Abstract
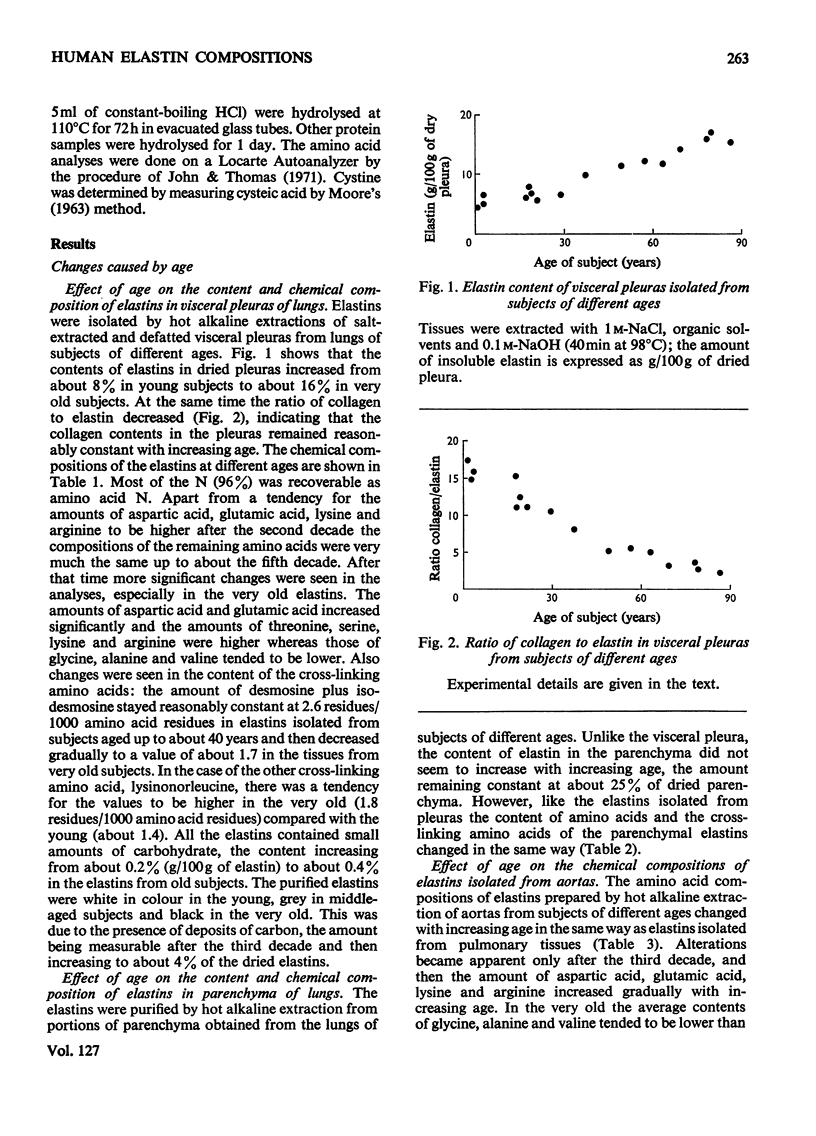
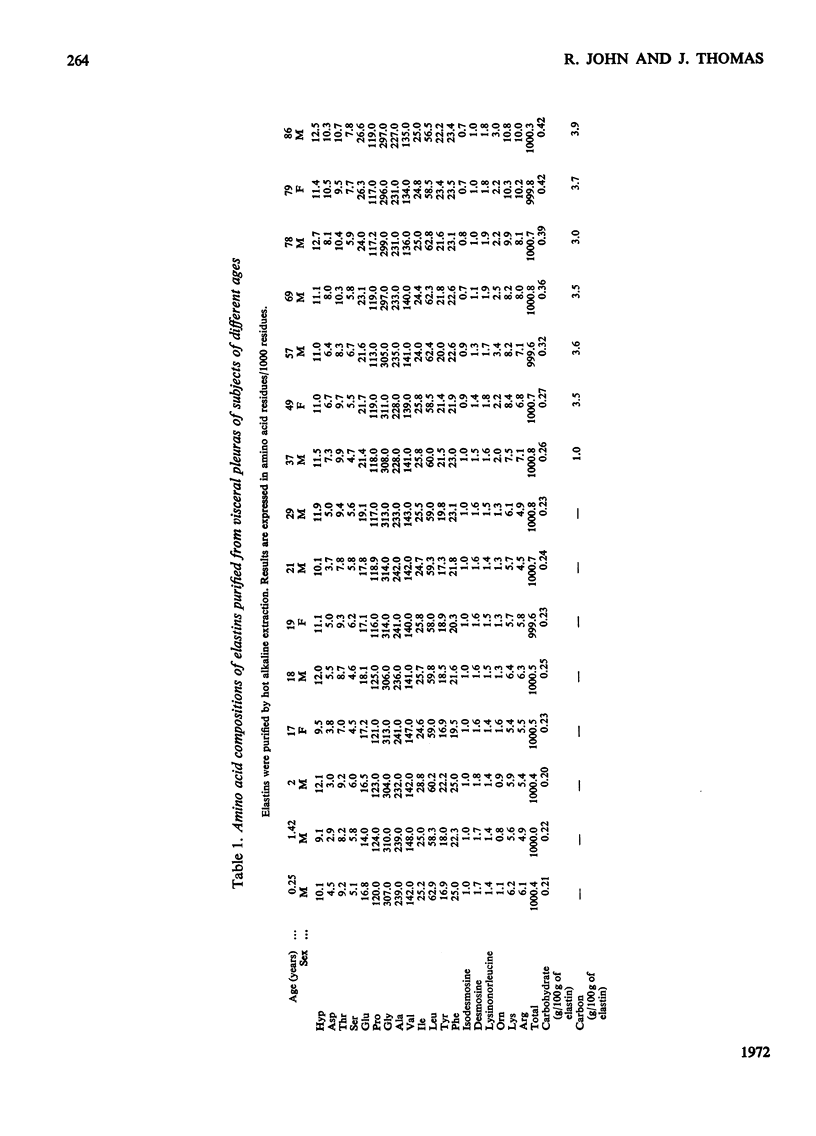
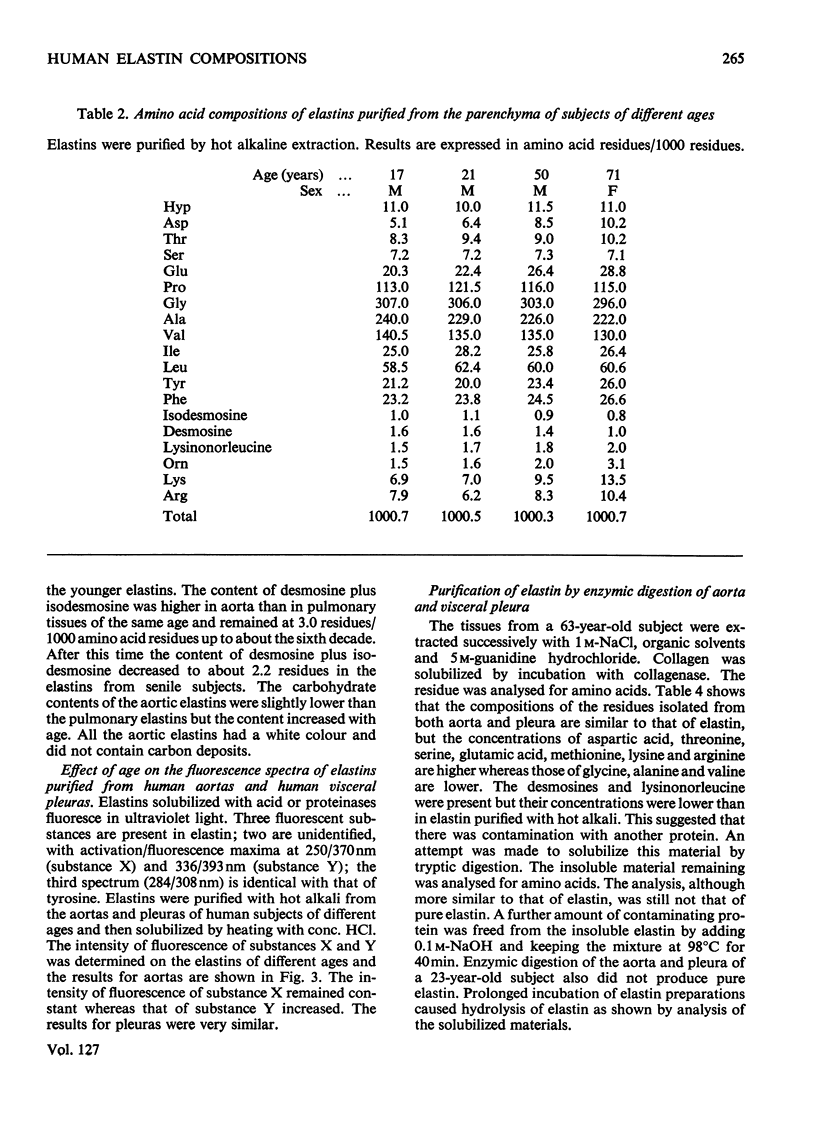
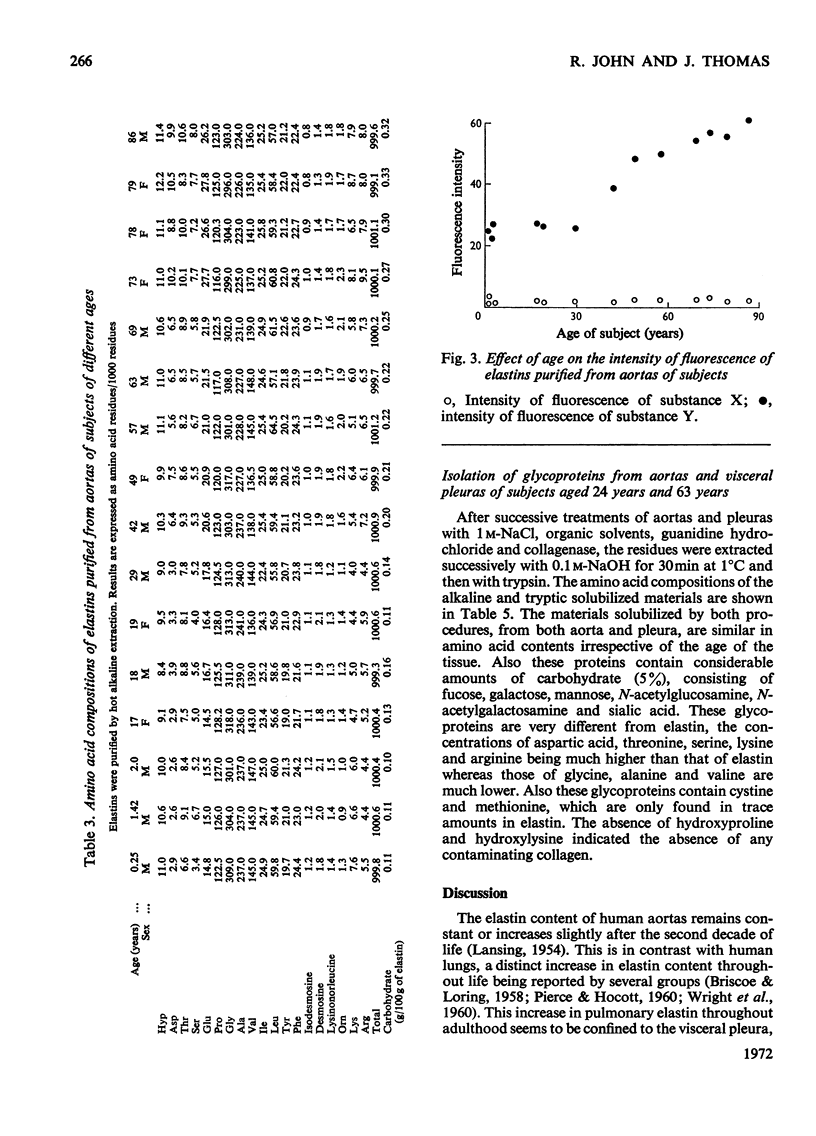
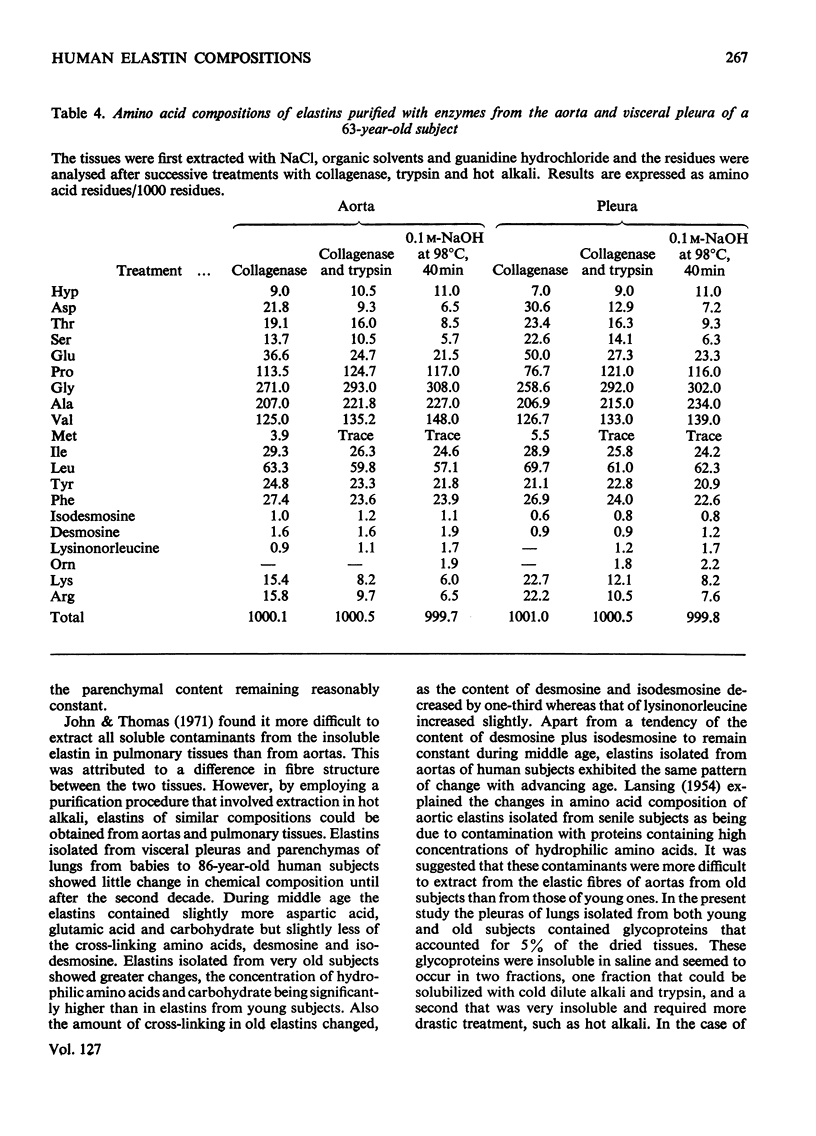
1. Elastins were isolated from the visceral pleuras and parenchymas of lungs of humans of different ages. 2. The elastin content of pleuras increased whereas that of parenchymas remained constant with increasing age. 3. The amino acid compositions and carbohydrate contents of elastins isolated from both pulmonary tissues changed in the same way with increasing age of the subjects. These changes were similar to those observed in elastins isolated from the aorta. 4. Similar glycoproteins were isolated from pleuras and aortas, and were more difficult to extract from the elastins of older subjects. Contamination with these glycoproteins was responsible for the changes in composition of elastin, as the age of the tissue from which it was extracted increased. 5. The amount of the cross-linking amino acids desmosine and isodesmosine was lower in elastins isolated from both aorta and pulmonary tissues of senile subjects than those from younger subjects.
