Abstract
Purpose
In early-stage, triple-negative breast cancer (TNBC), immune cell infiltration contributes to cancer cell survival, tumor invasion, and metastasis. High TNBC glucocorticoid receptor (GR) expression in early-stage TNBC is associated with poor long-term outcomes; it is unknown if high GR expression is associated with an immunosuppressed tumor microenvironment. We hypothesized that high tumor GR expression would be associated with an immune-suppressed tumor microenvironment, which could thus account for the poor prognosis observed in GR-positive TNBC.
Methods
Formalin fixed-paraffin embedded tissue (n = 47) from patients diagnosed with early-stage TNBC from The University of Chicago (2002–2014) were evaluated for both tumor cell anti-GR immunohistochemistry and for infiltrating immune cells by immunofluorescence. Multiplexed antibodies were used to enumerate CD8+, FOXP3+, and BATF3+ immune cells infiltrating within pan-cytokeratin positive tumor cell regions of interest, and nonparametric tests compared absolute counts of each of these tumor-infiltrating immune cell types.
Results
The average age of patients represented in this study was 52 years, and 63% self-identified as Black. There was no significant association between tumor GR expression and age, race, or clinical stage at diagnosis. Compared to GR-low tumors, high GR expression in early-stage, treatment-naïve TNBC was associated with relatively increased numbers of immunosuppressive FOXP3 + regulatory T cells (p = 0.046) and BATF3+immune cells (p = 0.021). While there was a positive correlation with high GR expression and CD8+ cell infiltration, it was not significant (p = 0.068). The ratio of CD8+/FOXP3+cells was also not significant (p = 0.24).
Conclusions
These data support the hypothesis that in early-stage TNBC, high GR expression is significantly associated with infiltration of immunosuppressive regulatory T cells, suggesting a tumor-intrinsic role in shaping the immunosuppressive immune cell milieu. Furthermore, suppression of GR activity may regulate the tumor immune microenvironment and improve long-term outcomes in GR-high TNBC.
Keywords: Triple-negative breast cancer (TNBC), Glucocorticoid receptor (GR), CD8+cytotoxic T-cells, FOXP3+, T-regulatory cells, Dendritic cells, BATF3+, Tumor immune microenvironment
Introduction
Breast cancer is the most common cancer in women worldwide [1]. Triple-negative breast cancer (TNBC) is defined by lack of estrogen receptor (ER), progesterone receptor (PR), and HER2 receptor expression [2, 3]. In general, TNBC exhibits higher proliferative indices, increased molecular heterogeneity, and exhibits high rates of locoregional and distant metastatic recurrence compared to other breast cancer subtypes [2–4]. The addition of anti-PD-1 therapy to chemotherapy for stage II–III TNBC improves both pathological complete response (pCR) rates as well as event-free survival (EFS). While PD-L1 positivity does predict benefit in advanced TNBC, biomarkers predictive of immunotherapy benefit in early TNBC remain elusive [5–7].
Cortisol (the human glucocorticoid stress hormone) and its canonical receptor (glucocorticoid receptor, GR) are critical regulators of the immune system [8, 9]. In addition, tumor cell GR expression and activation drives transcriptional pathways encoding proteins involved in TNBC cell survival, invasion, and anti-inflammatory pathways [10]. Our group has demonstrated that high tumor GR expression is associated with poor relapse-free survival in early-stage TNBC [11]. In contrast, within other breast cancer subtypes that have low presence of tumor infiltrating lymphocytes and do not respond to immune checkpoint blockade such as hormone receptor positive cancers [12], high tumor GR expression is associated with improved clinical outcomes [13, 14].
A recent study demonstrated that tumor cell-intrinsic high GR expression and activation in pancreatic cancer induces immune suppression in the surrounding tumor microenvironment [15]. We have shown that tumor cell intrinsic GR activation in ovarian cancer cells upregulates immunosuppressive cytokine secretion and drives myeloid-derived suppressor cell recruitment and differentiation [16]. While GR activation in T cells downregulates IFNγ and TNFα pathways leading to decreased T cell activation and GR activation suppresses macrophage and dendritic cell mediated antigen presentation [17, 18], little is known about how high GR expression and activity in TNBC affects the tumor immune microenvironment. While it is known that activation of the estrogen and progesterone receptors is associated with an immunosuppressive tumor microenvironment in hormone receptor-positive breast cancer [19, 20], the role of tumor cell-intrinsic GR activity, also a hormone receptor, has not been evaluated in TNBC.
In this study of treatment-naïve, early-stage TNBC [21, 22], we hypothesized that high GR expression would correlate with an immunosuppressed tumor microenvironment. In this study we quantified GR expression by immunohistochemistry (IHC) and evaluated the infiltration of three key immune cell populations, CD8+T cells, FOXP3+regulatory T cells (Treg), and BATF3+immune cells using multiplex immunohistochemistry as described below.
