Abstract
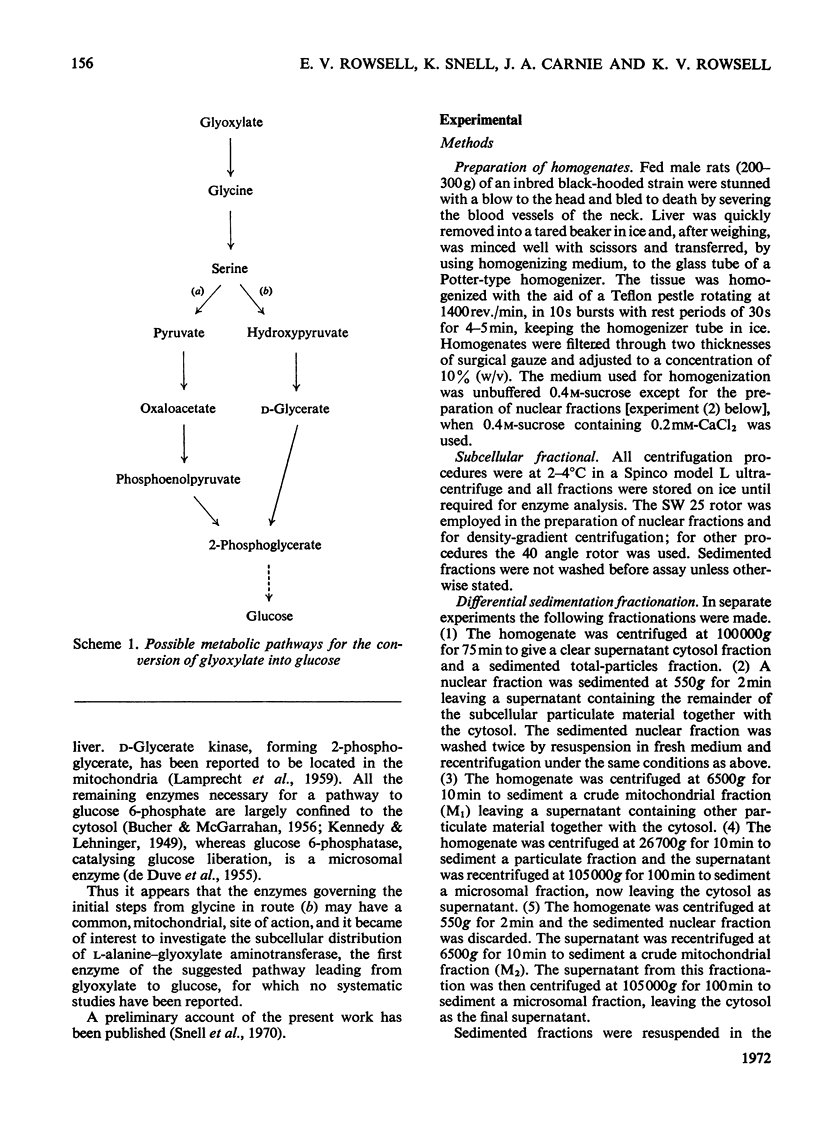
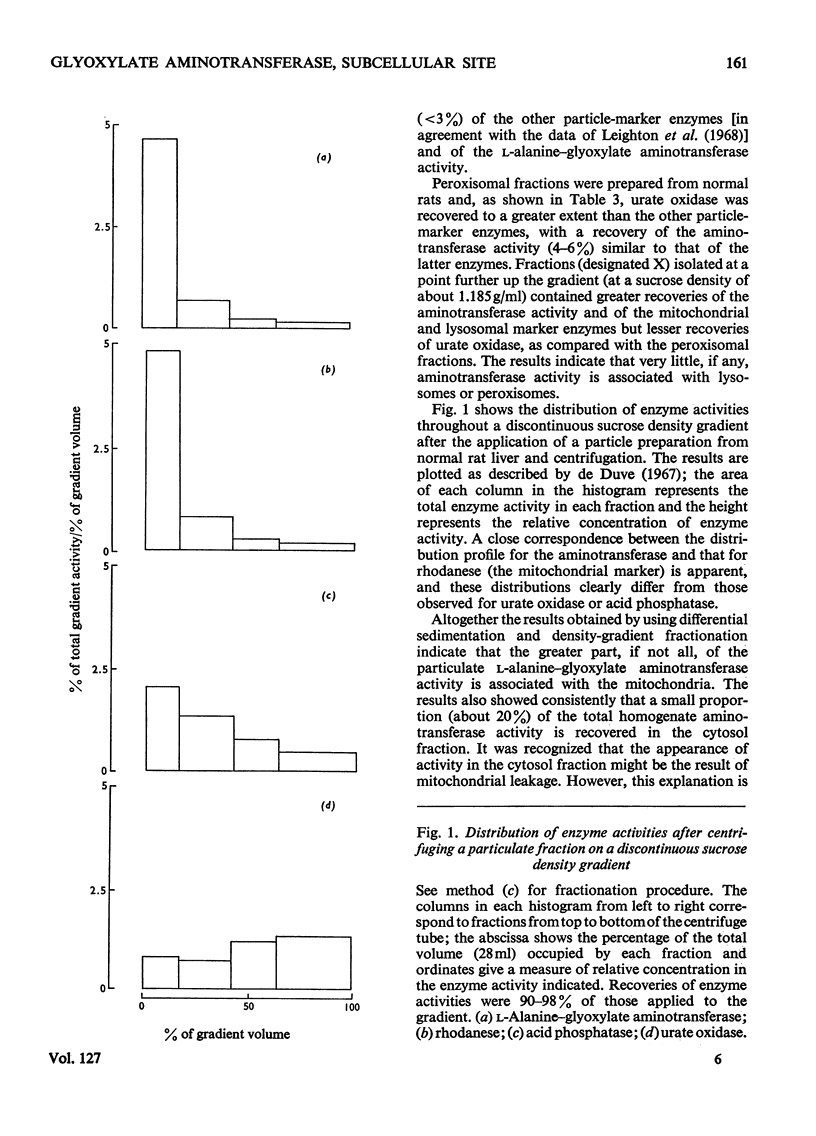
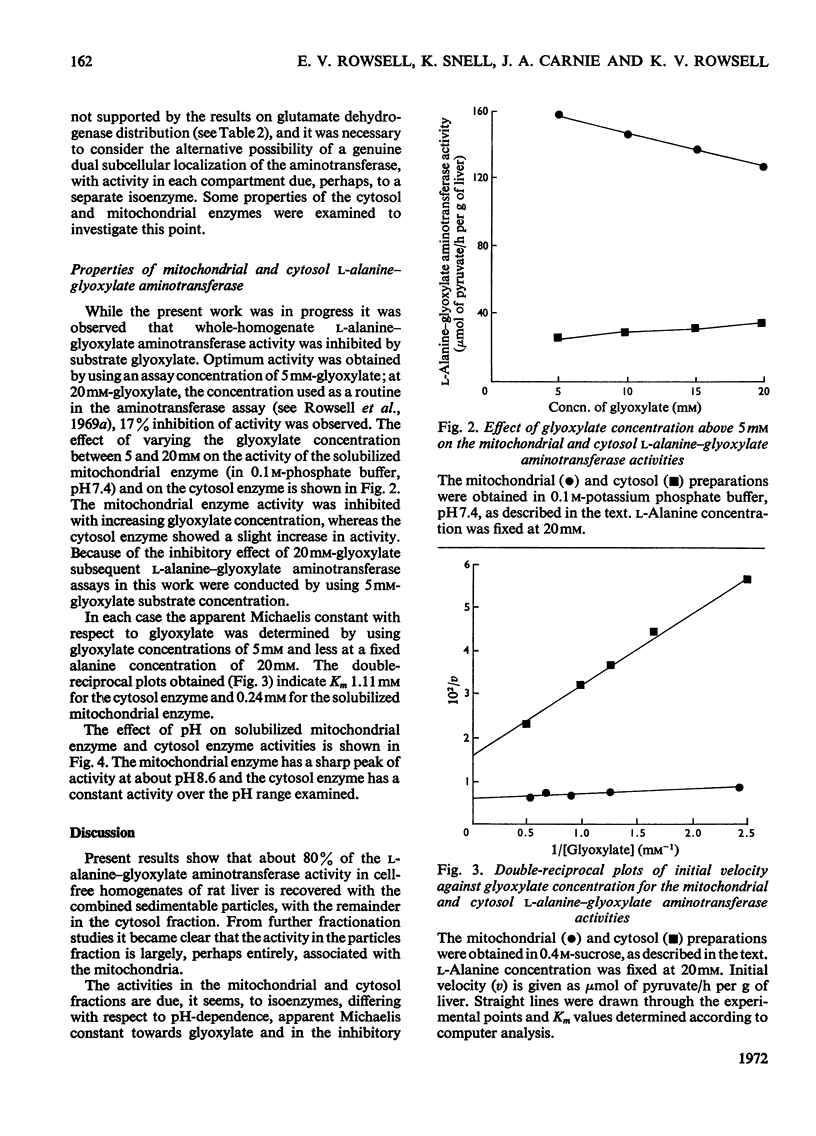
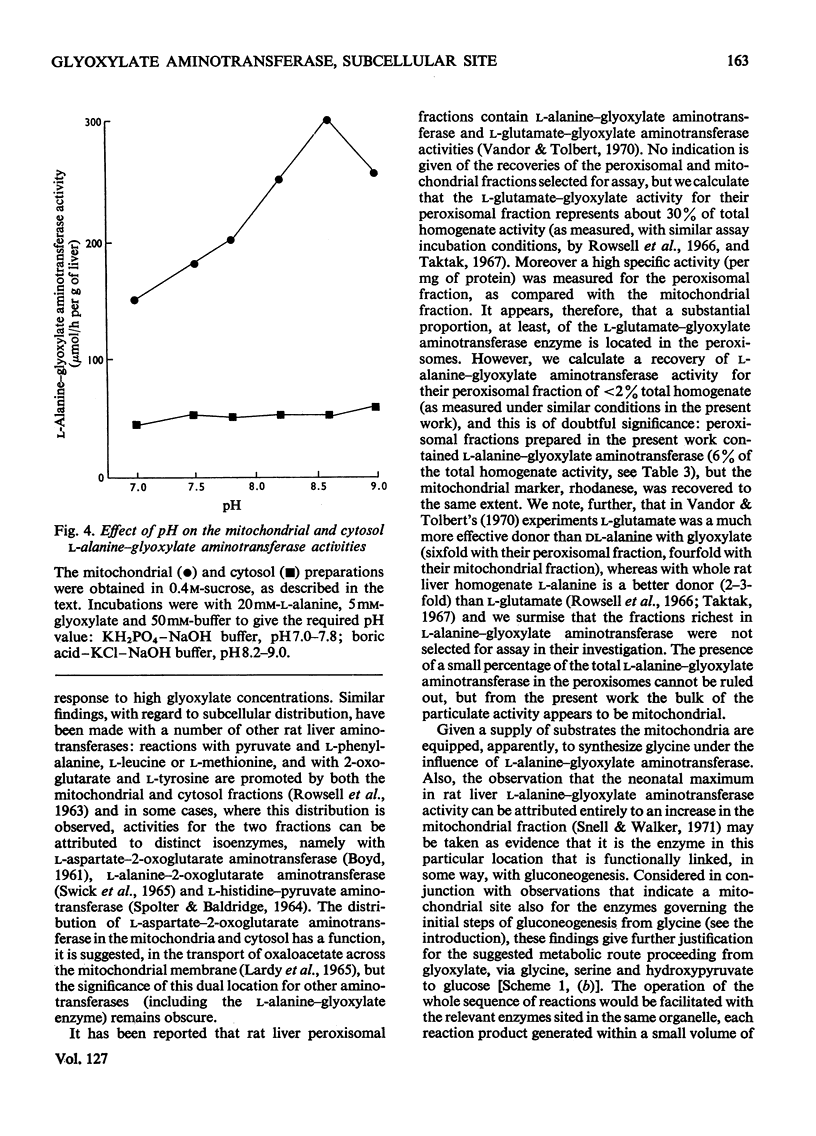
1. The distribution of l-alanine–glyoxylate aminotransferase activity between subcellular fractions prepared from rat liver homogenates was investigated. The greater part of the homogenate activity (about 80%) was recovered in the `total-particles' fraction sedimented by high-speed centrifugation and the remainder in the cytosol fraction. 2. Subfractionation of the particles by differential sedimentation and on sucrose density gradients revealed a specific association between the aminotransferase and the mitochondrial enzymes glutamate dehydrogenase and rhodanese. 3. The aminotransferase activities in the cytosol and the mitochondria are due to isoenzymes. The solubilized mitochondrial enzyme has a pH optimum of 8.6, an apparent Km of 0.24mm with respect to glyoxylate and is inhibited by glyoxylate at concentrations above 5mm. The cytosol aminotransferase shows no distinct pH optimum (over the range 7.0–9.0) and has an apparent Km of 1.11mm with respect to glyoxylate; there is no evidence of inhibition by glyoxylate. 4. The mitochondrial location of the bulk of the rat liver l-alanine–glyoxylate aminotransferase activity is discussed in relation to a pathway for gluconeogenesis involving glyoxylate.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAUFAY H., BENDALL D. S., BAUDHUIN P., DE DUVE C. Tissue fractionation studies. 