Abstract
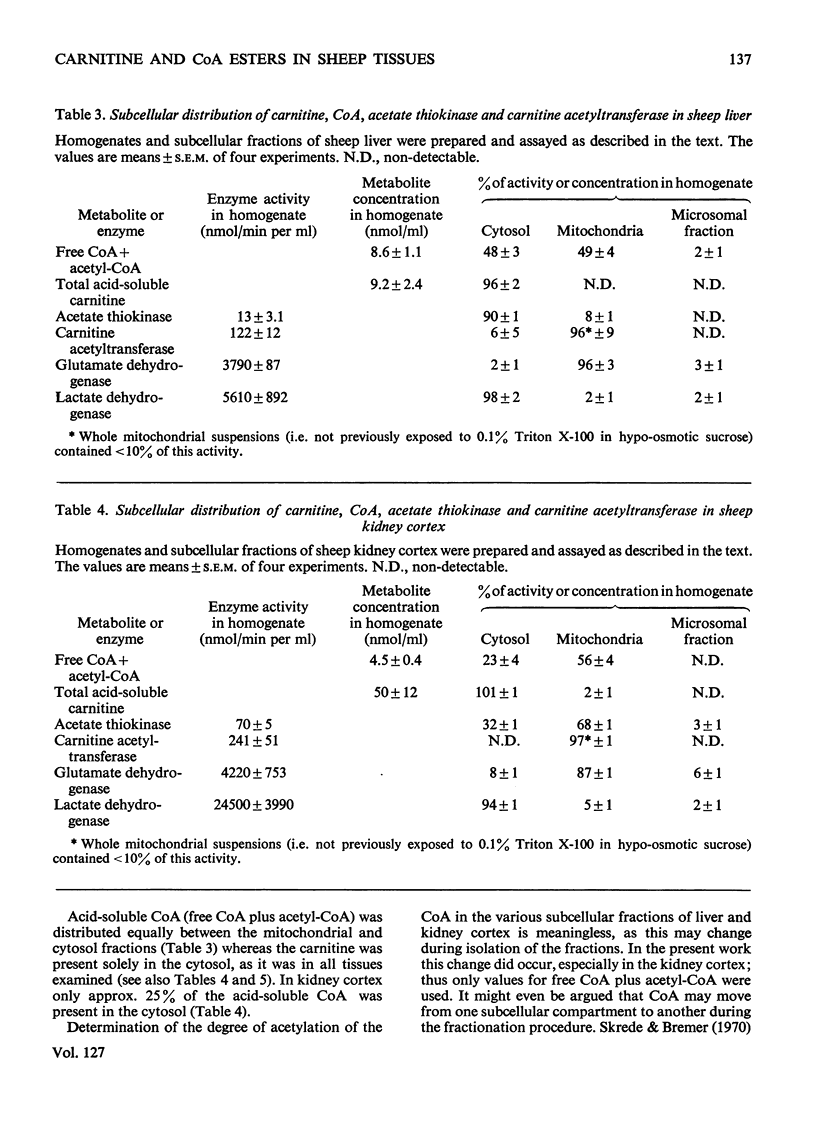
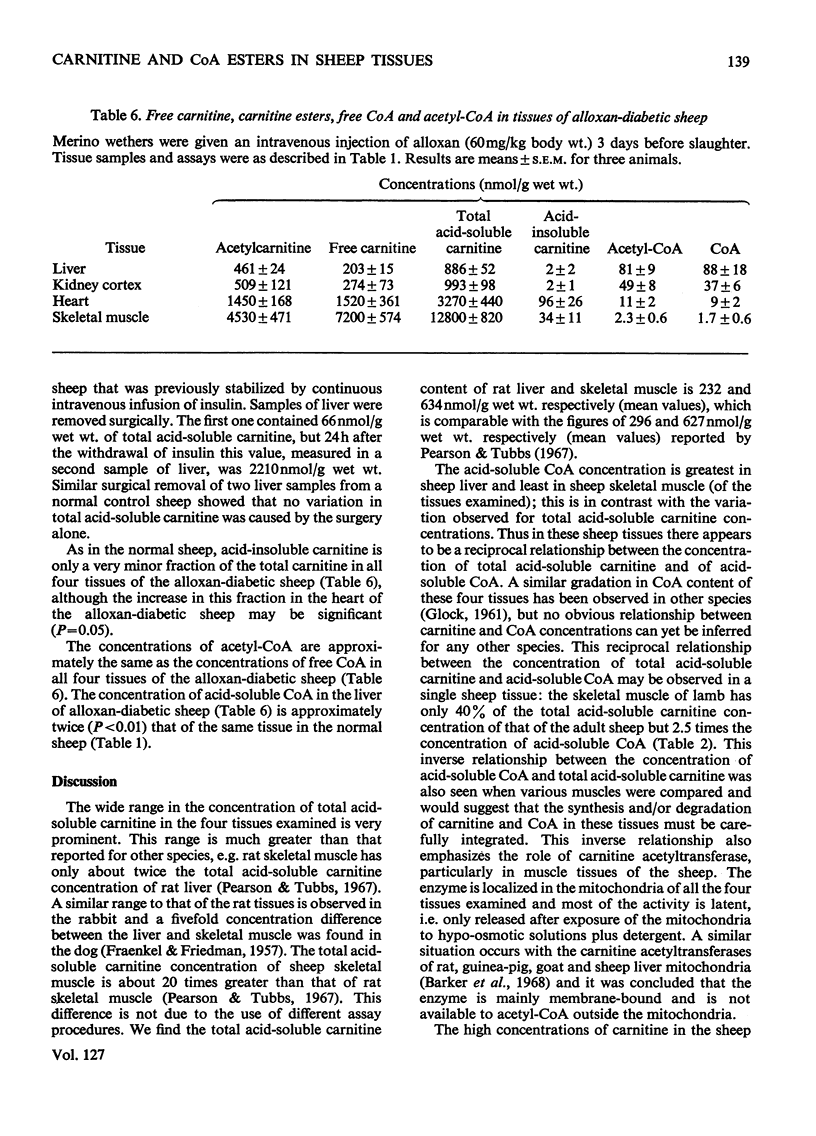
1. The total acid-soluble carnitine concentrations of four tissues from Merino sheep showed a wide variation not reported for other species. The concentrations were 134, 538, 3510 and 12900nmol/g wet wt. for liver, kidney cortex, heart and skeletal muscle (M. biceps femoris) respectively. 2. The concentration of acetyl-CoA was approximately equal to the concentration of free CoA in all four tissues and the concentration of acid-soluble CoA (free CoA plus acetyl-CoA) decreased in the order liver>kidney cortex>heart>skeletal muscle. 3. The total amount of acid-soluble carnitine in skeletal muscle of lambs was 40% of that in the adult sheep, whereas the concentration of acid-soluble CoA was 2.5 times as much. A similar inverse relationship between carnitine and CoA concentrations was observed when different muscles in the adult sheep were compared. 4. Carnitine was confined to the cytosol in all four tissues examined, whereas CoA was equally distributed between the mitochondria and cytosol in liver, approx. 25% was present in the cytosol in kidney cortex and virtually none in this fraction in heart and skeletal muscle. 5. Carnitine acetyltransferase (EC 2.3.1.7) was confined to the mitochondria in all four tissues and at least 90% of the activity was latent. 6. Acetate thiokinase (EC 6.2.1.1) was predominantly (90%) present in the cytosol in liver, but less than 10% was present in this fraction in heart and skeletal muscle. 7. In alloxan-diabetes, the concentration of acetylcarnitine was increased in all four tissues examined, but the total acid-soluble carnitine concentration was increased sevenfold in the liver and twofold in kidney cortex. 8. The concentration of acetyl-CoA was approximately equal to that of free CoA in the four tissues of the alloxan diabetic sheep, but the concentration of acid-soluble CoA in liver increased approximately twofold in alloxan-diabetes. 9. The relationship between CoA and carnitine and the role of carnitine acetyltransferase in the various tissues is discussed. The quantitative importance of carnitine in ruminant metabolism is also emphasized.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBERSON W. R., BAUER A. C., PHILPOTT D. E., ROISEN F. PROTEINS AND ENZYME ACTIVITIES OF PRESS JUICES, OBTAINED BY ULTRACENTRIFUGATION OF WHITE, RED AND HEART MUSCLES OF THE RABBIT. J Cell Physiol. 1964 Feb;63:7–24. doi: 10.1002/jcp.1030630103. [DOI] [PubMed] [Google Scholar]
- Allred J. B., Guy D. G. Determination of coenzyme A and acetyl CoA in tissue extracts. Anal Biochem. 1969 May;29(2):293–299. doi: 10.1016/0003-2697(69)90312-1. [DOI] [PubMed] [Google Scholar]