Materials and methods
TNBC data collection
We identified 47 patients with TNBC who received neoadjuvant chemotherapy at The University of Chicago between 2002 and 2014 and had pre-treatment tissue available for study. This research poses minimal risk, involves biospecimen information such that the identity of the human subjects cannot be readily identified, and is exempt under Code of Federal Regulations and Department of Health and Human Services (DHHS) requirements for informed consent as described under 45CFR46.104, §46.116, and for documentation of consent under §46.117.
GR IHC staining
Primary tumors were stained for GR expression using the anti-GR rabbit monoclonal XP antibody (Cell Signaling, 1:200 dilution). The weighted H-score was calculated as the percentage of positively stained nuclei in tumor cells multiplied by a weighted intensity of the anti-GR staining (H-score = ∑Pi * i where Pi is the percentage of stained cells in each intensity category, and i is the intensity for i = samples 0, 1, 2, 3) with a range of 0–300.
Multiplex immunohistochemistry
Multiplex immunofluorescent histochemistry was used to evaluate relative expression of immune cell specific markers of CD8 + T cells, FOXP3 + regulatory T cells (Treg), and BATF3 + immune cells in pan-cytokeratin positive tumor nests containing equidimensional regions of interests (ROIs) in the pretreatment biopsy specimens. The tumor nests were defined as areas of abundant tumor cells based on comparisons between the H&E and pan-cytokeratin stained slides. We determined 4–5 regions of interest (ROI) with the preset size of 931 µm × 698 µm that had the most abundant CD8 + T cells, FOXP3 + regulatory T cells (Treg), and BATF3 + immune cell infiltration and selected those ROIs within tumor nests to quantify relative immune cell numbers. Comparison of the mean number of each immune cell type per ROI, CD8+ /FOXP3+ immune cell ratios per ROI, and the total number of each cell type per ROI was performed with statistical analyses as further described below.
Formalin-fixed paraffin embedded (FFPE) slides were stained using Opal multiplex 6-plex kit (AKOYA Bioscience, Menlo Park, CA, USA) according to the manufacturer’s instruction. Primary antibodies included Batf3 (polyclonal [AF7437], R&D Systems, Minneapolis, MN, USA), CD8 (clone C8/144B, Santa Clara, CA, USA), Foxp3 (clone 236A/E7, Biocare Medical, Pacheco, CA, USA), PD-L1 (clone E1L3N, Cell Signaling, Danvers, MA, USA), and pan-cytokeratin (clone AE1/AE3, Waltham, MA, USA). Briefly, slides were baked for 1 h at 60 °C. After deparaffinization and rehydration, tissues were fixed with 10% neutral buffered formalin. Antigen retrieval was performed with pH9 buffer for 20 min at 110 ℃ in a pressure cooker followed by blocking. Tissues were then incubated with each primary antibody for 1 h at room temperature, followed by a horseradish peroxidase-conjugated secondary antibody for 10 min at room temperature. Signal amplification was achieved by the corresponding Opal fluorophore (AKOYA Bioscience, Menlo Park, CA, USA) reaction utilized tyramide signal amplification for 10 min at room temperature. The process from antigen retrieval to Opal fluorophore was repeated for each of the five target molecules. After staining the six target molecules, slides were counterstained with 4’, 6-diamidino-2-phenylindole (DAPI) and were mounted and cover slipped.
Image analysis
The stained slides were imaged using the Vectra® Polaris™ Automated Quantitative Pathology Imaging System (AKOYA Bioscience, Menlo Park, CA, USA) at 20 × resolutions with the following channels: DAPI, FITC, Cy3, Texas red and Cy5. On each scanned image, up to five regions of interest (ROI) with the preset size of 931 µm × 698 µm that had the most abundant CD8 + cell infiltration were selected in tumor nests. Selected ROIs were scanned at 20× magnification to make.im3 format image files for the following image analysis.
The scanned.im3 format image files were analyzed using inForm® Cell Analysis software (AKOYA Bioscience). An algorithm was developed to determine the following cell phenotypes using examples of each cell phenotype: CD8+, FOXP3+, BATF3+. Finally, batch analysis using the algorithm was performed for all the ROIs, outputting information including tumor area and phenotypes. Comparisons of the mean number of each immune cell type per ROI, CD8+ /FOXP3+ immune cell ratios per ROI, and percentages of each immune cell type of the total number of cells per ROI were performed with statistical analyses as described below.
Statistical methods
Clinical and demographic characteristics of patients were summarized by mean, standard deviation (SD), and range for continuous variables and frequency distributions for categorical data. Immune cell markers, values across multiple ROIs from the same patient were first averaged to obtain patient-level data. Subsequent analyses were then based upon relative percentages of each cell type as well as absolute infiltrate numbers. Because the latter distributions were positively skewed, a square root transformation was applied. The percentages of each immune cell type as well as the CD8+ T cell:Treg (CD8+ /FOXP3+) ratio were compared in GR-low, GR-moderate, and GR-high expressing tumors using a nonparametric trend test [23].
Results
Clinicopathologic characteristics of 47 human TNBC tumors and their correlation with GR expression
Patient demographics are depicted in Table 1. All tumor samples were treatment-naive biopsy specimens. The average age was 52 and 63% patients self-identified as Black. There were no significant associations between GR expression and age, race, or clinical stage at diagnosis.
