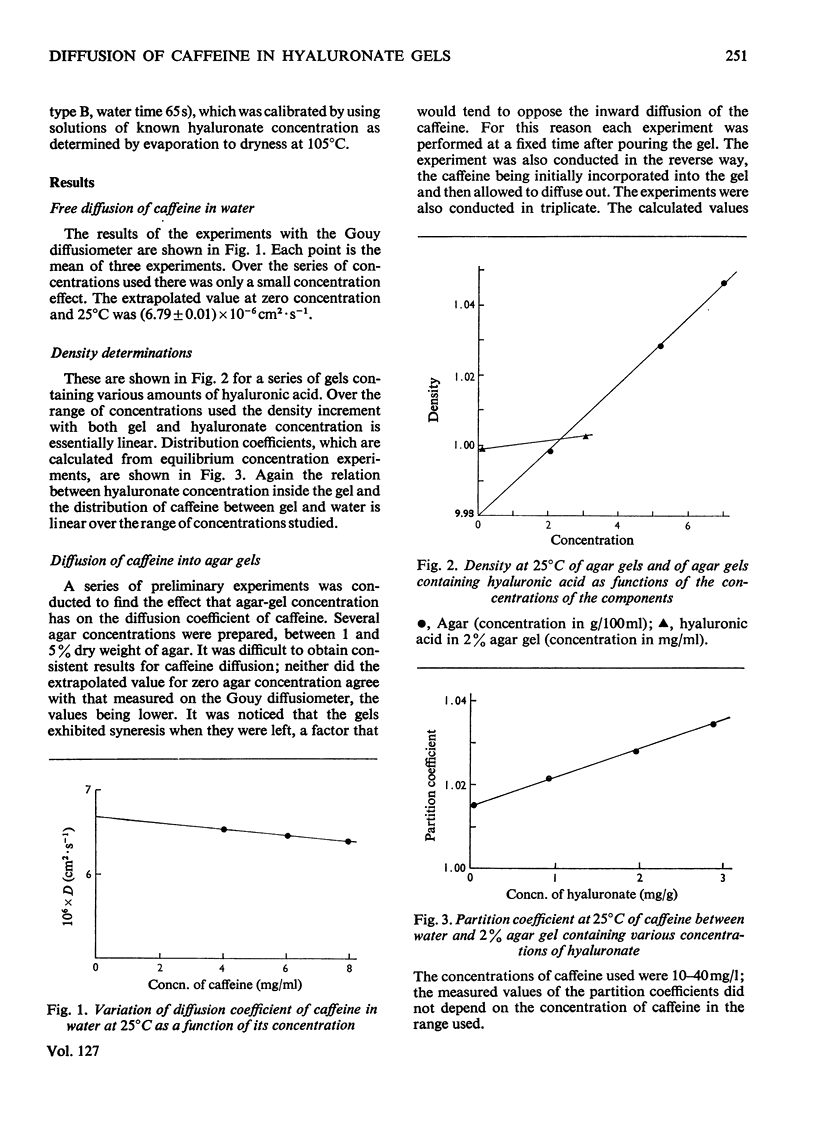
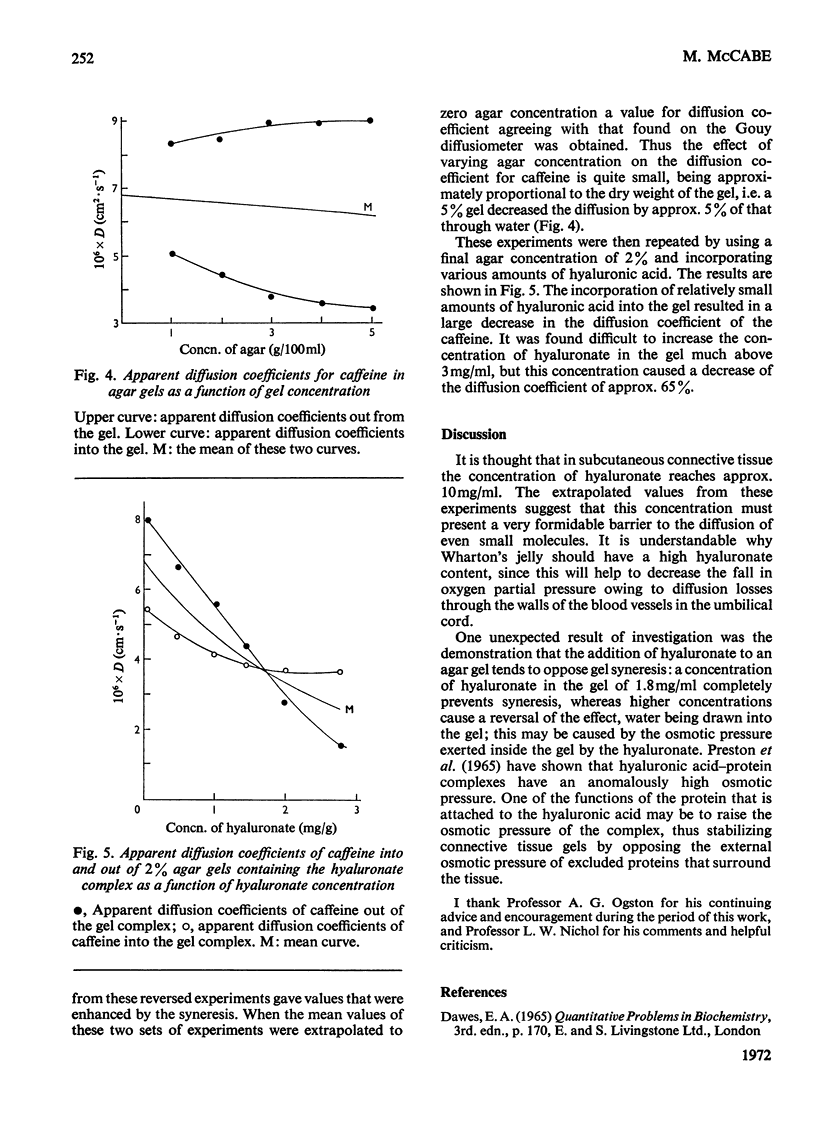
Abstract
A hyaluronic acid–protein complex was embedded into agar gel. This gel complex resembles in some respects the physiological situation in connective tissue, but still permits precise physicochemical measurements to be made. The diffusion coefficient of caffeine into and from such gels has been measured as a function of both agar and hyaluronate concentration. The value for the diffusion coefficient of caffeine was also measured by using a Gouy type diffusiometer. From both types of measurement the value for D (Fick) for caffeine when extrapolated to zero caffeine and agar concentrations agreed at (6.79±0.01)×10−6cm2·s−1 at 25°C. Although agar concentration had only a small effect on caffeine diffusion, hyaluronic acid caused a large decrease in caffeine diffusion co-efficient. The presence of the hyaluronic acid–protein complex within the gel tended to oppose gel syneresis, a concentration of 1.7mg/ml abolishing the effect and higher concentrations reversing it. The possible physiological implications of these results are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAY T. D. Connective tissue permeability and the mode of action of hyaluronidase. Nature. 1950 Nov 4;166(4227):785–786. doi: 10.1038/166785b0. [DOI] [PubMed] [Google Scholar]
- DAY T. D. The permeability of interstitial connective tissue and the nature of the interfibrillary substance. J Physiol. 1952 May;117(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- FESSLER J. H. Water and mucopolysaccharide as structural components of connective tissue. Nature. 1957 Feb 23;179(4556):426–427. doi: 10.1038/179426b0. [DOI] [PubMed] [Google Scholar]