12. Intracellular distribution of some dehydrogenases, alkaline deoxyribonuclease and iron in rat-liver tissue. Biochem J. 1959 Dec;73:623–628. doi: 10.1042/bj0730623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD J. W. The intracellular distribution, latency and electrophoretic mobility of L-glutamate-oxaloacetate transaminase from rat liver. Biochem J. 1961 Nov;81:434–441. doi: 10.1042/bj0810434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHER N. L., MCGARRAHAN K. The biosynthesis of cholesterol from acetate-1-C14 by cellular fractions of rat liver. J Biol Chem. 1956 Sep;222(1):1–15. [PubMed] [Google Scholar]
- Beaufay H., Jacques P., Baudhuin P., Sellinger O. Z., Berthet J., De Duve C. Tissue fractionation studies. 18. Resolution of mitochondrial fractions from rat liver into three distinct populations of cytoplasmic particles by means of density equilibration in various gradients. Biochem J. 1964 Jul;92(1):184–205. doi: 10.1042/bj0920184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B., THOMAS J. Studies on sulphatases. II. The assay of the arylsulphatase activity of rat tissues. Biochem J. 1953 Feb;53(3):452–457. doi: 10.1042/bj0530452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drahota Z., Hahn P., Honová E. Acetoacetate formation by livers of young and adult rats. Biol Neonat. 1965;9(1):124–131. doi: 10.1159/000239984. [DOI] [PubMed] [Google Scholar]