Table 1.
Clinicopathologic characteristics of 47 TNBC tumors and correlation to GR expression
| Characteristic | All tumors (n = 47) | Low GR (n = 8) | Moderate GR (n = 25) | High GR (n = 14) | p-valuea |
|---|---|---|---|---|---|
| Age | 0.54 | ||||
| Mean | 52.3 | 56.2 | 50.5 | 53.2 | |
| SD | 13.1 | 14.1 | 14.5 | 9.5 | |
| Range | 23–92 | 34–74 | 23–92 | 40–78 | |
| Race | 0.88 | ||||
| Black | 29 (63.0%) | 5 (62.5%) | 16 (66.7%) | 8 (57.1%) | |
| White | 16 (34.8%) | 3 (37.5%) | 7 (29.2%) | 6 (42.9%) | |
| Mixed | 1 (2.2%) | 0 (0.0%) | 1 (4.2%) | 0 (0.0%) | |
| Clinical stage at diagnosis | 0.54 | ||||
| I | 3 (6.7%) | 1 (12.5%) | 1 (4.4%) | 1 (7.1%) | |
| II | 18 (40.0%) | 3 (37.5%) | 7 (30.3%) | 8 (57.1%) | |
| III | 23 (51.1%) | 4 (50.0%) | 14 (60.9%) | 5 (35.7%) | |
| IV* | 1 (2.2%) | 0 (0.0%) | 1 (4.4%) | 0 (0.0%) |
aNonparametric trend test. *This tumor biopsy was from a breast mass that had direct dermal invasion and axillary lymphadenopathy at diagnosis and was initially misclassified as stage IV TNBC
High versus low TNBC GR expression is associated with differential immune cell infiltration
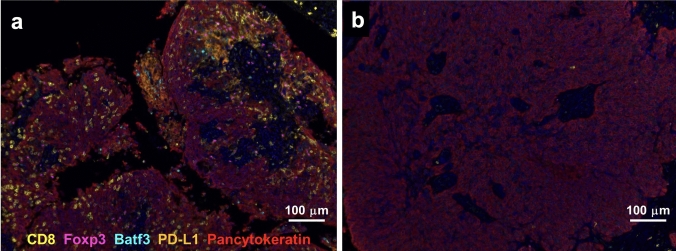
Immunohistochemistry was used to determine tumor cell GR expression and multiplex immunofluorescence was used to evaluate the tumor infiltrating immune cells adjacent to the TNBC cells. Samples were stained for GR in the pancytokeratin-positive tumor epithelial compartment (Fig. 1). GR immunoreactive tumor epithelial cells were scored using a rabbit monoclonal anti-GR antibody. N = 8 tumors scored as GR-low tumors (H-score < 100), 25 tumors scored as GR-moderate (H-score between 100 and 200), and 14 tumors scored as GR-high (H-score > 200) (Fig. 1). Slides were further stained with antibodies targeting various immune cell subgroups including CD8 (T cell lineage), FOXP3 (regulatory T cell lineage), and BATF3 (monocyte, macrophage, dendritic cell, or T/B lymphocyte lineages) to identify infiltrating immune cells in the tumor-proximal non-epithelial compartment of the tumor. PD-L1 cells were not counted due to lack of specific staining in any distinct cell type. Distinct infiltrating immune cell profiles within tumors were observed. For example, Fig. 2a shows an example of a TNBC with an abundance of all stained immune cell-lineages while Fig. 2b represents a TNBC with relatively low expression of immune cells (Fig. 2).
Fig. 1.
Examples of TNBC specimens with discrete expression levels (low, moderate, high) of GR within epithelial tumor cells. Black bars represent 50 µm
Fig. 2.
Diversity of immune marker expression in TNBC tissue. Representative images of multiplexed immunofluorescence are shown for two representative chemotherapy-naïve TNBC biopsies. Tumor a (GR-high) has relatively high numbers of CD8 + , FOXP3 + and BATF3 + immune cells compared to GR-negative tumor b which has very low numbers of immune cell infiltration. Slides were stained with antibodies for pancytokeratin (red, to detect tumor cells), and immune markers CD8 (yellow), FOXP3 (purple), BATF3 (blue), and PD-L1 (orange). PD-L1 cells were not counted due to concerns of lack of specific staining in any distinct cell type
Highly GR-expressing TNBC cell tumors are associated with increased numbers of FOXP3+ and BATF3+ immune cell infiltration
We observed relatively high percentages of CD8+ , FOXP3+ , and BATF3+ individual immune cells (measured as the percent of a specific immune marker-stained cells with respect to the total cell number in a region of interest or ROI) in tumors nests with high GR expression (Fig. 3 and Table 2), representing the frequency a cell type is expressed in a tumor nest. There was a statistically significant positive correlation with high GR tumor cell expression and higher percentages of FOXP3+ immune cells and BATF3+ immune cells (Table 2, FOXP3+ p = 0.046, BATF3+ p = 0.021), demonstrating increased infiltration of regulatory T-cells and BATF3+ immune cells into the GR-high TNBC microenvironment. GR-high tumors also appeared to have somewhat higher CD8+ immune cell infiltration, although this association did not reach significance (Table 2, CD8 + p = 0.068). The CD8+/FOXP3+ immune cell ratio in GR-high compared to GR-low tumors was not statistically significant although the absolute percentage of FOXP3 + cells was significantly higher in GR-high versus GR-low or GR-medium cells (Table 2, p = 0.24).