Full text
PDF

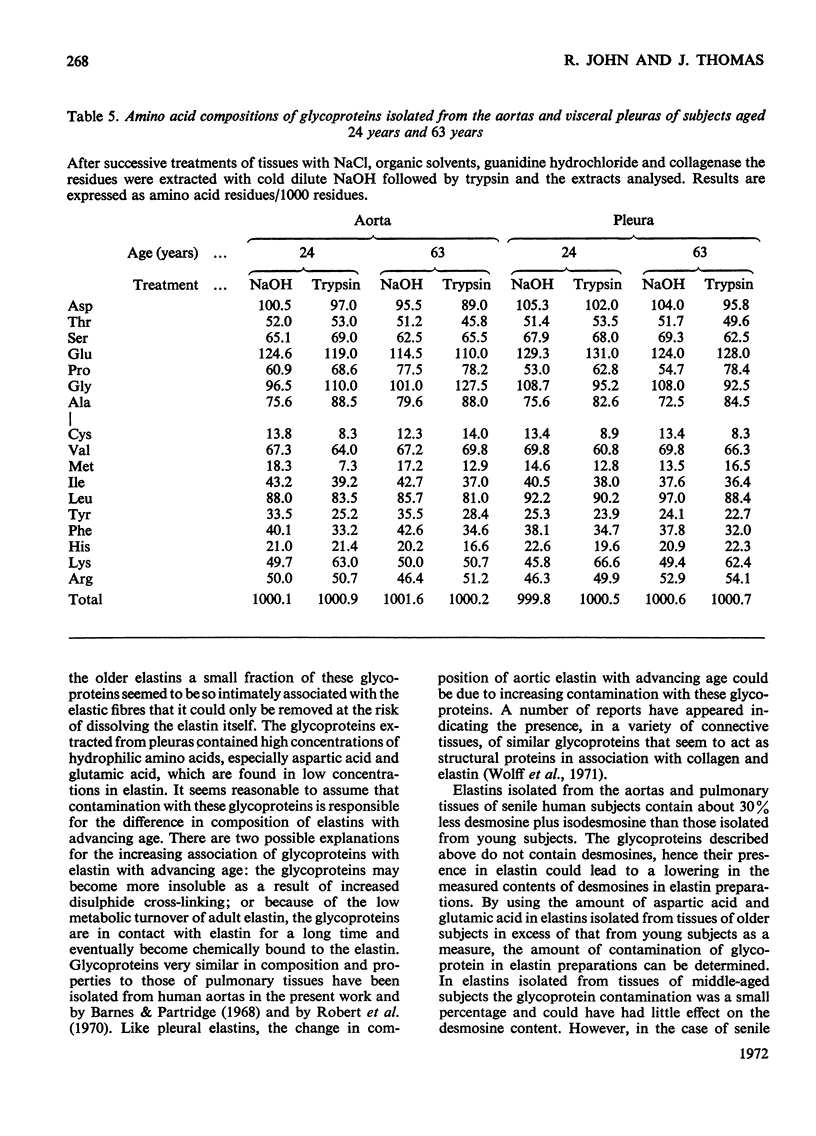

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRISCOE A. M., LORING W. E. Elastin content of the human lung. Proc Soc Exp Biol Med. 1958 Oct;99(1):162–164. doi: 10.3181/00379727-99-24280. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Partridge S. M. The isolation and characterization of a glycoprotein from human thoracic aorta. Biochem J. 1968 Oct;109(5):883–896. doi: 10.1042/bj1090883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomfield J., Farrar J. F. The fluorescent properties of maturing arterial elastin. Cardiovasc Res. 1969 Apr;3(2):161–170. doi: 10.1093/cvr/3.2.161. [DOI] [PubMed] [Google Scholar]
- FITZPATRICK M., HOSPELHORN V. D. Studies on human pulmonary connective tissue. I. Amino acid composition of elastins isolated by alkaline digestion. J Lab Clin Med. 1962 Nov;60:799–810. [PubMed] [Google Scholar]
- Fitzpatrick M., Hospelhorn V. D. Studies of human pulmonary connective tissue. II. Amino acid composition of residues following collagenase digestion of lung connective tissues. Am Rev Respir Dis. 1965 Nov;92(5):792–800. doi: 10.1164/arrd.1965.92.5.792. [DOI] [PubMed] [Google Scholar]
- Franzblau C., Sinex F. M., Faris B., Lampidis R. Identification of a new crosslinking amino acid in elastin. Biochem Biophys Res Commun. 1965 Dec 21;21(6):575–581. doi: 10.1016/0006-291x(65)90524-3. [DOI] [PubMed] [Google Scholar]
- LABELLA F. S., LINDSAY W. G. The structure of human aortic elastin as influenced by age. J Gerontol. 1963 Apr;18:111–118. doi: 10.1093/geronj/18.2.111. [DOI] [PubMed] [Google Scholar]
- LANSING A. I., ROSENTHAL T. B., ALEX M., DEMPSEY E. W. The structure and chemical characterization of elastic fibers as revealed by elastase and by electron microscopy. Anat Rec. 1952 Dec;114(4):555–575. doi: 10.1002/ar.1091140404. [DOI] [PubMed] [Google Scholar]
