Abstract
Here we analyzed the relative contributions of CD4+ regulatory T cells expressing Forkhead box protein P3 (FOXP3) and CD8+ regulatory T cells expressing killer cell immunoglobulin-like receptors to the control of autoreactive T and B lymphocytes in human tonsil-derived immune organoids. FOXP3 and GZMB respectively encode proteins FOXP3 and granzyme B, which are critical to the suppressive functions of CD4+ and CD8+ regulatory T cells. Using CRISPR–Cas9 gene editing, we were able to achieve a reduction of ~90–95% in the expression of these genes. FOXP3 knockout in tonsil T cells led to production of antibodies against a variety of autoantigens and increased the affinity of influenza-specific antibodies. By contrast, GZMB knockout resulted in an increase in follicular helper T cells, consistent with the ablation of CD8+ regulatory T cells observed in mouse models, and a marked expansion of autoreactive CD8+ and CD4+ T cells. These findings highlight the distinct yet complementary roles of CD8+ and CD4+ regulatory T cells in regulating cellular and humoral responses to prevent autoimmunity.
Subject terms: Autoimmunity, Immune tolerance
Here the authors use a tonsil organoid culture model system to investigate the roles of human CD4+ and CD8+ regulatory T cells in controlling self-reactive immune responses. CD4+ regulatory T cells were stronger regulators of autoreactive B cells, autoantibodies and antigen-specific antibody affinity, whereas CD8+ regulatory T cells predominantly controlled expansion of follicular helper cells and autoreactive CD4+ and CD8+ T cells.
Main
How the immune system maintains tolerance to self-antigens while having the ability to launch vigorous responses to foreign antigens has been a central question in immunology since Paul Ehrlich first raised it in 1901 (ref. 1). After some false starts, the modern era of investigations into this question emerged with analyses of B cell tolerance by Goodnow2 and the discovery, by Sakaguchi and colleagues, of a CD4+ T cell subset expressing the transcription factor FOXP3 that has been shown to have a major role in preventing autoimmunity3,4. More recently, a small subset of CD8+ T cells in mice was found by Cantor and colleagues to be another T cell subset involved in controlling autoreactivity5–7. Work by our group8,9 has shown that these T cells have a critical role in preventing autoimmunity following infection and have elevated levels in human autoimmune diseases. These CD8+ T cells express genes encoding members of the Ly49 family in mice and killer cell immunoglobulin-like receptors (KIRs) in humans and are notably active during infectious diseases; evidence suggests that they are specifically tasked with controlling self-reactive T cells that have been activated by infection8. Thus, at least two major types of regulatory T (Treg) cell maintain tolerance, but their specific roles have not been explored extensively.
To address this question, we used our recently established human tonsil organoid system10. In response to stimulation with influenza and other vaccines, this system recapitulates the processes of somatic hypermutation, affinity maturation and B cell maturation and produces antigen-specific antibodies10,11. Here, we disabled each of the tonsillar CD4+ and CD8+ Treg cells by knockout (KO) of key genes related to their functions and gauged their effects on self-reactive B and T cells. Ablating the functions of CD4+ Treg cells by knocking out the FOXP3 gene in tonsillar T cells resulted in production of autoantibodies upon stimulation with a panel of classical autoantigens. This was commensurate with the production of autoantibodies that is a hallmark of FOXP3 mutations in both humans and mice12,13, as well as with severe inflammation14. Notably, most tonsil organoids exhibited significant increases in the affinity of antigen-specific antibodies, indicating that FOXP3-expressing T cells also suppress generation of high-affinity antibodies. This could explain why most antibody affinities plateau in about the one-nanomolar range15, perhaps owing to cross-reactivity to self-antigens. To attenuate the function of CD8+ Treg cells, we knocked out the GZMB gene, which encodes granzyme B, a critical effector molecule of these cells16. This disruption had only a minor effect on autoantibody production but a much more pronounced effect on self-reactive CD8+ and CD4+ T cells. This finding for CD4+ T cells was correlated with the large increase in follicular helper T (TFH) cells seen in mice deficient in CD8+ Treg cells6, a phenotype we also observed in the tonsil organoids. These results suggest that the increase in TFH cells was due to more self-reactive T cells escaping this tolerance checkpoint. Unexpectedly, we found a notable rise in plasmablasts with GZMB KO, but this was dependent on TFH cells and was likely to have been an indirect effect. Last, we discovered a significant sex bias in these Treg cell ablations, with tonsils from women typically giving the strongest autoreactive responses. This bias was also observed in unmanipulated tonsillar B cells. This was consistent with the well-known sex biases seen in many autoimmune diseases17 and could be a useful diagnostic to identify women at risk.
We conclude that CD4+ and CD8+ Treg cells have overlapping yet distinct roles in regulating cellular and humoral responses and preventing autoimmunity. These genetic modifications of the tonsil organoids also demonstrate that we can model key features of how autoreactive B and T cells are controlled and, more broadly, quickly test hypotheses and define mechanisms in a purely human system.
Results
Tonsillar and blood Treg cells differ in frequency and phenotype
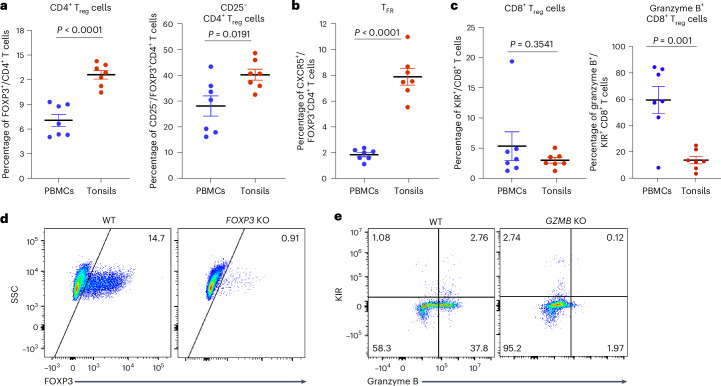
CD4+ and CD8+ Treg cells have been characterized in mice and human blood14,15 but less so in human tonsils. To address this gap, we first investigated and compared the percentages and phenotypes of CD4+ and CD8+ Treg cell subsets in tonsils and peripheral blood cells derived from the same individuals with sleep apnea, with an average age of approximately 40 years. The frequency of FOXP3+CD4+ Treg cells was significantly higher in tonsils compared with blood (Fig. 1a and Extended Data Fig. 1a). Although circulating CD4+ Treg cells usually express high surface levels of CD25 (ref. 18), we found that nearly half of the tonsillar CD4+ Treg cell population expressed low levels of CD25 (ref. 19) (Fig. 1a). In addition, there was a higher percentage of CD4+CXCR5+FOXP3+ follicular Treg cells in the tonsils compared with the blood (Fig. 1b and Extended Data Fig. 1b). The frequencies of CD8+ Treg cells expressing KIR were similar in tonsil and blood samples (Fig. 1c). However, CD8+ Treg cells from the blood displayed significantly higher levels of granzyme B expression (Fig. 1c).
Fig. 1. CD4+ and CD8+ Treg cell subsets in human tonsils showed percentage and phenotypic differences compared with peripheral blood.
a–c, Percentages of CD4+ Treg cells (CD4+FOXP3+) and CD25−CD4+ Treg cells (a), follicular Treg cells (TFR; CXCR5+FOXP3+) (b), and CD8+ Treg cells (KIR+CD8+) and granzyme+CD8+ Treg cells (c) from blood and tonsil samples from age- and gender-matched donors (n = 7). d,e, Representative FACS plots of FOXP3 expression in CD4+ T cells (d) and KIR and granzyme B expression in CD8+ T cells (e) from WT and KO tonsil cultures 5 days postelectroporation. PBMCs, peripheral blood mononuclear cells; SSC, side scatter. The mean ± s.e.m. is indicated. Significance in a–c was calculated using a two-sided unpaired t-test.
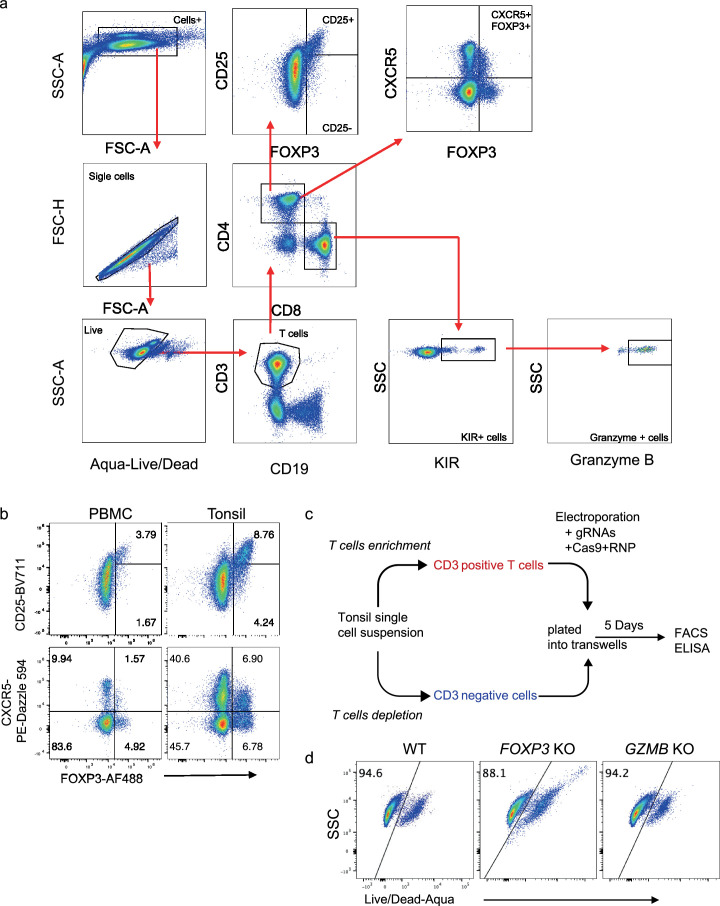
Extended Data Fig. 1. The cell subset percentage and phenotypic differences in tonsils compared to donor-matched peripheral blood and viability after knockouts.
a, A detailed flow gating strategy is used for the plots in Fig. 1a–c. b, A representative FACS profile showing CD25, CXCR5, and FOXP3 expression in PBMC and tonsil samples. c, A workflow of generating genetically modified tonsil organoids using Cas9-RNPs. d, A representative FACS profile showing Live/Dead staining of T cells from the WT, FOXP3 KO, and GZMB KO tonsil organoids.