Fig. 3.
GR expression in TNBC cells is associated with significantly increased FOXP3+ and BATF3+ immune cell numbers. Associations amongst a CD8+ immune cell, b FOXP3+ immune cell, c BATF3 + immune cell and d CD8+ /FOXP3+ immune cell ratios within GR-low, GR-moderate and GR-high chemotherapy naïve TNBC biopsy specimens. Percentages on the y-axes represent the specific immune cell type within the region of interest, with the denominator representing all cells counted by stained nuclei and represent frequency a cell type is expressed in a tumor nest. P-values: FOXP3+ p = 0.046, BATF3+ p = 0.021, CD8+ p = 0.68, CD8+ /FOXP3+ p = 0.24). Grey bars = GR-low; pink = GR-moderate; blue = GR-high
Table 2.
Association of GR expression in TNBC cells with immune cell percentage of total cell number in averaged ROIs
| Characteristic | All Tumors (n = 39) |
Low GR (n = 7) |
Moderate GR (n = 18) |
High GR (n = 14) |
p-value* |
|---|---|---|---|---|---|
| CD8+(%) | |||||
| Mean | 1.56 | 0.27 | 1.34 | 2.48 | 0.068 |
| SD | 3.40 | 0.32 | 2.08 | 5.11 | |
| Range | 0–19.86 | 0.01–0.94 | 0–7.80 | 0–19.86 | |
| FOXP3+(%) | |||||
| Mean | 0.83 | 0.40 | 0.77 | 1.13 | 0.046 |
| SD | 0.701 | 0.36 | 0.57 | 0.87 | |
| Range | 0–2.98 | 0.04–0.91 | 0.03–2.03 | 0–2.98 | |
| BATF3+(%) | |||||
| Mean | 0.065 | 0.028 | 0.058 | 0.092 | 0.021 |
| SD | 0.074 | 0.042 | 0.071 | 0.084 | |
| Range | 0–0.283 | 0–0.102 | 0–0.269 | 0–0.283 | |
| CD8 +/FOXP3+ ratio | |||||
| Mean | 1.27a | 0.81 | 1.24 | 1.55b | 0.24 |
| SD | 1.30 | 0.65 | 1.24 | 1.61 | |
| Range | 0–6.31 | 0.10–1.88 | 0–3.87 | 0.1–6.31 |
*Nonparametric trend test an=38, bn=13
Significant values (p-value < 0.05) are in bold text
GR-high TNBC correlates with increased regulatory T cells
Within the TCGA database via cBioportal [24–26], we found that NR3C1 mRNA expression was significantly correlated to FOXP3 expression (Fig. 4a and b).
Fig. 4.
GR-high TNBC correlates with increased regulatory T cells and a trend towards poor immunotherapy response. a FOXP3 mRNA expression displayed with quartiles of NR3C1 (encoding GR) mRNA expression based on TCGA data (n = 99 patients). b Correlation of NR3C1 encoding GR mRNA levels with FOXP3 mRNA levels in TNBC based on TCGA data (n = 99 patients)
Lack of correlation between GR expression or immune cells with pathological complete response
In our relatively small dataset, GR expression did not correlate with pCR, recurrence-free survival, or overall survival. pCR rate was 0% (0/7) in GR-low tumors, 30.4% (7/23) in GR-moderate tumors, and 28.6% (4/14) in GR-high tumors (Fisher’s exact p = 0.28, GR-low vs. GR-moderate/high p = 0.16). pCR was also not significantly associated with FOXP3+ (OR 1.33, p = 0.46), CD8+ (OR 0.99, p = 0.97), or BATF3+ cell percentages (OR 1.27, p = 0.54). Evaluation of survival outcomes was limited by the small dataset.
Discussion
High tumor GR expression in primary untreated TNBC has been shown to correlate with a shorter time to relapse in both adjuvant chemotherapy treated patients and patients with smaller tumors who did not receive chemotherapy, suggesting a role for GR activity in higher TNBC relapse risk [11, 27–29]. Whether tumor GR expression in chemotherapy-naive early-stage TNBC is accompanied by an immune-suppressive tumor-infiltrating immune microenvironment has not been previously examined. In our single-center, retrospective evaluation of untreated primary TNBCs, we observed higher percentages of tumor-infiltrating FOXP3+ cells and BATF3+ cells in GR-high compared to GR-low TNBC tumor nests, suggesting that an increased immunosuppressed microenvironment is associated with tumor GR expression.