- Eibl H., Lands W. E. A new, sensitive determination of phosphate. Anal Biochem. 1969 Jul;30(1):51–57. doi: 10.1016/0003-2697(69)90372-8. [DOI] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen T., Nyhan W. L., Rehberg M. L., Ando T. Metabolism of glyoxylate in nonketotic hyperglycinemia. Pediatr Res. 1969 Jul;3(4):269–274. doi: 10.1203/00006450-196907000-00001. [DOI] [PubMed] [Google Scholar]
- Hinton R. H., Burge M. L., Hartman G. C. Sucrose interference in the assay of enzymes and protein. Anal Biochem. 1969 May;29(2):248–256. doi: 10.1016/0003-2697(69)90308-x. [DOI] [PubMed] [Google Scholar]
- JOHNSON B. E., WALSH D. A., SALLACH H. J. CHANGES IN THE ACTIVITIES OF D-GLYCERATE AND D-3-PHOSPHOGLYCERATE DEHYDROGENASES IN THE DEVELOPING RAT LIVER. Biochim Biophys Acta. 1964 May 4;85:202–205. doi: 10.1016/0926-6569(64)90241-x. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Sato T., Kikuchi G. A new reaction for glycine biosynthesis. Biochem Biophys Res Commun. 1966 May 3;23(3):227–233. doi: 10.1016/0006-291x(66)90532-8. [DOI] [PubMed] [Google Scholar]
- LAMPRECHT W., DIAMANTSTEIN T., HEINZ F., BALDE P. [Phosphorylation of D-glyceric acid to 2-phospho-D-glyceric acid with glycerate kinase in the liver. I. On the biochemistry of fructose metabolism. II]. Hoppe Seylers Z Physiol Chem. 1959 Sep 30;316:97–112. doi: 10.1515/bchm2.1959.316.1.97. [DOI] [PubMed] [Google Scholar]