Efficient gene KO using Cas9–RNPs
Next, we aimed to ablate the functional Treg cell subsets to breach self-tolerance in human tonsil organoids10. Given that cell depletion using the CD25 surface marker becomes inefficient because more than half of the CD4+ Treg cell population expresses low levels of CD25, we decided to disrupt the suppressive function of the cells by knocking out key genes FOXP3 and GZMB using Cas9 nucleoprotein (RNP). The FOXP3 gene encodes an important transcription factor that stabilizes the suppressive function of CD4+ Treg cells20. GZMB encodes granzyme B, which is essential for the cytotoxic function of CD8+ T cells21 and has been shown to have high transcript and protein expression levels in activated KIR+CD8+ T cells22. We isolated total T cells from human tonsils and electroporated them with RNP complexes consisting of the Cas9 protein, FOXP3-targeting guide RNAs (gRNAs) or GZMB-targeting gRNAs (Extended Data Fig. 1c). gRNAs consisting of scrambled sequences were used as a control to generate wild-type (WT) T cells. To avoid the possibility of the attached beads interfering with the function of antigen-presenting cells (for example, B cells and myeloid cells), we purified the unlabeled CD3-negative cells separately from a new vial of tonsil cells. The CD3-negative cells and electroporated CD3-positive cells were enumerated, combined and plated into transwells at a density of 6 × 106 cells per organoid as before10. After 5 days, we assessed the viability and the KO efficiency by measuring intracellular protein levels via flow cytometry. T cells from WT and KO cultures maintained good viability (90% live cells) (Extended Data Fig. 1d). In FOXP3 KO tonsil organoids, the intracellular expression of FOXP3 markedly decreased from 14.7% to 0.91% (Fig. 1d). Similarly, in GZMB KO cultures, the intracellular expression of granzyme B in KIR+CD8+ T cells was significantly reduced, achieving an average KO efficiency of 95% (Fig. 1e). These results demonstrate that highly efficient gene KO can be achieved using Cas9–RNPs in human tonsillar T cells, while maintaining favorable cell viability.
KO tonsil organoids show inflammatory phenotypes
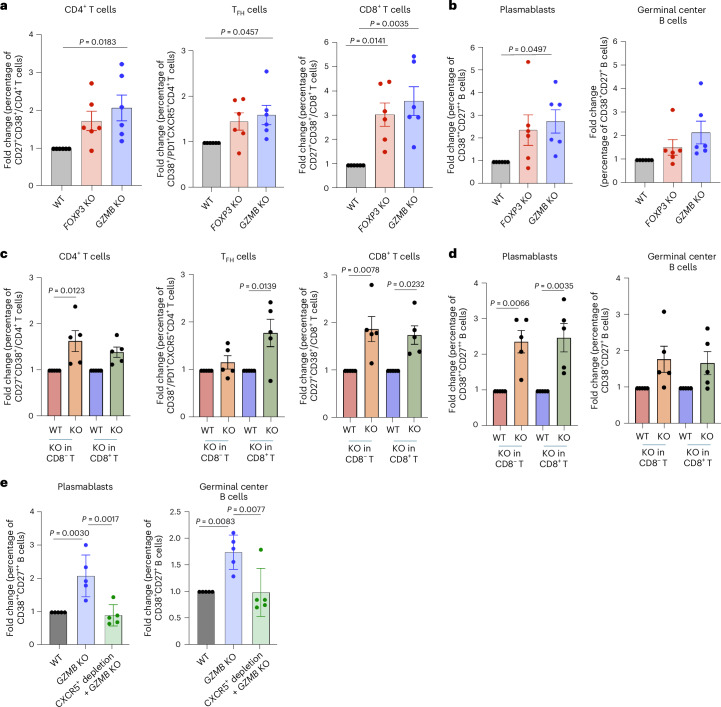
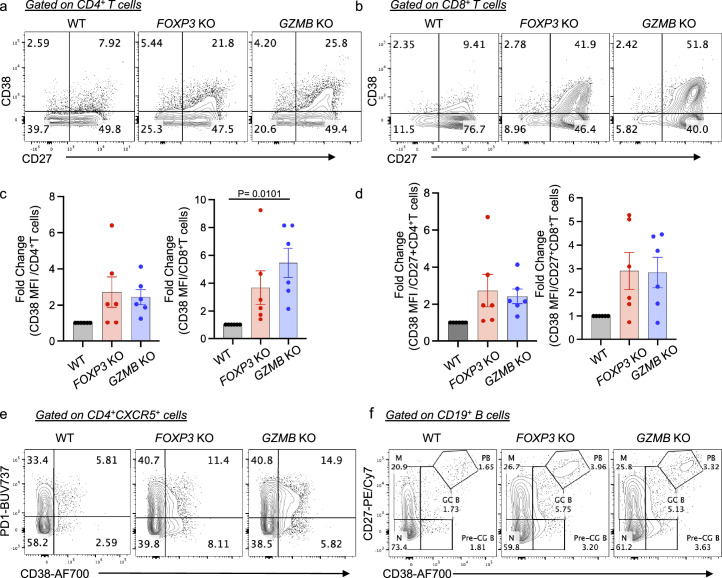
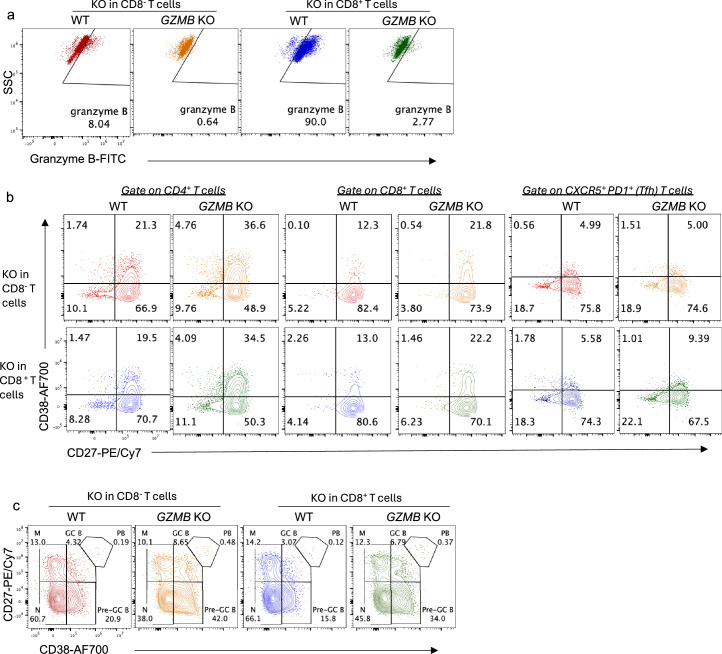
To investigate the impact of ablation of the FOXP3 and GZMB genes in T cells on the overall phenotype of tonsil organoids, we examined the percentages of different B and T cell subsets using surface markers associated with activation and differentiation. In the near absence of FOXP3 or GZMB gene in total T cells, tonsil organoids exhibited signs of inflammation, characterized by increases in percentages of cells expressing activation marker CD38 and costimulation molecule CD27 among both CD4+ and CD8+ T cells compared with WT (Fig. 2a and Extended Data Fig. 2a–d). In addition, there was a significant elevation in the percentage of activated TFH cells from GZMB KO tonsil organoids (Fig. 2a and Extended Data Fig. 2e). With both KOs, tonsil organoids showed an increased percentage of germinal center B cells (CD38+CD27+) and plasmablasts (CD38++CD27++) (Fig. 2b and Extended Data Fig. 2f) in most donors.
Fig. 2. Inflammatory T and B cell phenotypes in FOXP3 and GZMB KO tonsil organoids.
a,b, Statistical analysis of fold change in frequency of activated (CD27+CD38+) CD4+ and CD8+ T cells and TFH (CD38+PD1+CXCR5+CD4+) cells (a) and germinal center B cells and plasmablasts (b) in organoids with FOXP3 KO or GZMB KO T cells compared with WT (n = 6 donors). c,d, Statistical analysis of fold change in frequency of activated (CD27+CD38+) CD4+ and CD8+ T cells and TFH (CD38+PD1+CXCR5+CD4+) cells (c) and germinal center B cells (CD38+CD27+) and plasmablasts (CD38++CD27++) (d) in organoid culture with GZMB KO CD8+ cells or CD8− T cells compared with WT. e, Statistical analysis of fold change in percentage of germinal center B cells and plasmablasts in organoids with WT or GZMB KO in CD8+ T cells or in those with CXCR5+ depletion followed by GZMB KO in CD8+ T cells compared with WT controls (n = 5 donors). Mean ± s.e.m. is indicated. Statistical significance was calculated using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test.
Extended Data Fig. 2. Tonsil organoids with FOXP3 KO and GZMB KO total T cells displayed inflammatory phenotypes after 5-day culture.
a, b, Representative FACS plots showing the expression of CD38 and CD27 on CD4+ T cells (a) and CD8+ T cells (b) in organoids with WT, FOXP3 KO, and GZMB KO T cells. c, d Statistical analysis of the fold change in CD38 MFI of CD4+ T cells (c, left panel) and CD8+ T cells (c, right panel), as well as CD27+CD4+ T cells (d, left panel) and CD27+CD8+ T cells (d, right panel) in organoids with WT, FOXP3 KO, and GZMB KO T cells (n = 6). e Representative FACS plots showing the expression of PD1 and CD38 on Tfh cells (pre-gated on CD4+ CXCR5+ ) in WT and KO tonsil organoids after 5-day culture without stimulation. f, Representative FACS plot showing the expression of CD27 and CD38 on B cells (pre-gated on CD3-CD19+ ) in WT and KO tonsil organoids after 5-day culture without stimulation. The significance in (c) and (d) was calculated by one-way ANOVA followed by Tukey’s multiple comparisons test.
