Abstract
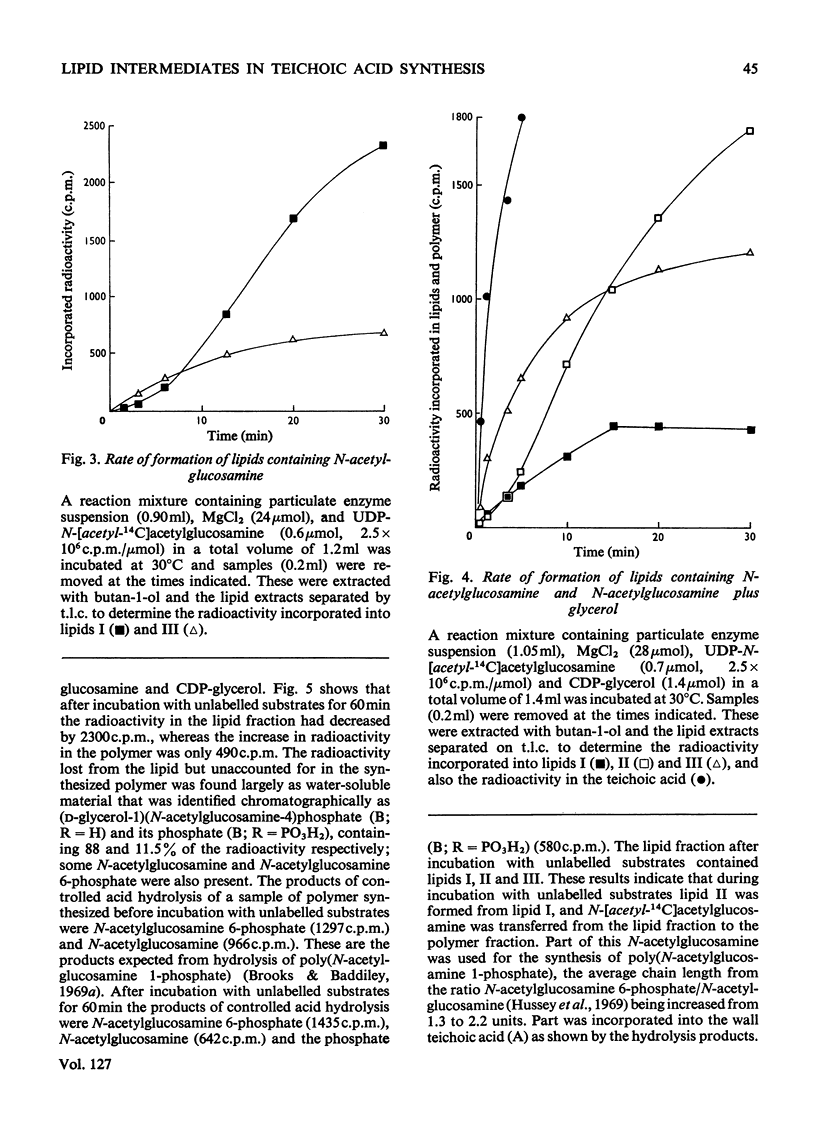
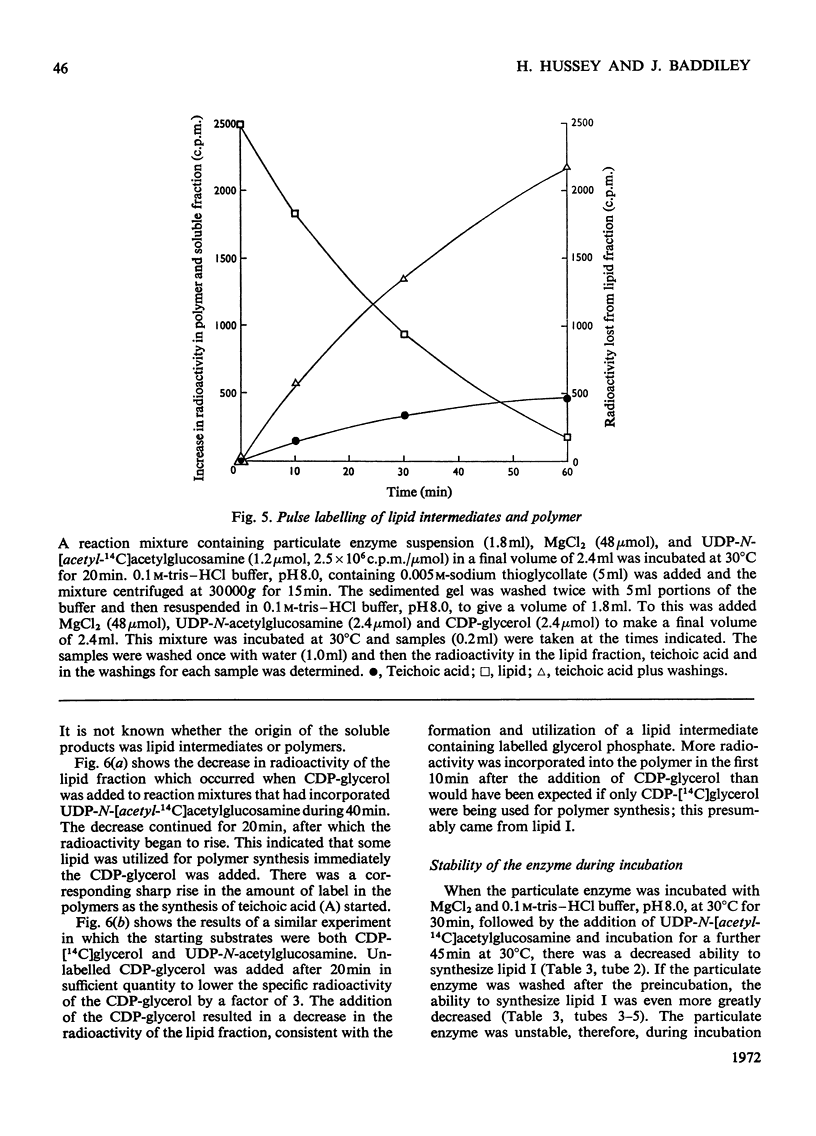
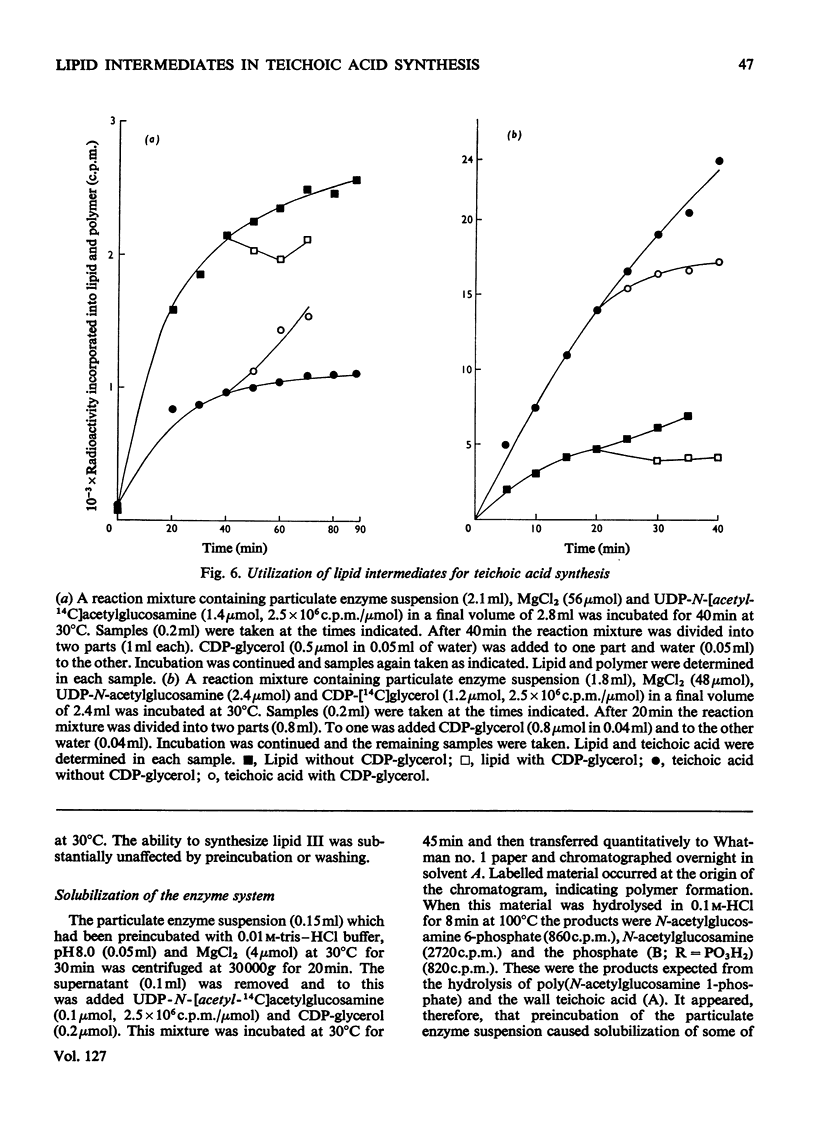
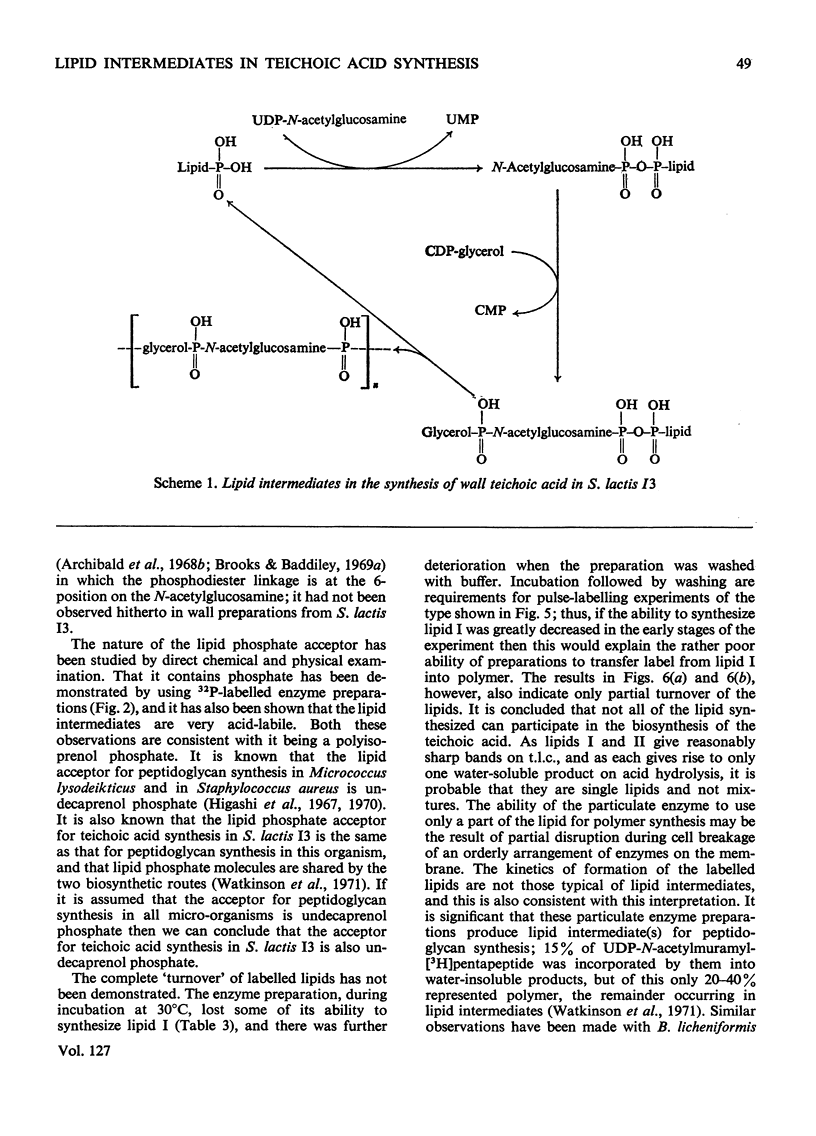
1. Particulate enzyme systems have been prepared from Staphylococcus lactis I3 which effect the synthesis of wall teichoic acid (a polymer containing a repeating unit in which d-glycerol 1-phosphate is attached to the 4-position on N-acetylglucosamine 1-phosphate) from the nucleotide precursors CDP-glycerol and UDP-N-acetylglucosamine. By using nucleotides labelled with 32P and 14C it has been shown that the synthesis proceeds via lipid intermediates. 2. Two intermediates have been found. In one of these N-acetylglucosamine 1-phosphate is present, whereas in the other the repeating unit of the teichoic acid occurs. 3. The simultaneous formation of the teichoic acid, a poly-(N-acetylglucosamine 1-phosphate) and an unidentified lipid, together with the poor ability of most particulate systems to synthesize polymer and the instability of the lipid intermediates themselves, have interfered with pulse-labelling experiments. Nevertheless, the biosynthetic sequence has been elucidated. It is concluded that the intermediates are derivatives of undecaprenol phosphate.