- Lent R., Franzblau C. Studies on the reduction of bovine elastin: evidence for the presence of delta-6,7-dehydrolysinonorleucine. Biochem Biophys Res Commun. 1967 Jan 10;26(1):43–50. doi: 10.1016/0006-291x(67)90250-1. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Fullmer H. M. Elastin: diminished reactivity with aldehyde reagents in copper deficiency and lathyrism. J Exp Med. 1966 Jun 1;123(6):1097–1108. doi: 10.1084/jem.123.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE J. A., HOCOTT J. B. Studies on the collagen and elastin content of the human lung. J Clin Invest. 1960 Jan;39:8–14. doi: 10.1172/JCI104030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge S. M., Elsden D. F. The chemistry of connective tissues. 7. Dissociation of the chondroitin sulphate-protein complex of cartilage with alkali. Biochem J. 1961 Apr;79(1):26–32. doi: 10.1042/bj0790026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert L., Robert B., Robert A. M. Molecular biology of elastin as related to aging and atherosclerosis. Exp Gerontol. 1970 Dec;5(4):339–356. doi: 10.1016/0531-5565(70)90017-3. [DOI] [PubMed] [Google Scholar]
- Sandberg L. B., Weissman N., Smith D. W. The purification and partial characterization of a soluble elastin-like protein from copper-deficient porcine aorta. Biochemistry. 1969 Jul;8(7):2940–2945. doi: 10.1021/bi00835a037. [DOI] [PubMed] [Google Scholar]
- Stegemann H., Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967 Nov;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- THOMAS J., ELSDEN D. F., PARTRIDGE S. M. PARTIAL STRUCTURE OF TWO MAJOR DEGRADATION PRODUCTS FROM THE CROSS-LINKAGES IN ELASTIN. Nature. 1963 Nov 16;200:651–652. doi: 10.1038/200651a0. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Weissmann S. X-RAY DIFFRACTION STUDIES OF HUMAN AORTIC ELASTIN RESIDUES. J Clin Invest. 1960 Nov;39(11):1657–1666. doi: 10.1172/JCI104189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff I., Fuchswans W., Weiser M., Furthmayr H., Timpl R. Acidic structural proteins of connective tissue. Characterization of their heterogeneous nature. Eur J Biochem. 1971 Jun 11;20(3):426–431. doi: 10.1111/j.1432-1033.1971.tb01409.x. [DOI] [PubMed] [Google Scholar]
- YEMM E. W., WILLIS A. J. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954 Jul;57(3):508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]