To further investigate the role of CD8+ Treg cells in the inflammatory phenotypes observed in organoids with GZMB KO T cells—particularly given prior reports of cytotoxic effects mediated by CD4+ Treg cells via granzyme B on autoreactive T cells23—we replicated experiments by knocking out GZMB in either CD8+ or CD8− T cells (Extended Data Fig. 3a). Our findings revealed significant expansion of CD4+ T cells, TFH cells, CD8+ T cells and plasmablasts within tonsil organoids containing GZMB KO CD8+ T cells (Fig. 2c,d and Extended Data Fig. 3b,c). These results were consistent with the phenotypes seen for total T cells. Notably, GZMB KO in CD8− T cells also affected inflammation, as we observed a similar increase in the percentage of the CD27+CD38+ population, especially in CD4+ T cells, CD8+ T cells and plasmablasts (Fig. 2c,d and Extended Data Fig. 3b,c).
Extended Data Fig. 3. Representative plots showing inflammatory phenotypes in tonsil organoids with GZMB KO in CD8+ and CD8- T cells.
a, Granzyme B expression in CD8- and CD8+ T cells 5 days post GZMB KO. b, Expression of CD38 and CD27 in CD4+ T cells, CD8+ T cells and Tfh cells from 5-day cultured tonsil organoids with GZMB KO in CD8- and CD8+ T cells. c, Expression of CD27 and CD38 in B cells from tonsil organoids with GZMB KO in CD8- and CD8+ T cells.
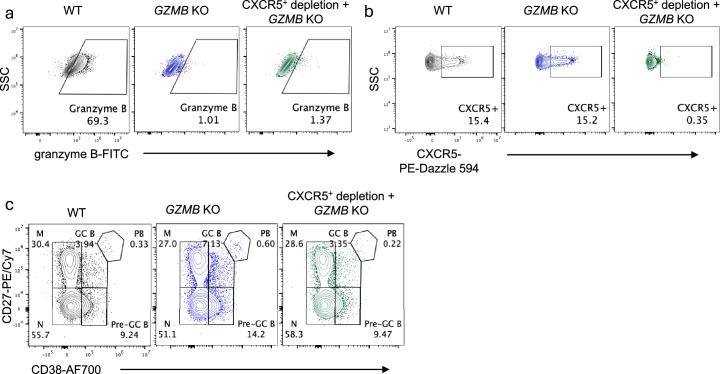
TFH cells play essential parts in the formation of germinal centers and direction of B cell differentiation by providing costimulatory signaling24–26. To further investigate whether the expansion of plasmablasts we observed in organoids with GZMB KO in CD8+ T cells was due to direct interaction between T and B cells or occurred indirectly via TFH cells, we examined B cell differentiation after efficient depletion of CXCR5+ T cells followed by KO of GZMB in CD8+ T cells (Extended Data Fig. 4a,b). When CXCR5+ T cells were depleted, while the number of CD8+ T cells was kept the same as in the WT, there was a significant reduction in the frequency of plasmablasts and germinal center B cells in GZMB KO tonsil organoids (Fig. 2e and Extended Data Fig. 4c). This finding suggests that the increased plasmablast levels require the presence of TFH cells and thus are likely to represent an indirect effect.
Extended Data Fig. 4. Representative plots showing plasmablast differentiation after CXCR5+ depletion and GZMB KO in CD8+ T cells of tonsil organoids.
a, Granzyme B expression in CD8+ T cells under the indicated conditions from tonsil organoids. b, CXCR5 expression in CD4+ T cells under indicated conditions from tonsil organoids. c, Expression of CD27 and CD38 in B cells from tonsil organoids with WT, GZMB KO, and CXCR5-depleted GZMB KO CD8+ T cells.
Differential autoantibody production in KO tonsil organoids
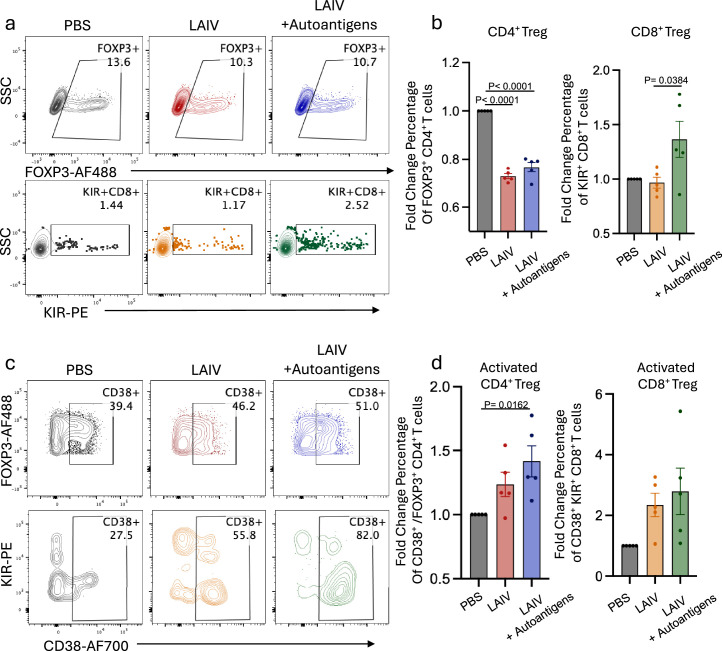
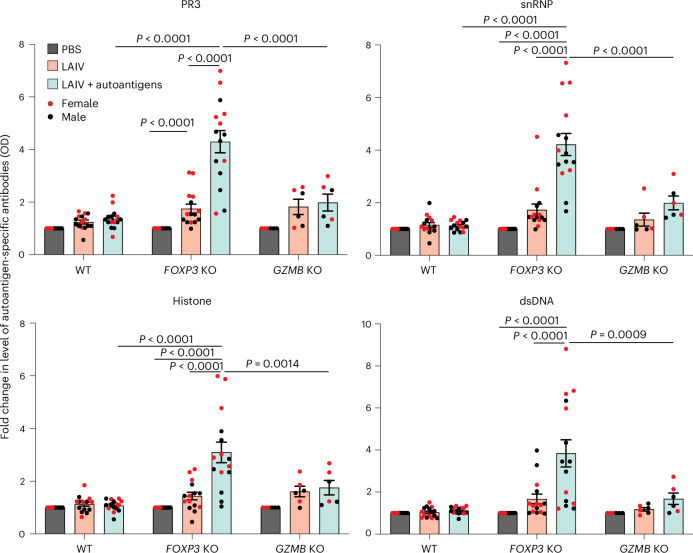
Despite inflammatory phenotypes being observed in B cells within both KO tonsil organoids, there was no significant change in autoantibody production compared with WT tonsil organoids, even with addition of the autoantigen to the culture (Extended Data Fig. 5). Previous studies have suggested that viral infection could be a primary factor in initiation of autoimmune diseases27, and mouse models have shown the development of autoimmune phenotypes following viral infection6. Thus, we investigated whether live attenuated influenza vaccine (LAIV) could induce an autoimmune response in the germinal center. The WT, FOXP3 KO and GZMB KO tonsil organoids were stimulated with phosphate-buffered saline (PBS), LAIV alone or a combination of LAIV and autoantigen cocktail. The autoantigen cocktail contained proteinase 3 (PR3), core histones, double-stranded DNA (dsDNA) and small nuclear ribonucleoproteins (snRNP), which are relatively common targets of autoantibodies in patients with autoimmune diseases28–30 and viral infections14. In WT tonsil organoids, LAIV stimulation led to a decreased percentage of CD4+ Treg cells and an increased percentage of CD8+ Treg cells (Extended Data Fig. 6a,b), both characterized by elevated CD38 expression (Extended Data Fig. 6c,d). However, no significant increase in autoantibodies was observed following LAIV stimulation (Fig. 3). By contrast, the FOXP3-deficient tonsil organoids showed up to seven-fold higher levels of autoantibodies compared with WT after stimulation with LAIV and autoantigens (Fig. 3). We also observed that most FOXP3 KO tonsil organoids with high autoantibody production were derived from female donors, whereas stimulated GZMB KO tonsil organoids secreted only minimal levels of autoantibodies. These results suggest that CD4+ Treg cells have a more prominent role in control of autoantibody responses compared with CD8+ Treg cells, consistent with FOXP3 deficiencies observed in mice and humans10,11.
Extended Data Fig. 5. PR3-Specific Autoantibody Production in Organoids with WT or FOXP3 KO T Cells.
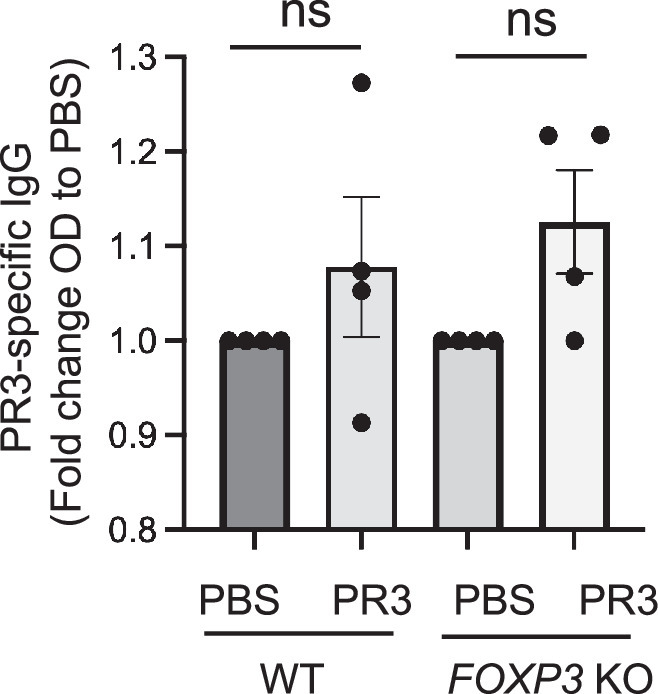
Ten-day cell supernatant from organoids with WT or FOXP3 KO T cells w as collected, and PR3-specific autoantibody was measured by ELISA (n = 4). The fold change in PR3-specific antibody (OD) between PBS and PR3 conditions was calculated. Significance was determined using one-way ANOVA followed by Tukey’s multiple comparisons test. ns, not significant.