Full text
PDF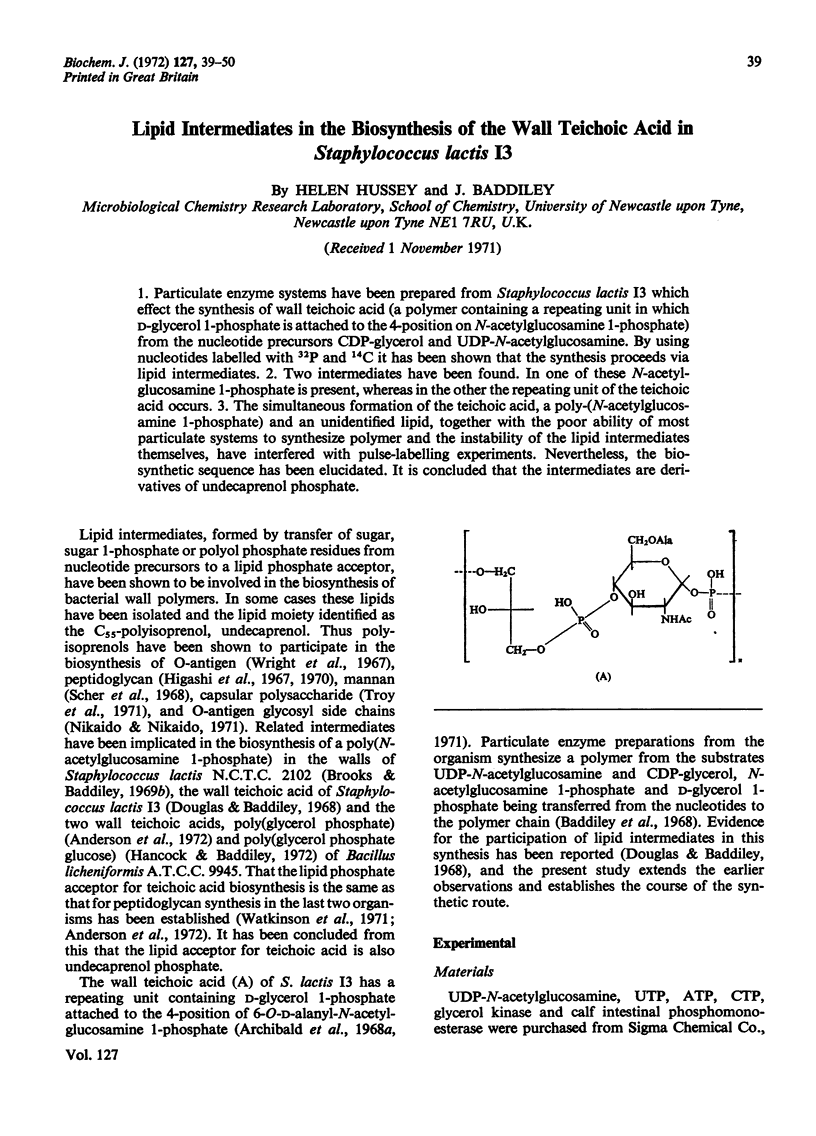




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Hussey H., Baddiley J. The mechanism of wall synthesis in bacteria. The organization of enzymes and isoprenoid phosphates in the membrane. Biochem J. 1972 Mar;127(1):11–25. doi: 10.1042/bj1270011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J., Button D. The glycerol teichoic acid of walls of Staphylococcus lactis I3. Biochem J. 1968 Dec;110(3):543–557. doi: 10.1042/bj1100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J., Heckels J. E., Heptinstall S. Further studies on the glycerol teichoic acid of walls of Staphylococcus lactis I3. Location of the phosphodiester groups and their susceptibility to hydrolysis with alkali. Biochem J. 1971 Nov;125(1):353–359. doi: 10.1042/bj1250353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddiley J., Blumsom N. L., Douglas L. J. The biosynthesis of the wall teichoic acid in Staphylococcus lactis I3. Biochem J. 1968 Dec;110(3):565–571. doi: 10.1042/bj1100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D., Baddiley J. A lipid intermediate in the synthesis of a poly-(N-acetylglucosamine 1-phosphate) from the wall of Staphylococcus lactis N.C.T.C. 2102. Biochem J. 1969 Nov;115(2):307–314. doi: 10.1042/bj1150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D., Baddiley J. The mechanism of biosynthesis and direction of chain extension of a poly-(N-acetylglucosamine 1-phosphate) from the walls of Staphylococcus lactis N.C.T.C. 2102. Biochem J. 1969 Jul;113(4):635–642. doi: 10.1042/bj1130635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHALVARDJIAN A., MORRIS L. J., HOLMAN R. T. Effect of dietary epoxyoleic acid upon rats. J Nutr. 1962 Jan;76:52–58. doi: 10.1093/jn/76.1.52. [DOI] [PubMed] [Google Scholar]
- Douglas L. J., Baddiley J. A lipid intermediate in the biosynthesis of a teichoic acid. FEBS Lett. 1968 Aug;1(2):114–116. doi: 10.1016/0014-5793(68)80034-1. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Hancock I. C., Baddiley J. Biosynthesis of the wall teichoic acid in Bacillus licheniformis. Biochem J. 1972 Mar;127(1):27–37. doi: 10.1042/bj1270027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Biosynthesis of the peptidoglycan of bacterial cell walls. XXI. Isolation of free C55-isoprenoid alcohol and of lipid intermediates in peptidoglycan synthesis from Staphylococcus aureus. J Biol Chem. 1970 Jul 25;245(14):3697–3702. [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey H., Brooks D., Baddiley J. Direction of chain extension during the biosynthesis of teichoic acids in bacterial cell walls. Nature. 1969 Feb 15;221(5181):665–666. doi: 10.1038/221665a0. [DOI] [PubMed] [Google Scholar]
- LECOCQ J., BALLOU C. E. ON THE STRUCTURE OF CARDIOLIPIN. Biochemistry. 1964 Jul;3:976–980. doi: 10.1021/bi00895a023. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuhashi M., Dietrich C. P., Strominger J. L. Incorporation of glycine into the cell wall glycopeptide in Staphylococcus aureus: role of sRNA and lipid intermediates. Proc Natl Acad Sci U S A. 1965 Aug;54(2):587–594. doi: 10.1073/pnas.54.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido K., Nikaido H. Glucosylation of lipopolysaccharide in Salmonella: biosynthesis nof O antigen factor n12 2 . II. Structure of the lipid intermediate. J Biol Chem. 1971 Jun 25;246(12):3912–3919. [PubMed] [Google Scholar]
- PALADINI A. C., LELOIR L. F. Studies on uridine-diphosphate-glucose. Biochem J. 1952 Jun;51(3):426–430. doi: 10.1042/bj0510426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Scher M., Lennarz W. J., Sweeley C. C. The biosynthesis of mannosyl-1-phosphoryl-polyisoprenol in Micrococcus lysodeikticus and its role in mannan synthesis. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1313–1320. doi: 10.1073/pnas.59.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Troy F. A., Frerman F. E., Heath E. C. The biosynthesis of capsular polysaccharide in Aerobacter aerogenes. J Biol Chem. 1971 Jan 10;246(1):118–133. [PubMed] [Google Scholar]
- Watkinson R. J., Hussey H., Baddiley J. Shared lipid phosphate carrier in the biosynthesis of teichoic acid and peptidoglycan. Nat New Biol. 1971 Jan 13;229(2):57–59. doi: 10.1038/newbio229057a0. [DOI] [PubMed] [Google Scholar]
- Wright A., Dankert M., Fennessey P., Robbins P. W. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]