Treg cell activity, and DC dysfunction has been associated with immune evasion in breast cancer through several mechanisms [30–35]. Enhanced infiltration of T regulatory cells facilitates tumor escape from immunosurveillance by suppressing CD8+ T effector cells [32, 35]. In all breast cancers, high FOXP3+ Treg cell densities are related to worse patient outcomes [36], including a poor tumor response to chemotherapy [37, 38]. and predicting late relapse in high risk patients [39]. In breast cancer, DC dysfunction is implicated as a driver of metastasis [40–42], and DC infiltration has been associated with adverse outcomes [43]. Because we found that GR-high versus GR-moderate or GR-low TNBC tumors are positively associated with increased Treg infiltration, GR-high TNBC outcomes may be related to increased immune evasion [11]. Prior work suggests the association between TNBC and Treg and DC function could be mediated by paracrine TGF-β or G-CSF activation [44]. In addition to promoting immunosuppressive Treg cell differentiation, G-CSF can inhibit DC differentiation [45, 46]. We have previously shown that TNBC cell TGFB2 is a direct target gene of GR activation [47], and others have shown that transforming growth factor beta receptor 1 (TGFBR1) is significantly co-expressed with NR3C1 (GR) gene expression driving TNBC invasion, migration and aggressive cell phenotypes [48].
Increased infiltration of FOXP3+ T-regs and BATF3+ immune cells (potentially immature DCs) were significantly associated with GR-high TNBC in our study (p-values of 0.046 and 0.021, respectively) which has also been examined by other groups using publicly available single cell datasets and cohorts [49]. Our analyses demonstrate the importance of using protein expression to evaluate tumor-intrinsic GR expression and immune cell subsets since some deconvolution algorithms such as CIBERSORT may be less efficient in distinguishing between gene expression within cancer and immune cell populations [50].
There was a trend toward increased percentages CD8+ T-cells in GR-high tumor nests, although this association did not reach significance, and the ratio of CD8+ compared to FOXP3+ T-reg cells was similar between GR-high and GR-low tumors. Higher abundance of tumor infiltrating lymphocytes (TILs) in early-stage TNBC has been associated with significantly improved survival (invasive disease-free survival, recurrence free survival, distant recurrence free survival, and overall survival) [51, 52]. Furthermore, while in early-stage TNBC the presence of TILs has been associated with improved response to neoadjuvant chemotherapy [52–54], the TIL subtype (cytotoxic CD8+ cells or T-reg FOXP3+ cells) and the ratio amongst TIL subtypes correlates with different clinical outcomes and responses. Cytotoxic CD8 + TILs and the ratio of CD8+ to T-reg cells are associated with improved responses to neoadjuvant chemotherapy and improved survival; high T-reg TILs have been associated with worse outcomes in other solid tumors including bladder cancer [55, 56]. In TNBC, a higher CD8+ /FOXP3+ ratio has been associated with higher pathologic complete response rates to neoadjuvant chemotherapy [57]. In our data, the CD8+ numbers of infiltrating cells observed in GR-high tumors may be insufficient to improve clinical outcomes, especially given the context of a the highest percentage of T-reg immune cell infiltration in the highest GR-expressing TNBCs. PD-L1 cells were not counted due to lack of specific staining in tumor cells and very minimal staining in PD-L1+ immune cells.
Limitations of this study include the small sample size that preclude associations to clinicopathologic and clinical outcomes and need for deeper immune cell characterization. The value of the study was to define a previously unknown association between GR-high early-stage TNBC, immunosuppressive regulatory T cells, and BATF3 + immune cell subsets, which serves as a starting point for several hypothesis-generating ideas. GR-high TNBC cells are hypothesized to favor an immunosuppressive microenvironment with immunosuppressive Tregs, exhausted CD8 + T cells, and immature BATF3+ DC infiltration, which is being tested in functional and mechanistic studies. BATF3 is necessary, but not sufficient, for the development of classical dendritic cells that cross-present antigens to generate tumor antigen-specific T lymphocytes. For example, WDFY4 knockout mice have shown defective priming, even with intact BATF3 [58]. Further studies using scRNA seq or functional assays will confirm the functional state of the BATF3+ DCs, T-reg cells and CD8+ T cells we observed in this cohort. More extensive multiplex staining should next be performed to characterize infiltrating immune cell subsets with deeper resolution. Evaluation of RFS and OS in larger datasets will be important to evaluate the association between clinical outcomes and tumor GR expression along with the presence of immunosuppressive cell infiltrates in GR-high versus GR-low primary TNBC.
In summary, we describe for the first time the positive relationship between high GR expression in primary TNBC tumors and increased frequency of immunosuppressive infiltrating cells (FOXP3+ and BATF3+) in the tumor microenvironment. Our results of increased FOXP3+ and BATF3+ infiltrating cells in GR-high TNBC support our original hypothesis that GR activation may promote an immunosuppressed tumor microenvironment. GR antagonism could reduce immunosuppression in the tumor microenvironment by both inhibiting GR + paracrine effects on immunosuppressive cells and antagonizing the immunosuppressive effects of cortisol on infiltrating immune cells.
Acknowledgements
We are grateful to The University of Chicago Comprehensive Cancer Center Human Tissue Core for processing the patient samples. The results in Fig. 4 are in whole or part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga.