Extended Data Fig. 6. CD4+ and CD8 + Tregs markers and phenotypes in WT organoids stimulated with LAIV or LAIV plus autoantigens.
a, Representative FACS plots showing CD4+ Tregs (FOXP3+ in CD4+ T cells) or CD8+ Tregs (KIR+ in CD8+ T cells) in WT tonsil organoids after 5 days of culture. b, Fold change in the percentage of FOXP3+CD4+ T cells and KIR+CD8+ T cells in WT tonsil organoids stimulated with LAIV or LAIV plus autoantigens (n = 5 donors). c, Representative FACS plots showing CD38 expression on CD4+ Tregs (pre-gated on FOXP3+CD4+ T cells) or KIR+CD8+ Tregs (pre-gated on KIR+CD8+ T cells) in WT tonsil organoids after 5 days of culture. d, Fold change in the percentage of activated CD38+ FOXP3+CD4+ T cells and CD38+ KIR+CD8+ T cells in WT tonsil organoids stimulated with LAIV or LAIV plus autoantigens (n = 5 donors). Significance in (b) and (d) was calculated by one-way ANOVA followed by Tukey’s multiple comparisons test.
Fig. 3. Differential autoantigen-specific antibody production in FOXP3 KO and GZMB KO tonsil organoids after stimulation.
Autoantibodies specific to PR3, dsDNA, snRNP and core histone were detected in the supernatant of 10-day cultures under the indicated conditions (n = 15 for FOXP3 KO; n = 6 donors for GZMB KO). The fold change in optical density (OD) values compared with PBS was calculated. The mean ± s.e.m. is indicated. Statistical significance was calculated by two-way ANOVA followed by Tukey’s multiple comparisons test.
Autoreactive B cells in FOXP3 KO organoids expanded after stimulation
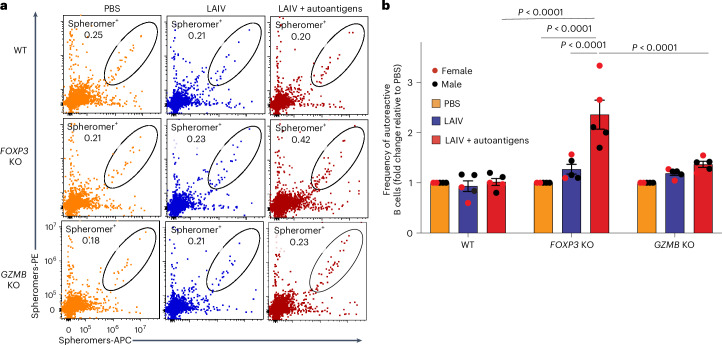
The higher levels of autoantibody production in the FOXP3 KO tonsil organoids led us to hypothesize that the frequency of autoreactive B cells would also be elevated following stimulation. To test this hypothesis, we generated a pool of three ‘spheromers’, which are highly sensitive multimer reagents based on modified maxi-ferritin31, specifically targeting the autoantigens PR3, snRNP-C and core histone. In the presence of LAIV, the autoantigen cocktail profoundly stimulated the expansion of self-antigen-specific B cells in FOXP3 KO tonsil organoids (Fig. 4a,b), consistent with the observed autoantibody levels. Notably, the top two FOXP3 KO tonsil organoids that produced the higher frequency of autoreactive B cells after LAIV plus autoantigen stimulation were also derived from female donors (Fig. 4b). By contrast, knocking out GZMB in tonsillar T cells had only a minor impact on the frequencies of autoreactive B cells (Fig. 4a,b), confirming that CD4+ Treg cells modulate autoantibody secretion by constraining the activation of autoreactive B cells.
Fig. 4. Autoreactive B cells expanded from FOXP3 KO tonsil organoids after stimulation with LAIV and autoantigens.
a, Representative FACS plot showing costained spheromer-positive B cells specific to PR3, snRNP-C and core histone (H3.1) after 7 days of culture. b, Fold change in the percentage of costained B cells between stimulation and PBS conditions in tonsils with WT, FOXP3 KO and GZMB KO T cells 7 days poststimulation (n = 5 donors). The mean ± s.e.m. is indicated. Statistical significance was calculated using two-way ANOVA followed by Tukey’s multiple comparisons test.
Differential autoreactive T cell response in KO organoids
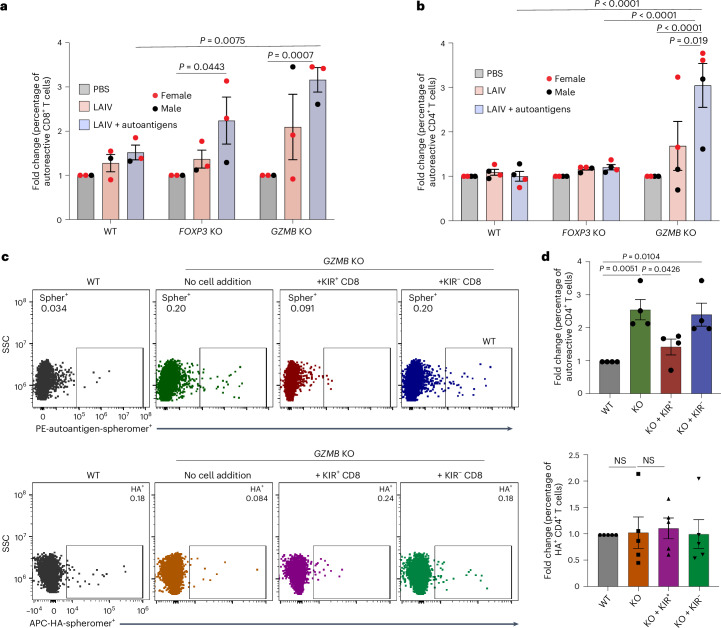
Although GZMB KO tonsil organoids generated a mild autoantibody response (Fig. 3), in most donors we observed an increased percentage of activated T cells compared with WT tonsil organoids (Fig. 2a). This suggests that CD8+ Treg cells may exhibit a more robust suppressive response against autoreactive T cell responses. To test this hypothesis, we analyzed the effects of CD8+ Treg cells on CD8+ T cells specific to self-peptides derived from fructose bisphosphate aldolase, keratin, Y-chromosome-encoded SMCY, preproinsulin (PPI) and glutamic acid decarboxylase 65. These self-peptides have previously been detected in the blood of healthy humans, and SMCY-specific CD8+ T cells, in particular, are strictly regulated in males32. To detect CD8+ T cells specific to these self-peptides, we generated HLA-A*0201 spheromers and also used a peptide from the hepatitis C virus as a negative control. We stimulated the tonsil organoids with WT, FOXP3 KO and GZMB KO T cells derived from HLA-A*0201 donors with PBS, LAIV, or LAIV supplemented with the autoantigen-derived peptides. After 7 days poststimulation, two of three FOXP3 KO tonsil organoids showed a two-fold increase in the frequency of self-specific CD8+ T cells. All three GZMB KO tonsil organoids showed an almost-three-fold increase with statistical significance in self-peptide-specific CD8+ T cells after stimulation with LAIV and the autoantigen peptide pool (Fig. 5a).
Fig. 5. Differential CD4+ and CD8+ T cell responses to autoantigens in FOXP3 KO and GZMB KO tonsil organoids after stimulation with LAIV and autoantigen stimulation.
a, HLA-A*0201 tonsil organoids under the indicated WT or KO conditions were cultured for 7 days and then collected for spheromer staining. The percentage of autoreactive CD8+ T cells was determined after gating CD8+CD3+ T cells and human papillomavirus spheromer-negative T cells. The fold change in the percentage of autoreactive T cells under stimulated conditions compared with the control group (treated with PBS) was calculated (n = 3 donors). b, HLA-DRB1*04:01 tonsil organoids with WT, FOXP3 KO or GZMB KO total CD3+ T cells (n = 4 donors) were cultured for 7 days, followed by staining with self-peptide-specific or HA-specific spheromers. The fold change in the percentage of autoreactive-specific CD4+ T cells compared with the PBS condition was calculated. c, KIR+CD8+ T cells were depleted, followed by GZMB KO in CD8+ T cells. Subsequently, KIR+CD8+ or KIR−CD8+ T cells were reintroduced into the culture before stimulation with LAIV and autoantigens. Control cultures (WT) received LAIV stimulation without depletion or gene KO. Representative FACS plots show the percentages of spheromer-positive autoreactive CD4+ T cells (upper panel) or HA-specific CD4+ T cells (lower panel) from tonsil organoids under the indicated conditions. d, Fold change in the frequency of autoreactive and HA-specific CD4+ T cells compared with WT (n = 5 donors). Mean ± s.e.m. is indicated. Statistical significance in a, b and d was calculated using two-way ANOVA followed by Tukey’s multiple comparisons test. Spher, spheromer; NS, not significant.
We next investigated the regulation of autoreactive CD4+ T cells by Treg cell subsets. We selected self-peptides from gp100, fibrinogen or PPI, as these are relevant in antitumor responses and autoimmunity33–36. These antigens can be recognized by CD4+ T cells in healthy individuals expressing HLA-DRB1*04:01 (ref. 34). Influenza hemagglutinin (HA)-specific spheromer reagents were included in the staining panel to assess the suppression by CD4+ and CD8+ Treg cells of autoreactive T cells compared with other activated T cells. Notably, we observed a statistically significant expansion of CD4+ T cells specific to self-antigens, particularly in tonsil organoids with GZMB KO T cells stimulated by LAIV and autoreactive peptides (Fig. 5b). Whereas HA-reactive CD4+ T cells expanded after LAIV stimulation, there was no significant difference in the percentage of HA-specific CD4+ T cells across the WT, FOXP3 KO and GZMB KO cultures (Extended Data Fig. 7). Furthermore, the majority of expanded autoreactive CD4+ T cells in the GZMB KO stimulated tonsil organoids were derived from female donors (Fig. 5b). By contrast, knocking out FOXP3 did not affect the frequency of CD4+ T cells specific to self-antigens (Fig. 5b).