- Krogh A. The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. J Physiol. 1919 May 20;52(6):391–408. doi: 10.1113/jphysiol.1919.sp001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGMUIR I. S., ROUGHTON F. J. W. The diffusion coefficients of carbon monoxide and nitrogen in haemoglobin solutions. J Physiol. 1952 Oct;118(2):264–275. doi: 10.1113/jphysiol.1952.sp004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGSTON A. G., SHERMAN T. F. Effects of hyaluronic acid upon diffusion of solutes and flow of solvent. J Physiol. 1961 Apr;156:67–74. doi: 10.1113/jphysiol.1961.sp006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGSTON A. G., STANIER J. E. Further observations on the preparation and composition of the hyaluronic acid complex of ox synovial fluid. Biochem J. 1952 Sep;52(1):149–156. doi: 10.1042/bj0520149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston A. G., Stanier J. E. On the state of hyaluronic acid in synovial fluid. Biochem J. 1950 Mar;46(3):364–376. doi: 10.1042/bj0460364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAULSON S., SYLVEN B., HIRSCH C., SNELLMAN O. Biophysical and physiological investigations on cartilage and other mesenchymal tissues. III. The diffusion rate of various substances in normal bovine Nucleus Pulposus. Biochim Biophys Acta. 1951 Jul;7(2):207–213. doi: 10.1016/0006-3002(51)90019-4. [DOI] [PubMed] [Google Scholar]
- Preston B. N., Davies M., Ogston A. G. The composition and physicochemical properties of hyaluronic acids prepared from ox synovial fluid and from a case of mesothelioma. Biochem J. 1965 Aug;96(2):449–474. doi: 10.1042/bj0960449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienits K. G. The acid mucopolysaccharides of the sexual skin of apes and monkeys. Biochem J. 1960 Jan;74(1):27–38. doi: 10.1042/bj0740027. [DOI] [PMC free article] [PubMed] [Google Scholar]