Author contribution
MDM, CS, MD, NC, RFS, RN, SDC wrote the main manuscript text and prepared the figures. DND and KH prepared Figs. 1–2. MDM prepared Fig. 3. DND, KH, AB, KH (K.Hall), MDM, MD, CS, NC, PS, SDC, RFS and RN collected and analyzed the data. TK performed the statistical analyses. All authors reviewed the manuscript.
Funding
This research was funded by The University of Chicago Comprehensive Cancer Center (NIH Grant P30CA014599-42S2) support to the Human Tissue Core and Biostatistics Core (RN and SDC). This research was also funded by a Cancer Prevention and Research Institute of Texas Scholar Award to SDC (CPRIT Grant ID RR190037).
Data availability
The data generated and analyzed in this study available within the article. Raw data that support the findings of this study are available due to but are available to corresponding author on reasonable request.
Declarations
Competing interests
Margarite Matossian, Christine Shiang, Deniz Nesli Dolcen, Ken Hatogai, Katie Hall, Theodore Karrison and Anna Biernacka, Poornima Saha declare they have no financial interests. Randy F. Sweis reports consulting fees from, Astellas, AstraZeneca, Aveo, BMS, EMD Serono, Editas, Exelixis, Gilead, Eisai, Janssen, Loxo, Lilly, Mirati, Pfizer, Silverback, and Seattle Genetics. Randy F. Sweis reports research support (to institution) from Ascendis, ALX Oncology, Astellas, AstraZeneca, Bayer, BMS, CytomX, Eisai, Genentech/Roche, Gilead, Immunocore, Jounce, Loxo, Lilly, Merck, Moderna, Mirati, Novartis, Pfizer, Pionyr, Pyxis, Scholar Rock, QED Therapeutics. Randy F. Sweis reports the following patents: Neoantigens in Cancer, PCT/US2020/031357. Rita Nanda has served on advisory boards for Astrazeneca, BeyondSpring, Daiichi Sankyo, Exact Sciences, Fujifilm, GE, Gilead, Guardant Health, Infinity, iTeos, Merck, Moderna, Novartis, OBI, Oncosec, Pfizer, Sanofi, Seagen, Stemline, and she has research funding from Arvinas, AstraZeneca, BMS, Corcept Therapeutics, Genentech/Roche, Gilead, GSK, Merck, Novartis, OBI Pharma, OncoSec, Pfizer, Relay, Seattle Genetics, Sun Pharma, Taiho. Nan Chen has received consultant fees from Stemline Therapeutics, Guardant Health, Novartis, and Seagen. Nan Chen has received institutional research funding from Eli Lilly. S.D. Conzen is a co-inventor of patents issued to The University of Chicago for methods and compositions related to GR antagonists and breast cancer.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Margarite D. Matossian, Christine Shiang, Deniz Nesli Dolcen and Marie Dreyer have contributed equally to this work.
Contributor Information
Rita Nanda, Email: rnanda@bsd.uchicago.edu.
Suzanne D. Conzen, Email: Suzanne.Conzen@UTSouthwestern.edu
References
- 1.Giaquinto AN et al (2022) Breast cancer statistics, 2022. CA 72(6):524–541. 10.3322/caac.21754 [DOI] [PubMed] [Google Scholar]
- 2.Brewster AM, Chavez-MacGregor M, Brown P (2014) Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol 15(13):e625–e634. 10.1016/S1470-2045(14)70364-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N (2009) Triple-negative breast cancer—current status and future directions. Ann Oncol 20(12):1913–1927. 10.1093/annonc/mdp492 [DOI] [PubMed] [Google Scholar]
- 4.Manjunath M, Choudhary B (2021) Triple-negative breast cancer: a run-through of features, classification and current therapies (review). Oncol Lett 22(1):512. 10.3892/ol.2021.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid P et al (2020) Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol 31(5):569–581. 10.1016/j.annonc.2020.01.072 [DOI] [PubMed] [Google Scholar]
- 6.Schmid P et al (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382(9):810–821. 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 7.Nanda R et al (2020) Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol 6(5):676. 10.1001/jamaoncol.2019.6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadmiel M, Cidlowski JA (2013) Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci 34(9):518–530. 10.1016/j.tips.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moran TJ, Gray S, Mikosz CA, Conzen SD (2000) The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res 60(4):867–872 [PubMed] [Google Scholar]
- 10.Mayayo-Peralta I, Zwart W, Prekovic S (2021) Duality of glucocorticoid action in cancer: tumor-suppressor or oncogene? Endocr Relat Cancer 28(6):R157–R171. 10.1530/ERC-20-0489 [DOI] [PubMed] [Google Scholar]
- 11.Pan D, Kocherginsky M, Conzen SD (2011) Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Can Res 71(20):6360–6370. 10.1158/0008-5472.CAN-11-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg J et al (2021) The immunology of hormone receptor positive breast cancer. Front Immunol 12:674192. 10.3389/fimmu.2021.674192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abduljabbar R et al (2015) Clinical and biological significance of glucocorticoid receptor (GR) expression in breast cancer. Breast Cancer Res Treat 150(2):335–346. 10.1007/s10549-015-3335-1 [DOI] [PubMed] [Google Scholar]