Extended Data Fig. 7. The percentage of HA-specific CD4+ T cells in organoids with WT, FOXP3 KO, or GZMB KO T cells.
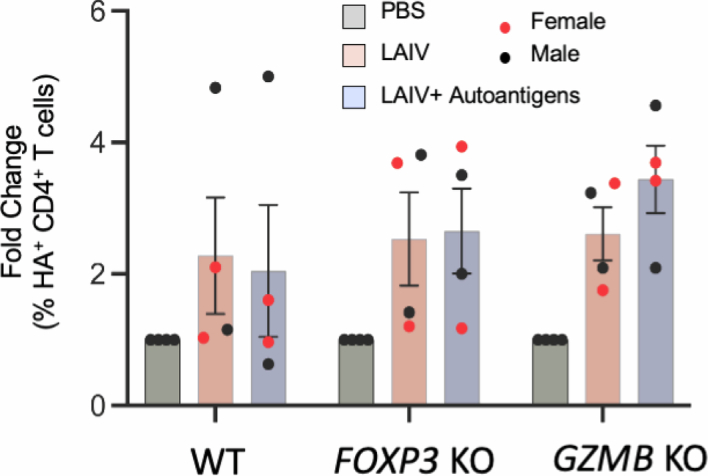
HLA-DRB1*04:01 tonsil organoids with WT, FOXP3 KO or GZMB KO total CD3+ T cells (n = 4 donors) were cultured for 7 days, followed by staining with HA-specific spheromer. The fold change in the percentage of HA-specific CD4+ T cells relative to the PBS condition was calculated. Mean ± s.e.m is indicated. Significances were calculated by two-way ANOVA followed by Tukey’s multiple comparisons test. All p-values are greater than 0.05.
To further confirm that the increase in autoreactive CD4+ T cells from GZMB KO organoids was specifically due to functional impairment of KIR+CD8+ T cells, we depleted KIR+CD8+ T cells from tonsils derived from DRB1*04:01+ donors, followed by KO of GZMB in CD8+ T cells. KIR+ and KIR−CD8+ T cells were then reintroduced into the culture and supplemented with selected self-peptides from gp100, fibrinogen, or PPI and LAIV cocktail. In the absence of KIR+CD8+ T cells and granzyme B, the cocktail profoundly stimulated the expansion of autopeptide-specific CD4+ T cells. Reintroducing KIR+CD8+ T cells from the same donor significantly reduced the percentage of autoreactive T cells, whereas introducing an equal amount of KIR−CD8+ T cells did not (Fig. 5c,d). By contrast, there was no significant effect on the percentage of HA+ T cells under comparable conditions. These results suggest that CD8+ Treg cells are a potent suppressor of autoreactive CD4+ T cells.
Overall, our results demonstrate that CD4+ and CD8+ Treg cells have distinct areas of responsibility for suppression of self-reactive lymphocytes, albeit with some overlap.
FOXP3 KO enhances higher antibody affinity
As FOXP3 is critical in regulation of autoantibody production, we investigated whether it could also affect antibody responses against foreign antigens. Taking advantage of the fact that we had used LAIV as an adjuvant in these experiments, we measured the half-life (t1/2) of the elicited antibodies against recombinant influenza hemagglutinin (HA). The tonsil organoids with WT, FOXP3 KO or GZMB KO T cells were stimulated with PBS or LAIV for 10 days. Supernatants from the organoid cultures were collected for the biolayer interferometry assays. Knocking out FOXP3 in tonsillar T cells led to an increase in the t1/2 of the antibodies, such that it was up to 18-fold higher than that in the donor-matched WT condition (Fig. 6), indicating that FOXP3-expressing T cells enable production of antibodies with much higher binding affinities in most tonsil donors. The effect of GZMB KO was less dramatic, with an average two-fold improvement in five donors. These data indicate that CD4+ Treg cells not only regulate autoreactive B cells but also have an impact on B cells in general, effectively limiting their affinity to the typical range, at or near 1 nM.
Fig. 6. FOXP3 KO enhances antibody affinity, whereas GZMB KO shows only a limited effect.
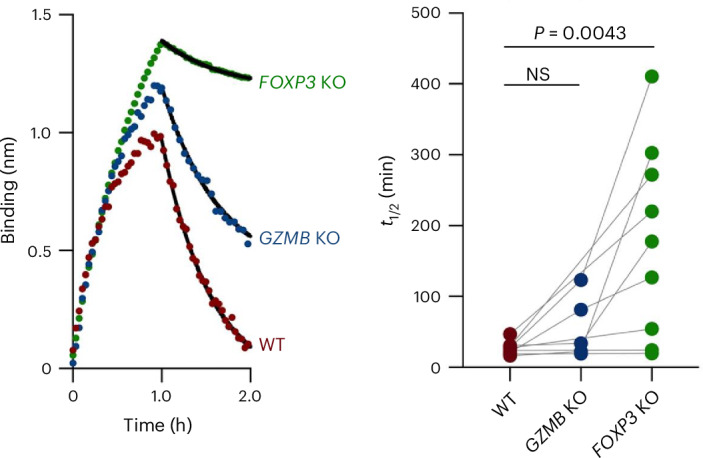
Cell supernatants from LAIV-stimulated WT, GZMB KO and FOXP3 KO tonsil cultures were collected after 10 days of culture. Biolayer interferometry assay was performed to measure the binding affinity of antibodies to HA CA/09 hemagglutinin. An overlay of binding traces under the indicated condition is shown. The t1/2 was calculated from the off-rates (koff) using the equation for first-order rate kinetics: t1/2 = 0.693/koff. Statistical significance was determined using one-way ANOVA followed by Tukey’s multiple comparisons test. NS, not significant.
Higher autoreactivity in organoids from women compared with men
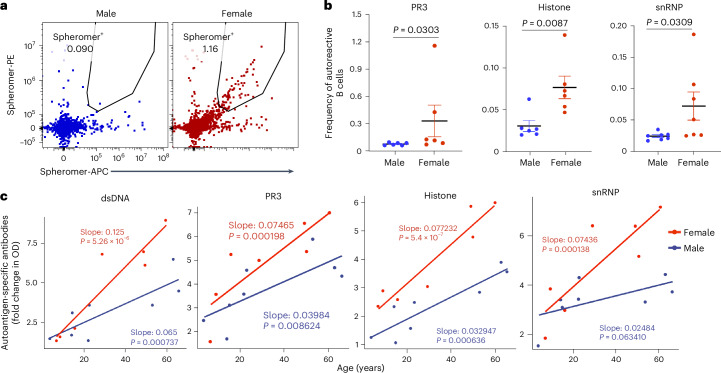
In recent years, risk factors associated with autoimmune diseases, such as sex and aging, have garnered significant attention37,38. Using our tonsil organoid system, we explored some factors that might be associated with self-reactive antibodies. A previous study showed that early immature B cells in healthy donors could produce self-reactive antibodies39. Thus, we investigated whether autoreactive B cells were also present in tonsils and whether there was a correlation with sex. For this purpose, we used biotinylated spheromers specific to PR3, snRNP or core histone, coupled with PE- or APC-conjugated streptavidin, and costained tonsil samples from age-matched male and female donors. In most donors, we successfully detected B cells specific to these autoantigens, constituting approximately 0.01% to 0.2% of B cells. Notably, these higher levels were found only in the female donors (Fig. 7a,b). Next, we investigated whether sex and chronological age contributed to the autoantibody production we observed in stimulated tonsil organoids with FOXP3 KO T cells. Indeed, we found a strong correlation between autoantibody production and age, with higher levels of autoantibodies detected in donors above 40 years of age (Fig. 7c). These results highlight the unique and representative nature of tonsil cells and organoids as a valuable system for hypothesis testing and mechanistic studies related to human autoimmune diseases.
Fig. 7. Tonsillar B cells show a higher frequency of autoreactivity in women versus men, regardless of attenuating Treg cells.
a, Representative FACS plots showing spheromer (PR3)-positive tonsillar B cells from age-matched male and female donors. b, Dot plots illustrating the percentage of B cells specific to PR3, histone and snRNP from age-matched donors (n = 6). c, Autoantibodies specific to PR3, dsDNA, snRNP and core histone were detected by ELISA in the supernatant of 10-day cultures from organoids with WT and FOXP3 KO T cells (n = 15) stimulated with LAIV and autoantigen pools. The fold change in optical density compared with PBS was analyzed using a linear regression model to predict the correlations between autoantibody levels, age and sex. The regression model coefficients (slopes) represent the effect of age on the response variable for each sex, with P values indicating statistical significance. Model statistics (F-statistic, P value and adjusted R2) are reported for each autoantibody: dsDNA: F(2,12) = 30.08, P = 2.115 × 10−5, adjusted R2 = 0.806; PR3: F(2,12) = 13.97, P = 0.0007357, adjusted R2 = 0.649; histone: F(2,12) = 46.49, P = 2.231 × 10−6, adjusted R2 = 0.8666; snRNP: F(2,12) = 15.58, P = 0.0004622, adjusted R2 = 0.6756. In b, significance was calculated using one-sided Mann–Whitney test; mean ± s.e.m. is indicated.