- Lardy H. A., Paetkau V., Walter P. Paths of carbon in gluconeogenesis and lipogenesis: the role of mitochondria in supplying precursors of phosphoenolpyruvate. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1410–1415. doi: 10.1073/pnas.53.6.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAITRA U., DEKKER E. E. PURIFICATION AND PROPERTIES OF RAT LIVER 2-KETO-4-HYDROXYGLUTARATE ALDOLASE. J Biol Chem. 1964 May;239:1485–1491. [PubMed] [Google Scholar]
- McMullen A. I., McSweeney G. P. The biosynthesis of rubber: Incorporation of isopentenyl pyrophosphate into purified rubber particles by a soluble latex serum enzyme. Biochem J. 1966 Oct;101(1):42–47. doi: 10.1042/bj1010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowsell E. V., Snell K., Carnie J. A., Al-Tai A. H. Liver-L-alanine-glyoxylate and L-serine-pyruvate aminotransferase activities: an apparent association with gluconeogenesis. Biochem J. 1969 Dec;115(5):1071–1073. doi: 10.1042/bj1151071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPOLTER H., BALDRIDGE R. C. MULTIPLE FORMS OF HISTIDINE-PYRUVATE TRANSAMINASE IN RAT LIVER. Biochim Biophys Acta. 1964 Aug 19;90:287–290. doi: 10.1016/0304-4165(64)90191-6. [DOI] [PubMed] [Google Scholar]
- SWANSON M. A. Phosphatases of liver. I. Glucose-6-phosphatase. J Biol Chem. 1950 Jun;184(2):647–659. [PubMed] [Google Scholar]
- SWICK R. W., BARNSTEIN P. L., STANGE J. L. THE METABOLISM OF MITOCHONDRIAL PROTEINS. I. DISTRIBUTION AND CHARACTERIZATION OF THE ISOZYMES OF ALANINE AMINOTRANSFERASE IN RAT LIVER. J Biol Chem. 1965 Aug;240:3334–3340. [PubMed] [Google Scholar]