- 14.West DC et al (2016) GR and ER coactivation alters the expression of differentiation genes and associates with improved ER+ breast cancer outcome. Mol Cancer Res 14(8):707–719. 10.1158/1541-7786.MCR-15-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng Y et al (2021) Glucocorticoid receptor regulates PD-L1 and MHC-I in pancreatic cancer cells to promote immune evasion and immunotherapy resistance. Nat Commun 12(1):7041. 10.1038/s41467-021-27349-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taya M, LeBlanc E, Bennett L, Conzen SD (2023) Abstract 654: ovarian cancer-cell glucocorticoid receptor activity modulates cytokine secretion promoting infiltration of immunosuppressive cells into the tumor microenvironment. Cancer Res 83(7_Supplement):654. 10.1158/1538-7445.AM2023-654 [Google Scholar]
- 17.Shimba A, Ikuta K (2020) Control of immunity by glucocorticoids in health and disease. Semin Immunopathol 42(6):669–680. 10.1007/s00281-020-00827-8 [DOI] [PubMed] [Google Scholar]
- 18.Rozkova D, Horvath R, Bartunkova J, Spisek R (2006) Glucocorticoids severely impair differentiation and antigen presenting function of dendritic cells despite upregulation of Toll-like receptors. Clin Immunol 120(3):260–271. 10.1016/j.clim.2006.04.567 [DOI] [PubMed] [Google Scholar]
- 19.Moulton VR (2018) Sex hormones in acquired immunity and autoimmune disease. Front Immunol 9:2279. 10.3389/fimmu.2018.02279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman ML et al (2019) Progesterone receptor attenuates STAT1-mediated IFN signaling in breast cancer. J Immunol 202(10):3076–3086. 10.4049/jimmunol.1801152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belova L, Delgado B, Kocherginsky M, Melhem A, Olopade OI, Conzen SD (2009) Glucocorticoid receptor expression in breast cancer associates with older patient age. Breast Cancer Res Treat 116(3):441–447. 10.1007/s10549-008-0136-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saha P, Turk A, Lurain K, Baker G, Conzen S, Nanda R (2016) Abstract P3-07-24: the role of glucocorticoid receptor (GR) expression in predicting pathological complete response (pCR) to neoadjuvant chemotherapy in triple-negative breast cancer (TNBC). Cancer Res 76(4_Supplement):P3-07–24. 10.1158/1538-7445.SABCS15-P3-07-24 [Google Scholar]
- 23.Cuzick J (1985) A wilcoxon-type test for trend. Statist Med 4(1):87–90. 10.1002/sim.4780040112 [DOI] [PubMed] [Google Scholar]
- 24.Cerami E et al (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2(5):401–404. 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao J et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal”. Sci Signal. 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Bruijn I et al (2023) Analysis and visualization of longitudinal genomic and clinical data from the AACR project GENIE biopharma collaborative in cBioPortal. Can Res 83(23):3861–3867. 10.1158/0008-5472.CAN-23-0816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakour N, Moriarty F, Moore G, Robson T, Annett SL (2021) Prognostic significance of glucocorticoid receptor expression in cancer: a systematic review and meta-analysis. Cancers 13(7):1649. 10.3390/cancers13071649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elkashif A et al (2020) Glucocorticoid receptor expression predicts good outcome in response to taxane-free, anthracycline-based therapy in triple negative breast cancer. J Oncol 2020:1–10. 10.1155/2020/3712825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skor MN et al (2013) Glucocorticoid receptor antagonism as a novel therapy for triple-negative breast cancer. Clin Cancer Res 19(22):6163–6172. 10.1158/1078-0432.CCR-12-3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jhunjhunwala S, Hammer C, Delamarre L (2021) Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer 21(5):298–312. 10.1038/s41568-021-00339-z [DOI] [PubMed] [Google Scholar]
- 31.Zebley CC, Zehn D, Gottschalk S, Chi H (2024) T cell dysfunction and therapeutic intervention in cancer. Nat Immunol. 10.1038/s41590-024-01896-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gil Del Alcazar CR, Alečković M, Polyak K (2020) Immune escape during breast tumor progression. Cancer Immunol Res 8(4):422–427. 10.1158/2326-6066.CIR-19-0786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu C, Jiang A (2018) Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front Immunol 9:3059. 10.3389/fimmu.2018.03059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattiuz R et al (2021) Type 1 conventional dendritic cells and interferons are required for spontaneous CD4 + and CD8 + T-cell protective responses to breast cancer. Clin Transl Immunol. 10.1002/cti2.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paluskievicz CM, Cao X, Abdi R, Zheng P, Liu Y, Bromberg JS (2019) T regulatory cells and priming the suppressive tumor microenvironment. Front Immunol 10:2453. 10.3389/fimmu.2019.02453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmoud SMA et al (2011) An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res Treat 127(1):99–108. 10.1007/s10549-010-0987-8 [DOI] [PubMed] [Google Scholar]