Discussion
Although we have learned a great deal about the characteristics and importance of FOXP3-expressing CD4+ T cells in enforcing self-tolerance40–44, it has only recently emerged that a small subset of CD8+ T cells, characterized by Ly49 expression in mice and KIR expression in humans, also plays an important part7–9. These CD8+ Treg cells are particularly active in the intestines during acute celiac disease and are elevated in patients with other autoimmune diseases, as well as during infections in humans and mice8. Conditional KO of the predominant Ly49 gene in mice leads to autoimmune sequelae following either influenza or lymphocytic choriomeningitis virus infection8. The importance of both Treg cell types in controlling self-reactivity raises the question of what their specific roles might be. To address this question, we made use of our recent demonstration that a type of organoid made from human tonsil cells could reproduce many of the hallmarks of an adaptive immune response in vitro, especially when stimulated with various vaccines10,11. The single-cell suspensions of tonsil cells as a starting point for organoid culture provide an ideal platform for using CRISPR–Cas9 gene editing to disable CD4+ and CD8+ Treg cells to analyze their relative roles in controlling autoreactive T and B cells.
Knocking out FOXP3 resulted in expansion of self-reactive B cells and production of autoantibodies. Autoantibodies are a characteristic of FOXP3 disruption in humans and mice45,46; thus, this effect in the tonsil organoids phenocopies the in vivo results. However, it was surprising that despite both FOXP3 and GZMB KO resulting in similar levels of TFH cells and plasma cells, the latter had only a minimal impact on autoantigen-specific antibody production. Given that CD8+KIR+ Treg cells are known to target TFH cells, as observed in our study and others7,47, but do not affect antibody production, we speculate that the effects of CD4+ Treg cells on autoantibody production are exerted through direct interaction with B cells. This is supported by previous work showing that CD4+ Treg cells are present in follicles and T–B borders in human tonsils and can directly suppress B cell class switch recombination48, as well as inhibiting autoantibody-producing B cells in vitro49. Although FOXP3 ablation in human Treg cells does not affect CD25 and CTLA-4 expression50, other signaling molecules may be involved, considering the potential impact of FOXP3 on genome architectural functions and transcriptional regulation51. Given the diverse FOXP3+ Treg cell population52, understanding which subsets contribute to autoantibody production and B cell regulation is crucial.
We also observed substantial increases in the bulk serological affinity of influenza-specific antibodies in most of the FOXP3-deficient organoids and to a lesser extent in GZMB KOs; this is reminiscent of the increased affinity for self-specific antibodies in AIRE-deficient individuals53,54 and indicates that disrupting T cell tolerance allows the emergence of higher-affinity antibodies. This is likely to relate to the phenomenon by which the higher the affinity of an antibody, the greater the chances of pathological cross-reactivity to one or more self-antigens55,56. This result also suggests a reason that most monoclonal antibodies typically fall within the low nanomolar range and rarely higher54.
Our results highlight the differential roles of CD4+ and CD8+ Treg cells in regulating autoimmunity, potentially by targeting different cellular activities and involvement in different stages of autoimmune development. GZMB KO allowed both autoreactive CD4+ and CD8+ T cells to proliferate, demonstrating that CD8+ Treg cells are the major factor controlling T cell tolerance, especially for autoreactive CD4+ T cells. This CD4+ effect was earlier observed by the marked increase in TFH cells in mice deficient in CD8+ Treg cells6,8, suggesting that more TFH cells were allowed to expand than normal when CD8+ Treg cell activity was absent or attenuated. In addition, previous work with patients infected with SARS-CoV-2 or influenza has demonstrated increased proportions of CD8+ Treg cells during these infections but no change in CD4+ Treg cells8,9. Infection is a crucial trigger for the breakdown of tolerance, with many autoimmunity patients reporting an infection just before the onset of clinical symptoms57. Thus, the disruption of CD8+ Treg cell control could be a key first step in triggering clinical autoimmunity. However, as autoantibodies can precede autoimmune disease by years58–61, there is likely to be a loss of CD4+ Treg cell control earlier.
In summary, we have developed human tonsil organoid models of autoimmunity using CRISPR–Cas9 gene editing techniques. By performing targeted KO, we investigated the distinct roles of two different Treg cell subsets. Knocking out FOXP3 in the CD4+ Treg cell within the tonsil organoids resulted in a drastic increase in autoreactive B cells, autoantibodies and antigen-specific antibody affinity. On the other hand, knocking out GZMB in T cells led to dysfunction of CD8+ Treg cells, promoting the expansion of TFH cells and autoreactive CD4+ and CD8+ T cells, and enhancing B cell differentiation. These findings align with observations made in mouse models and patients with defective CD4+ and CD8+ Treg cells. Further exploration of the tonsil organoid model holds significant potential for identifying new mechanisms and translating them into therapeutic approaches for autoimmune diseases.
Methods
Human samples and tonsil processing
The collection and processing of tonsil samples from children and adult volunteers were covered by institutional review board (IRB) protocols 30837 and 60741, approved by the Stanford University IRB. Blood from healthy donors was requested from the Stanford Blood Center under IRB protocol 40146. Written informed consent was obtained from adult participants and from the legal guardians of children aged 0–17 years. No participant compensation was provided for the participants. Any individuals who were taking systemic immunomodulatory drugs, had a history of immunosuppressive or autoimmune diseases, or had a serious active infection at the time of the procedure were excluded.
The procedures for tonsil samples followed those described in a previous study10. Tonsil samples were collected immediately after surgery and incubated in Ham’s F-12 medium (Gibco) containing Normocin (InvivoGen), penicillin and streptomycin for at least 1 h at 4 °C before processing. Afterward, tonsil tissue was manually sectioned into small pieces (~5 mm × 5 mm × 5 mm) and passed through a 100-mM filter using a syringe plunger to obtain a cell suspension. The filter was repeatedly washed with complete medium to help filter the cells. Complete medium included RPMI supplemented with Glutamax (Thermo Fisher), 10% fetal bovine serum (FBS), 1× penicillin–streptomycin, 1× Normocin (InvivoGen), 1× insulin–transferrin–selenium supplement (Gibco), 1× nonessential amino acids and 1× sodium pyruvate. Debris was reduced by Ficoll density gradient separation. Collected cells were washed with PBS, enumerated and frozen into cryovials in FBS + 10% dimethyl sulfoxide. Frozen aliquots were stored at −140 °C until use.
Blood processing
Blood was diluted with cell culture medium and 2% FBS in a 1:1 volume ratio. This diluted blood was then carefully placed on top of a density gradient medium (Ficoll-Paque, GE Healthcare) and centrifuged at 800g for 20 min without the brake. For collection of peripheral blood mononuclear cells, a pipette was inserted directly through the upper plasma layer into the interface containing the mononuclear cells. The collected cells were washed twice with medium, frozen in aliquots using FBS with 10% dimethyl sulfoxide and stored at −140 °C until needed.
Cas9–RNP assembly and electroporation
Cas9–RNPs (Integrated DNA Technologies) were prepared by incubating 20 µM Cas9 with 20 µM gRNA (Synthego) at a 1:1 ratio at 37 °C for 15 min, resulting in a final concentration of 10 µM. The sequences of oligonucleotides are provided in Supplementary Table 1. Electroporation was performed using reagents from a P3 Primary Cell 4D-Nucleofector X Kit S (Lonza) according to the manufacturer’s instructions. Target T cells were gently suspended in P3 buffer supplemented with supplement 1 reagent at a density of 10–20 million cells per 20 μl. The Cas9–RNPs and T cells were mixed gently in the P3 buffer. This mixture was then transferred to a 4D-Nucleofector cuvette from Lonza Bioscience and subjected to electroporation using code EH105. Following electroporation, the cuvette was placed in the tissue culture incubator at 37 °C for 30 min to allow the cells to recover. Once recovered, the cells were ready to be cultured in transwells.
Cell sorting and organoid assembly
For KO experiments involving total T cells, T cells were isolated from tonsil single-cell suspensions using a Human Pan T Cell Isolation Kit (Miltenyi Biotec) for electroporation. CD3− cells were obtained by depleting CD3+ T cells using CD3 MicroBeads, human (Miltenyi Biotec). Organoids were reassembled by combining KO CD3+ T cells with CD3− cells at the same donor-specific cell ratio and cultured at a density of 6 × 106 cells in 100 µl per transwell.
For GZMB KO experiments in CD8+ T cells, untouched CD8+ T cells were isolated using a CD8+ T Cell Isolation Kit, human (Miltenyi Biotec) for electroporation. The CD8− cell fraction was collected by staining the tonsil single-cell suspension with CD8-PE antibody for 30 min at 4 °C, followed by depletion with anti-PE MicroBeads (Miltenyi Biotec). KO organoids were reassembled by combining CD8+ T cells with CD8− cells at the same donor-specific cell ratio and cultured in transwells.
For GZMB KO experiments in CD8− T cells, untouched CD3+ T cells were first isolated using a Pan T Cell Isolation Kit, human (Miltenyi Biotec), followed by CD8+ T cell depletion using a REAlease CD8 MicroBead Kit, human (Miltenyi Biotec). After electroporation and a 30-min recovery, the CD3− cells, CD8+ T cells (beads removed according to the manufacturer’s instructions) and GZMB KO CD8− T cells were recombined at the same donor-specific cell ratio and cultured at 6 × 106 cells in 100 µl per transwell.
For CXCR5 depletion followed by GZMB KO in CD8+ T cells, tonsil single-cell suspensions were stained with anti-human CD8-PE (BioLegend) and CXCR5-PE (BioLegend) antibodies at room temperature in the dark for 30 min. PE-positive cells were depleted using anti-PE MicroBeads (Miltenyi Biotec) according to the manufacturer’s instructions. Untouched CD8+ T cells were isolated using a CD8+ T Cell Isolation Kit, human (Miltenyi Biotec), followed by electroporation. Finally, the KO CD8+ T cells were combined with the CD8−CXCR5− cell population at the same donor-specific cell ratio.