- Barker P. J., Fincham N. J., Hardwick D. C. The availability of carnitine acetyltransferase in mitochondria from guinea-pig liver and other tissues. Biochem J. 1968 Dec;110(4):739–746. doi: 10.1042/bj1100739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode C., Stähler E., Kono H., Goebell H. Effects of ethanol on free coenzyme A, free carnitine and their fatty acid esters in rat liver. Biochim Biophys Acta. 1970 Sep 8;210(3):448–455. doi: 10.1016/0005-2760(70)90041-x. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B., PERRY S. V. Biochemical and osmotic properties of skeletal muscle mitochondria. Nature. 1954 Jun 5;173(4414):1094–1095. doi: 10.1038/1731094a0. [DOI] [PubMed] [Google Scholar]
- Cook R. M., Liu S. C., Quraishi S. Utilization of volatile fatty acids in ruminants. 3. Comparison of mitochondrial acyl coenzyme A synthetase activity and substrate specificity in different tissues. Biochemistry. 1969 Jul;8(7):2966–2969. doi: 10.1021/bi00835a042. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Coore H. G., Martin B. R., Randle P. J. Insulin activates pyruvate dehydrogenase in rat epididymal adipose tissue. Nat New Biol. 1971 May 26;231(21):115–116. doi: 10.1038/newbio231115a0. [DOI] [PubMed] [Google Scholar]
- Erfle J. D., Fisher L. J., Sauer F. Carnitine and acetylcarnitine in the milk of normal and ketotic cows. J Dairy Sci. 1970 Apr;53(4):486–489. doi: 10.3168/jds.S0022-0302(70)86236-1. [DOI] [PubMed] [Google Scholar]
- FRITZ I. B., SCHULTZ S. K., SRERE P. A. Properties of partially purified carnitine acetyltransferase. J Biol Chem. 1963 Jul;238:2509–2517. [PubMed] [Google Scholar]
- JACOBSON K. B. Effect of substrate structure on activity of pigeon liver acetyl transferase. J Biol Chem. 1961 Feb;236:343–348. [PubMed] [Google Scholar]
- MARQUIS N. R., FRITZ I. B. ENZYMOLOGICAL DETERMINATION OF FREE CARNITINE CONCENTRATIONS IN RAT TISSUES. J Lipid Res. 1964 Apr;5:184–187. [PubMed] [Google Scholar]
- Mayfield E. D., Bensadoun A., Johnson B. C. Acetate metabolism in ruminant tissues. J Nutr. 1966 Jun;89(2):189–196. doi: 10.1093/jn/89.2.189. [DOI] [PubMed] [Google Scholar]
- Mehlman M. A., Kader M. M., Therriault D. G. Metabolism, turnover time, half life, body pool of carnitine-14C in normal, alloxan diabetic and insulin treated rats. Life Sci. 1969 May 15;8(10):465–472. doi: 10.1016/0024-3205(69)90243-4. [DOI] [PubMed] [Google Scholar]
- PEARSON D. J., TUBBS P. K. ACETYL-CARNITINE IN HEART AND LIVER. Nature. 1964 Apr 4;202:91–91. doi: 10.1038/202091a0. [DOI] [PubMed] [Google Scholar]