- 37.Aruga (2009) A low number of tumor-infiltrating FOXP3-positive cells during primary systemic chemotherapy correlates with favorable anti-tumor response in patients with breast cancer. Oncol Rep. 10.3892/or_00000434 [PubMed] [Google Scholar]
- 38.Ladoire S et al (2008) Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating Foxp3+ regulatory T cells. Clin Cancer Res 14(8):2413–2420. 10.1158/1078-0432.CCR-07-4491 [DOI] [PubMed] [Google Scholar]
- 39.Bates GJ et al (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. JCO 24(34):5373–5380. 10.1200/JCO.2006.05.9584 [DOI] [PubMed] [Google Scholar]
- 40.Mansfield AS, Heikkila P, von Smitten K, Vakkila J, Leidenius M (2011) Metastasis to sentinel lymph nodes in breast cancer is associated with maturation arrest of dendritic cells and poor co-localization of dendritic cells and CD8+ T cells. Virchows Arch 459(4):391–398. 10.1007/s00428-011-1145-3 [DOI] [PubMed] [Google Scholar]
- 41.Gadalla R et al (2019) Tumor microenvironmental plasmacytoid dendritic cells contribute to breast cancer lymph node metastasis via CXCR4/SDF-1 axis. Breast Cancer Res Treat 174(3):679–691. 10.1007/s10549-019-05129-8 [DOI] [PubMed] [Google Scholar]
- 42.Verronèse E et al (2016) Immune cell dysfunctions in breast cancer patients detected through whole blood multi-parametric flow cytometry assay. OncoImmunology 5(3):e1100791. 10.1080/2162402X.2015.1100791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treilleux I et al (2004) Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res 10(22):7466–7474. 10.1158/1078-0432.CCR-04-0684 [DOI] [PubMed] [Google Scholar]
- 44.Dolcen DN et al (2019) Abstract 2812: triple-negative breast cancer cell-intrinsic glucocorticoid receptor activity and modulation of the immune microenvironment. Cancer Res 79(13):2812. 10.1158/1538-7445.AM2019-281230967398 [Google Scholar]
- 45.Hiam-Galvez KJ, Allen BM, Spitzer MH (2021) Systemic immunity in cancer. Nat Rev Cancer 21(6):345–359. 10.1038/s41568-021-00347-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar A, Taghi Khani A, Sanchez Ortiz A, Swaminathan S (2022) GM-CSF: a double-edged sword in cancer immunotherapy. Front Immunol 13:901277. 10.3389/fimmu.2022.901277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.West DC et al (2018) Discovery of a glucocorticoid receptor (GR) activity signature using selective GR antagonism in ER-negative breast cancer. Clin Cancer Res 24(14):3433–3446. 10.1158/1078-0432.CCR-17-2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez Kerkvliet C et al (2020) Glucocorticoid receptors are required effectors of TGFβ1-induced p38 MAPK signaling to advanced cancer phenotypes in triple-negative breast cancer. Breast Cancer Res 22(1):39. 10.1186/s13058-020-01277-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gandhi S et al (2020) Contribution of immune cells to glucocorticoid receptor expression in breast cancer. IJMS 21(13):4635. 10.3390/ijms21134635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso-Moreda N, Berral-González A, De La Rosa E, González-Velasco O, Sánchez-Santos JM, De Las Rivas J (2023) Comparative analysis of cell mixtures deconvolution and gene signatures generated for blood, immune cancer cells. IJMS 24(13):10765. 10.3390/ijms241310765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leon-Ferre RA et al (2024) Tumor-infiltrating lymphocytes in triple-negative breast cancer. JAMA 331(13):1135. 10.1001/jama.2024.3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park JH et al (2019) Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol 30(12):1941–1949. 10.1093/annonc/mdz395 [DOI] [PubMed] [Google Scholar]
- 53.Loi S et al (2022) Tumor infiltrating lymphocyte stratification of prognostic staging of early-stage triple negative breast cancer. NPJ Breast Cancer 8(1):3. 10.1038/s41523-021-00362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dieci MV et al (2023) Incorporating weekly carboplatin in anthracycline and paclitaxel-containing neoadjuvant chemotherapy for triple-negative breast cancer: propensity-score matching analysis and TIL evaluation. Br J Cancer 128(2):266–274. 10.1038/s41416-022-02050-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatogai K, Sweis RF (2020) The tumor microenvironment of bladder cancer, in tumor microenvironments in organs. In: Birbrair A (ed) Advances in experimental medicine and biology, vol 1296. Springer, Cham, pp 275–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baras AS et al (2016) The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. OncoImmunology 5(5):e1134412. 10.1080/2162402X.2015.1134412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asano Y et al (2016) Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg 103(7):845–854. 10.1002/bjs.10127 [DOI] [PubMed] [Google Scholar]
- 58.Theisen DJ et al (2018) WDFY4 is required for cross-presentation in response to viral and tumor antigens. Science 362(6415):694–699. 10.1126/science.aat5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and analyzed in this study available within the article. Raw data that support the findings of this study are available due to but are available to corresponding author on reasonable request.