Cell culture and stimulation
For culture of cryopreserved cells, aliquots were thawed into complete medium, enumerated and resuspended to 6 × 107 cells per ml for larger cultures or 2 × 107 cells per ml for smaller cultures. Then, 6 × 106 cells in 100 μl were plated into each 12-mm cell culture insert, which had a 0.4-μm pore size (Millipore Sigma). 1 ml complete medium supplemented with 1 ng ml−1 IL-21 was added to the lower chamber. 1 μl LAIV (Intranasal FluMist Quadrivalent 2022–2023) was added per well, equivalent to 1.6 × 104 to 1.6 × 105 fluorescent focus units per strain. 1 μg ml−1 autoantigens were added in some experiments. 1 μg ml−1 of recombinant human B cell-activating factor (BioLegend) may be added to the culture after 4 days. Cell cultures were incubated at 37 °C, 5% CO2 with humidity, and supplemented with additional medium to the lower wells as necessary.
CD8+KIR+ functional assay
CD8+ T cells were purified from tonsil cells using CD8 microbeads (Miltenyi Biotec) per the manufacturer’s instructions and stained with flow antibodies, and live CD8+KIR+ or CD8+KIR− T cells were sorted out by flow cytometry (BD). Sorted KIR−CD8+ T cells were added back to CD8− tonsil cells, mixed with Cas9 protein and GZMB gRNA or scrambled gRNA (as WT) to perform KO. After being allowed to rest for 30 min postelectroporation, equal numbers of KIR+ or KIR−CD8+ T cells were added to the culture of KIR− tonsil cells at a 1:50 ratio. A total of 6 × 106 cells per condition in 100 μl were plated into each 12-mm cell culture insert, which had a 0.4-μm pore size (Millipore Sigma). Then, 1 ml complete medium supplemented with 1 μl LAIV (Intranasal FluMist Quadrivalent 2022–2023, equivalent to 1.6 × 104 to 1.6 × 105 fluorescent focus units per strain) and 10 μg ml−1 autopeptides was added to the lower chamber. After day 4, 300 U ml−1 of IL-2 and 1 μg ml−1 of recombinant human B cell-activating factor (BioLegend) were added to the culture. Cell cultures were incubated at 37 °C, 5% CO2 with humidity, and supplemented with additional medium to the lower wells as necessary.
Flow cytometry
Culture organoids were resuspended by rinsing the membrane with medium and collected from the transwells. Cells were washed with fluorescence-activated cell sorting (FACS) buffer (PBS + 0.1% bovine serum albumin, 0.05% sodium azide and 2 mM EDTA) and treated with Fc receptor block (BioLegend, 10 μg ml−1) in FACS buffer for 10 min, followed by staining with live/dead Aqua Zombie stain (Thermo Fisher) and antibodies against surface markers (30 min, 4 °C). Antibodies against surface markers include anti-human CD3 (BUV805, Clone UCHT1, BD) and anti-human CD19 (BUV737, Clone HIB19, BD), anti-human CD4 (BV650, Clone OKT4, BioLegend), anti-human CD8 (BV421, Clone BPA-T8, BioLegend), anti-human CXCR5 (PE-Dazzle 594, Clone J252D4, BioLegend), anti-human PD1 (APC, Clone EH12.2H7, BioLegend), anti-human CD38 (Alexa Fluor 700, Clone HIT2, BioLegend), anti-human CD27 (Pecy7, Clone 0323, BioLegend), anti-human KIR3DL1 (PE, Clone DX9, BioLegend), anti-human KIR2DL2/L3/S2 (PE, Clone DX27, BioLegend), anti-human KIR2DL5 (PE, Clone UP-R1, BioLegend), anti-human CD25 (BV711, Clone M-A251, BioLegend) and anti-human KIR2DL2/L3 (PE, DX27, BioLegend). For intracellular staining, the cells were fixed and permeabilized with the Intracellular Fixation & Permeabilization Buffer Set (eBioscience) followed by staining with anti-human FOXP3 (AF647 or AF488, Clone 259D, BioLegend) or anti-granzyme B antibody (FITC, Clone QA16A02, BioLegend) (30 min, 4 °C). All analyzer data were collected on BD FACSymphony or Agilent NovoCyte Penteon instruments and analyzed using FlowJo (TreeStar) and BD FACSDiva software.
Antibody detection by enzyme-linked immunosorbent assay
For detection of influenza-specific antibodies, enzyme-linked immunosorbent assay (ELISA) plates (Costar) were coated with 0.1 μg per well of 2022–2023 Fluzone Quadrivalent inactivated influenza vaccine (Sanofi). For detection of autoantigen-specific antibodies, ELISA plates (Costar) were coated with 0.1 μg PR3, snRNP, core histone or dsDNA per well as the capture antigen. Plates were coated with capture antigen overnight, followed by blocking reagents for 2 h. Then, cell supernatants from tonsil culture were added to the coated, blocked plates. After the plates had been washed with washing buffer, horseradish peroxidase-conjugated anti-human secondary antibodies to IgM/IgG/IgA (Sigma) were added to the plate for 1 h, followed by TMB substrate solution (Thermo Scientific). Sulfuric acid was added to stop the reaction, and the plates were read at 450 nm.
Protein expression, purification and biotinylation
We performed protein production and purification for generating spheromers following a previous study31. Briefly, 20 ml of Expi293F cells were subcultured at a density of 3 × 106 viable cells per ml in Expi293 expression medium (Thermo Fisher Scientific) and transfected with expression plasmids using ExpiFectamine 293 transfection reagent according to the manufacturer’s instructions. The cells were supplemented with enhancers after 18 h and further incubated for 4 days. The supernatant was collected by centrifugation (2,000g, 30 min, 4 °C) and gently mixed with buffer-equilibrated nickel-nitrilotriacetic acid (Ni-NTA) beads (Qiagen) overnight at 4 °C. Then, the Ni-NTA beads were collected and washed with 20 mM imidazole in HEPES-buffered saline (pH 7.2). The bound protein was eluted using 200 mM imidazole in HEPES-buffered saline (pH 7.2). The proteins were further purified using Amicon Ultra centrifugal units (Millipore Sigma) based on their molecular size. The eluted fractions were analyzed for purity using sodium dodecyl sulfate polyacrylamide gel electrophoresis. The protein was buffer-exchanged to remove the imidazole and biotinylated using a BirA biotin-protein ligase reaction kit (Avidity) according to the manufacturer’s recommendations. The biotinylated proteins were subsequently purified using Amicon Ultra centrifugal units (Millipore Sigma) based on their molecular weight. The eluted fractions were analyzed for purity and biotinylation using sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Assembly of the peptide-MHC–spheromer complex and autoantigen–spheromer complex
The assembly was started by generating a semisaturated SAv–peptide-MHC2 (pMHC2) complex. First, 1 μM pMHC-I monomers (NIH tetramer core facility), pMHC-I monomers (NIH tetramer core facility) or autoantigens (generated in-house) were incubated with 0.45 μM streptavidin–fluorophore (BioLegend) at a 1:0.45 ratio at room temperature for 30 min protected from light. The sequences of monomers are provided in Supplementary Table 2. Then, the mixture was incubated with an engineered maxi-ferritin scaffold31 at room temperature for 1 h protected from light with gentle rotation. Molar excess of d-biotin was added to saturate any free biotin-binding sites on streptavidin of pMHC2. The assembled spheromer was stored at 4 °C for later use.
Binding affinity measurement
The binding affinity of full-length H1 California/04/2009 influenza hemagglutinin (CA/09 HA) to the antibodies secreted into the culture media of the indicated human tonsil organoids on day 10 after LAIV stimulation was measured by biolayer interferometry using an Octet QK instrument (Pall ForteBio). The antigen (H1 CA/09 HA) diluted in PBST (PBS with 0.05% Tween 20, pH 7.4) was captured using Ni-NTA biosensors. The ligand-bound biosensors were dipped into a serially diluted culture supernatant. The association and dissociation were both monitored for 1 h. Double referencing was performed using unliganded biosensors and an irrelevant Escherichia coli maltose-binding protein. The t1/2 was calculated from the off-rate (koff) using the equation for first-order rate kinetics, t1/22 = 0.693/koff. Each binding interaction was performed in duplicate.
Statistical analysis
Most statistical analyses except for those shown in Fig. 7 were performed using GraphPad Prism (v.10). All results are presented as mean ± s.e.m. The significance of the differences between groups were analyzed as described in the figure legends. To assess the relationship between autoantibody levels and donor age and sex, a linear regression analysis was conducted for each autoantibody (dsDNA, PR3, histone and snRNP). Optical density fold changes from ELISA assays were used as the response variable, with age and sex interaction terms as predictors. The model estimated the coefficients (slopes) for age effects in males and females separately. Model fit was evaluated using the F-statistic, P value and adjusted R2 value. Statistical significance was determined for each predictor, and residuals were assessed for model accuracy. All analyses were performed using R.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41590-024-02062-x.
Supplementary information
Donor sex, age and ethnicity reported in the study.
List of genes targeted by gRNA sequences and negative control sequence.
List of peptide sequences used as epitopes for spheromers to measure percentages of CD8+ and CD4+ cells from stimulated tonsil organoids.
Source data
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Acknowledgements
We thank all the volunteers and patients for their participation in this study. We also thank L. Chen and L. Kamalyan for tonsil processing, J. Li for providing the autoreactive peptides, the Stanford Shared FACS Facility for assistance in flow cytometric analysis, and D. Fernandez and O. N. Pattelli from Stanford ChEM-H Macromolecular Structure Knowledge Center for their guidance in protein production and purification. Finally, we thank the participants for donating their tissues for the tonsil organoids study. HLA typing was performed at the Stanford Functional Genomics Facility. This work was supported by the Lundbeck Foundation’s Danish American Research Exchange Fellowship to M.G. and grants from the Howard Hughes Medical Institute, Open Philanthropy, The Bill and Melinda Gates Foundation, and NIAID (AI057229) to M.M.D.
Extended data
Author contributions
Project conceptualization and study design were performed by X.C. and M.M.D. Experiments were performed by X.C., M.G. and K.R.K. Data analyses were performed by X.C. and M.M.D. E.S. assisted with tonsil and peripheral blood mononuclear cell sample databases and data collection, processing and banking. V.M. performed the biolayer interferometry experiments and analysis. R.C. provided tonsil samples. X.C. and M.M.D. wrote the paper with input from all authors.
