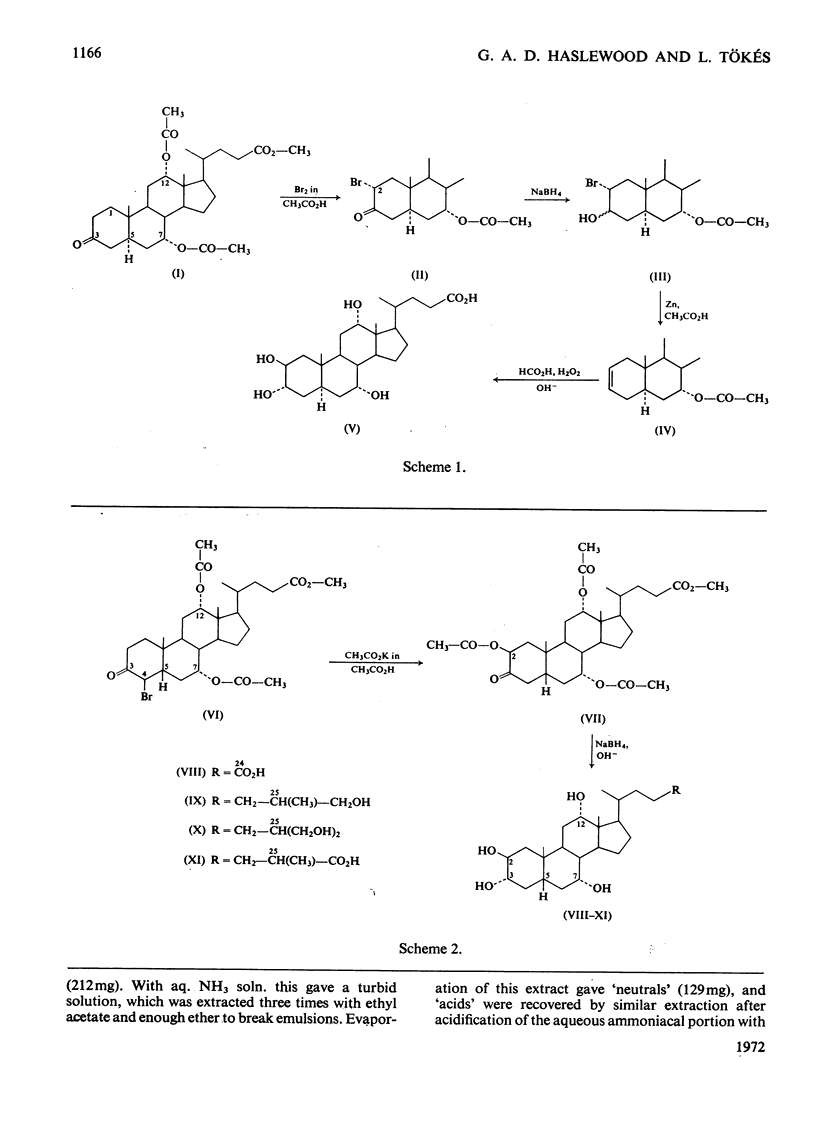




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. G., Haslewood G. A. Comparative studies of bile salts. 5 alpha-Chimaerol, a new bile alcohol from the white sucker Catostomus commersoni Lacépède. Biochem J. 1970 Feb;116(4):581–585. doi: 10.1042/bj1160581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. D., Aplin R. T. Mass spectrometric studies on bile acids: the differentiation between chenodeoxycholic acid and deoxycholic acid and the identification of 3alpha, 7alpha-dihydroxy-5beta-cholestanoic acid in alligator bile. Steroids. 1966 Oct;8(4):565–579. doi: 10.1016/0039-128x(66)90051-1. [DOI] [PubMed] [Google Scholar]
- Djerassi C., Tökés L. Mass spectrometry in structural and stereochemical problems. 93. Further observations on the importance of interatomic distance in the McLafferty rearrangement. Synthesis and fragmentation behavior of deuterium-labeled 12-keto steroids. J Am Chem Soc. 1966 Feb 5;88(3):536–544. doi: 10.1021/ja00955a027. [DOI] [PubMed] [Google Scholar]
- Haslewood G. A. Bile salts of germ-free domestic fowl and pigs. Biochem J. 1971 Jun;123(1):15–18. doi: 10.1042/bj1230015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra M. N., Elliott W. H. Bile acids. 23. A new direct synthesis of allocholic acid and its 3 beta isomer. J Org Chem. 1968 Jan;33(1):175–181. doi: 10.1021/jo01265a033. [DOI] [PubMed] [Google Scholar]