- Pearson D. J., Tubbs P. K. Carnitine and derivatives in rat tissues. Biochem J. 1967 Dec;105(3):953–963. doi: 10.1042/bj1050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd D., Garland P. B. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J. 1969 Sep;114(3):597–610. doi: 10.1042/bj1140597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrede S., Bremer J. The compartmentation of CoA and fatty acid activating enzymes in rat liver mitochondria. Eur J Biochem. 1970 Jul;14(3):465–472. doi: 10.1111/j.1432-1033.1970.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Snoswell A. M., Henderson G. D. Aspects of carnitine ester metabolism in sheep liver. Biochem J. 1970 Aug;119(1):59–65. doi: 10.1042/bj1190059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABOR H., MEHLER A. H., STADTMAN E. R. The enzymatic acetylation of amines. J Biol Chem. 1953 Sep;204(1):127–138. [PubMed] [Google Scholar]
- Taylor P. H., Wallace J. C., Keech D. B. Gluconeogenic enzymes in sheep liver. Intracellular localization of pyruvate carboxylase and phosphoenolpyruvate carboxykinase in normal, fasted and diabetic sheep. Biochim Biophys Acta. 1971 May 18;237(2):179–191. [PubMed] [Google Scholar]
- Tubbs P. K., Garland P. B. Membranes and fatty acid metabolism. Br Med Bull. 1968 May;24(2):158–164. doi: 10.1093/oxfordjournals.bmb.a070619. [DOI] [PubMed] [Google Scholar]
- Tubbs P. K., Garland P. B. Variations in tissue contents of coenzyme A thio esters and possible metabolic implications. Biochem J. 1964 Dec;93(3):550–557. doi: 10.1042/bj0930550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Walter P., Anabitarte M. On the use of glutamate dehydrogenase as a mitochondrial marker enzyme for the determination of the intracellular distribution of rat liver pyruvate carboxylase. FEBS Lett. 1971 Jan 30;12(5):289–292. doi: 10.1016/0014-5793(71)80201-6. [DOI] [PubMed] [Google Scholar]
- Yates D. W., Garland P. B. Carnitine palmitoyltransferase activities (EC 2.3.1.-) of rat liver mitochondria. Biochem J. 1970 Sep;119(3):547–552. doi: 10.1042/bj1190547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. W., Garland P. B. The partial latency and intramitochondrial distribution of carnitine-palmitoyltransferase (e.c.2.3.1.-), and the CoASH and carnitine permeable space of rat liver mitochondria. Biochem Biophys Res Commun. 1966 May 25;23(4):460–465. doi: 10.1016/0006-291x(66)90750-9. [DOI] [PubMed] [Google Scholar]
- de Jong J. W., Hülsmann W. C. Effects of nagarse, adenosine and hexokinase on palmitate activation and oxidation. Biochim Biophys Acta. 1970 Sep 8;210(3):499–501. doi: 10.1016/0005-2760(70)90050-0. [DOI] [PubMed] [Google Scholar]