Peer review
Peer review information
Nature Immunology thanks Bing Su and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: S. Houston, in collaboration with the Nature Immunology team.
Data availability
All data are available in Figs. 1–7, Supplementary Information and Extended Data Figs. 1–7. Source data are provided with this paper.
Code availability
Code for statistical analysis using R and existing software packages has been described in the Methods section. No custom code was used in this study.
Competing interests
M.M.D., X.C., M.G. and V.M. are inventors on a patent application (PCT/US2023/010431) on the systems and methods incorporating modified T cells described in this work. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xin Chen, Mustafa Ghanizada.
Extended data
is available for this paper at 10.1038/s41590-024-02062-x.
Supplementary information
The online version contains supplementary material available at 10.1038/s41590-024-02062-x.
References
- 1.Valent, P. et al. Paul Ehrlich (1854-1915) and his contributions to the foundation and birth of translational medicine. J. Innate Immun.8, 111–120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodnow, C. C. et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature334, 676–682 (1988). [DOI] [PubMed] [Google Scholar]
- 3.Asano, M., Toda, M., Sakaguchi, N. & Sakaguchi, S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med.184, 387–396 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baecher-Allan, C. & Hafler, D. A. Human regulatory T cells and their role in autoimmune disease. Immunol. Rev.212, 203–216 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Jiang, H., Zhang, S. I. & Pernis, B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science256, 1213–1215 (1992). [DOI] [PubMed] [Google Scholar]
- 6.Kim, H. J. et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science350, 334–339 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim, H. J., Verbinnen, B., Tang, X., Lu, L. & Cantor, H. Inhibition of follicular T-helper cells by CD8+ regulatory T cells is essential for self tolerance. Nature467, 328–332 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li, J. et al. KIR+CD8+ T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science376, eabi9591 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saligrama, N. et al. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature572, 481–487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagar, L. E. et al. Modeling human adaptive immune responses with tonsil organoids. Nat. Med.27, 125–135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kastenschmidt, J. M. et al. Influenza vaccine format mediates distinct cellular and antibody responses in human immune organoids. Immunity56, 1910–1926.e7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadaschik, E. N. et al. Regulatory T cell-deficient scurfy mice develop systemic autoimmune features resembling lupus-like disease. Arthritis Res. Ther.17, 35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuda, M. et al. The spectrum of autoantibodies in IPEX syndrome is broad and includes anti-mitochondrial autoantibodies. J. Autoimmun.35, 265–268 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Chang, S. E. et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun.12, 5417 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulsen, T. R., Jensen, A., Haurum, J. S. & Andersen, P. S. Limits for antibody affinity maturation and repertoire diversification in hypervaccinated humans. J. Immunol.187, 4229–4235 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Choi, S. J. et al. KIR+CD8+ and NKG2A+CD8+ T cells are distinct innate-like populations in humans. Cell Rep.42, 112236 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Rubtsova, K., Marrack, P. & Rubtsov, A. V. Sexual dimorphism in autoimmunity. J. Clin. Invest.125, 2187–2193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dieckmann, D., Plottner, H., Berchtold, S., Berger, T. & Schuler, G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med.193, 1303–1310 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baecher-Allan, C. M. & Hafler, D. A. The purification and functional analysis of human CD4+CD25high regulatory T cells. Curr. Protoc. Immunol.72, 7.4B.1–7.4B.12 (2006). [DOI] [PubMed]
- 20.Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science10.1126/science.1079490 (2003). [DOI] [PubMed]
- 21.Salti, S. M. et al. Granzyme B regulates antiviral CD8+ T cell responses. J. Immunol.187, 6301–6309 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, J. et al. KIR+CD8+ T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science10.1126/science.abi9591 (2022). [DOI] [PMC free article] [PubMed]
- 23.Loebbermann, J. et al. Regulatory T cells expressing granzyme B play a critical role in controlling lung inflammation during acute viral infection. Mucosal Immunol.5, 161–172 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ise, W. et al. T follicular helper cell-germinal center B cell interaction strength regulates entry into plasma cell or recycling germinal center cell fate. Immunity48, 702–715.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Mintz, M. A. & Cyster, J. G. T follicular helper cells in germinal center B cell selection and lymphomagenesis. Immunol. Rev.296, 48–61 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shulman, Z. et al. T follicular helper cell dynamics in germinal centers. Science341, 673–677 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Getts, D. R., Chastain, E. M., Terry, R. L. & Miller, S. D. Virus infection, antiviral immunity, and autoimmunity. Immunol. Rev.255, 197–209 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieker, J. et al. Autoantibodies against modified histone peptides in SLE patients are associated with disease activity and lupus nephritis. PLoS ONE11, e0165373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kattah, N. H., Kattah, M. G. & Utz, P. J. The U1-snRNP complex: structural properties relating to autoimmune pathogenesis in rheumatic diseases. Immunol. Rev.233, 126–145 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Primo, V. C. et al. Anti-PR3 immune responses induce segmental and necrotizing glomerulonephritis. Clin. Exp. Immunol.159, 327–337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallajosyula, V. et al. CD8+ T cells specific for conserved coronavirus epitopes correlate with milder disease in patients with COVID-19. Sci. Immunol.10.1126/sciimmunol.abg5669 (2021). [DOI] [PMC free article] [PubMed]
- 32.Yu, W. et al. Clonal deletion prunes but does not eliminate self-specific αβ CD8+ T lymphocytes. Immunity42, 929–941 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielen, M. M. et al. Antibodies to citrullinated human fibrinogen (ACF) have diagnostic and prognostic value in early arthritis. Ann. Rheum. Dis.64, 1199–1204 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su, L. F., Kidd, B. A., Han, A., Kotzin, J. J. & Davis, M. M. Virus-specific CD4+ memory-phenotype T cells are abundant in unexposed adults. Immunity38, 373–383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan, J. et al. Safety and immunogenicity of a human and mouse gp100 DNA vaccine in a phase I trial of patients with melanoma. Cancer Immun.9, 5 (2009). [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, L., Nakayama, M. & Eisenbarth, G. S. Insulin as an autoantigen in NOD/human diabetes. Curr. Opin. Immunol.20, 111–118 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goronzy, J. J. & Weyand, C. M. Immune aging and autoimmunity. Cell. Mol. Life Sci.69, 1615–1623 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitacre, C. C. Sex differences in autoimmune disease. Nat. Immunol.2, 777–780 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science10.1126/science.1086907 (2003). [DOI] [PubMed]
- 40.Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol.4, 330–336 (2003). [PubMed] [Google Scholar]
- 41.Kim, J. M. & Rudensky, A. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol. Rev.212, 86–98 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi, S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol.6, 345–352 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Yang, S., Fujikado, N., Kolodin, D., Benoist, C. & Mathis, D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science348, 589–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng, Y. & Rudensky, A. Y. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol.8, 457–462 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Hoshino, A. et al. Identification of autoantibodies using human proteome microarrays in patients with IPEX syndrome. Clin. Immunol.203, 9–13 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Kinnunen, T. et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood121, 1595–1603 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarron, M. J. & Marie, J. C. TGF-beta prevents T follicular helper cell accumulation and B cell autoreactivity. J. Clin. Invest.124, 4375–4386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim, H. W., Hillsamer, P., Banham, A. H. & Kim, C. H. Cutting edge: direct suppression of B cells by CD4+CD25+ regulatory T cells. J. Immunol.10.4049/jimmunol.175.7.4180 (2005). [DOI] [PubMed]
- 49.Iikuni, N., Lourenço, E. V., Hahn, B. H. & La, C. Cutting edge: regulatory T cells directly suppress B cells in systemic lupus erythematosus. J. Immunol.10.4049/jimmunol.0901163 (2009). [DOI] [PMC free article] [PubMed]
- 50.Lam, A. J. et al. Optimized CRISPR-mediated gene knockin reveals FOXP3-independent maintenance of human Treg identity. Cell Rep.36, 109494 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Zhang, W. et al. FOXP3 recognizes microsatellites and bridges DNA through multimerization. Nature624, 433–441 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le Coz, C. et al. Human T follicular helper clones seed the germinal center-resident regulatory pool. Sci. Immunol.8, eade8162 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis, M. M. In praise of descriptive science: a breath of fresh AIRE. Cell166, 530–531 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer, S. et al. AIRE-deficient patients harbor unique high-affinity disease-ameliorating autoantibodies. Cell166, 582–595 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabia, L. A., Desai, A. A., Jhajj, H. S. & Tessier, P. M. Understanding and overcoming trade-offs between antibody affinity, specificity, stability and solubility. Biochem. Eng. J.137, 365–374 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suurmond, J. & Diamond, B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J. Clin. Invest.125, 2194–2202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sundaresan, B., Shirafkan, F., Ripperger, K. & Rattay, K. The role of viral infections in the onset of autoimmune diseases. Viruses10.3390/v15030782 (2023). [DOI] [PMC free article] [PubMed]
- 58.Arbuckle, M. R. et al. Development of anti-dsDNA autoantibodies prior to clinical diagnosis of systemic lupus erythematosus. Scand. J. Immunol.54, 211–219 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med.349, 1526–1533 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Leslie, D., Lipsky, P. & Notkins, A. L. Autoantibodies as predictors of disease. J. Clin. Invest.108, 1417–1422 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olsen, N. J., Okuda, D. T., Holers, V. M. & Karp, D. R. Editorial: understanding the concept of pre-clinical autoimmunity. Front. Immunol.13, 983310 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Donor sex, age and ethnicity reported in the study.
List of genes targeted by gRNA sequences and negative control sequence.
List of peptide sequences used as epitopes for spheromers to measure percentages of CD8+ and CD4+ cells from stimulated tonsil organoids.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Data Availability Statement
All data are available in Figs. 1–7, Supplementary Information and Extended Data Figs. 1–7. Source data are provided with this paper.
Code for statistical analysis using R and existing software packages has been described in the Methods section. No custom code was used in this study.