- Sato T., Kochi H., Motokawa Y., Kawasaki H., Kikuchi G. Glycin metabolism by rat liver mitochondria. I. Synthesis of two molecules of glycine from one molecule each of serine, bicarbonate and ammonia. J Biochem. 1969 Jan;65(1):63–70. [PubMed] [Google Scholar]
- Thompson J. S., Richardson K. E. Isolation and characterization of an L-alanine: glyoxylate aminotransferase from human liver. J Biol Chem. 1967 Aug 25;242(16):3614–3619. [PubMed] [Google Scholar]
- Vandor S. L., Tolbert N. E. Glyoxylate metabolism by isolated rat liver peroxisomes. Biochim Biophys Acta. 1970 Sep 22;215(3):449–455. doi: 10.1016/0304-4165(70)90095-4. [DOI] [PubMed] [Google Scholar]
- WEBB J. M., LEVY H. B. A sensitive method for the determination of deoxyribonucleic acid in tissues and microorganisms. J Biol Chem. 1955 Mar;213(1):107–117. [PubMed] [Google Scholar]
- WEINHOUSE S., FRIEDMANN B. Metabolism of labeled 2-carbon acids in the intact rat. J Biol Chem. 1951 Aug;191(2):707–717. [PubMed] [Google Scholar]
- WEISSBACH A., SPRINSON D. B. The metabolism of 2-carbon compounds related to glycine. II. Ethanolamine. J Biol Chem. 1953 Aug;203(2):1031–1037. [PubMed] [Google Scholar]
- Yeung D., Stanley R. S., Oliver I. T. Development of gluconeogenesis in neonatal rat liver. Effect of triamcinolone. Biochem J. 1967 Dec;105(3):1219–1227. doi: 10.1042/bj1051219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yielding K. L. Modification by sucrose of the catalytic activity and physical properties of glutamic dehydrogenase. Biochem Biophys Res Commun. 1970 Feb 20;38(4):546–551. doi: 10.1016/0006-291x(70)90615-7. [DOI] [PubMed] [Google Scholar]


