Abstract
Oral submucous fibrosis (OSF), characterized by excessive deposition of extracellular matrix (ECM) that causes oral mucosal tissue sclerosis, and even cancer transformation, is a chronic, progressive fibrosis disease. However, despite some advancements in recent years, no targeted antifibrotic strategies for OSF have been approved; likely because the complicated mechanisms that initiate and drive fibrosis remain to be determined. In this review, we briefly introduce the epidemiology and etiology of OSF. Then, we highlight how cell-intrinsic changes in significant structural cells can drive fibrotic response by regulating biological behaviors, secretion function, and activation of ECM-producing myofibroblasts. In addition, we also discuss the role of innate and adaptive immune cells and how they contribute to the pathogenesis of OSF. Finally, we summarize strategies to interrupt key mechanisms that cause OSF, including modulation of the ECM, inhibition of inflammation, improvement of vascular disturbance. This review will provide potential routes for developing novel anti-OSF therapeutics.
Subject terms: Oral diseases, Mechanisms of disease
Introduction
Oral submucous fibrosis (OSF) is a chronic, progressive disease of the oral mucosa that results in fibrosis and leads to limitation in mouth opening, burning sensation, xerostomia, dysphagia, and even facial deformity, which seriously influences patients’ quality of life. It is worth noting that OSF has a potential tendency for malignant transformation. The World Health Organization (WHO) has listed OSF as one of the oral potentially malignant disorders (OPMDs) and ~3%–10% of patients with OSF may eventually develop oral cancer.1,2 However, despite substantial progress in the understanding of the pathobiology of various organ fibrosis, the investigation of OSF was largely deficient due to the lack of attention to this disease and the limitations in the field of research. What is consistent with other organ fibrosis diseases is that huge obstacles remain between the identification of ideal antifibrotic strategies and the implementation of this concept into clinical application.
Currently, the exploration of OSF has been carried out for half a century and has been mainly focused on etiology and pathogenesis3–21 (Fig. 1). Relative studies of patients with OSF along with experimental models of OSF in rodents have preliminarily demonstrated potential mechanisms leading to OSF. These include activation of fibroblast, damage to the epithelial barrier, injury of endothelial cell, dysregulation of immune responses, microvascular lesions, and the roles of oxidative stress, senescence, and autophagy. However, the underlying molecular mechanisms remain controversial. In general, strategies for individual and precise therapy of OSF are the ultimate goals to be explored. This review summarizes mechanisms and antifibrotic strategies outlined in the existing studies. The improvement in comprehending possible mechanisms including the role of oral microbiota, immune regulation, and the promising innovations will be discussed to introduce novel perspectives for the therapy of OSF.
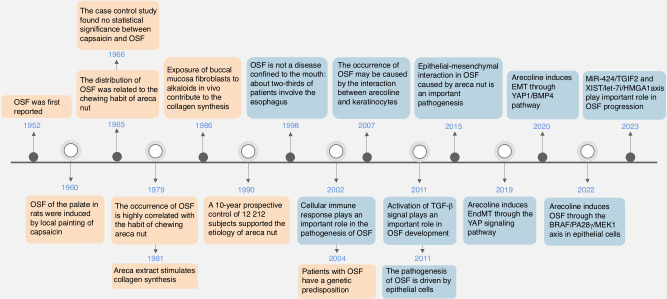
Fig. 1.
Timeline for key events in studies of OSF research. Orange boxes summarize etiological studies of OSF; Blue boxes show relevant studies of OSF pathogenesis
Epidemiology and Etiology
According to epidemiological statistics, there are about 600 million people with the habit of chewing areca nut (AN) in the world, and ~5% of AN chewers are OSF patients.22,23 Numerous studies from different countries supported the correlation between AN chewing and OSF.24–26 It is reported that the prevalence rate of OSF in China is above 1.0%.27 The prevalence rate of other south-east Asia countries such as India and Vietnam is 0.04%–6.4% and 0.15%–14.6%, respectively.26,28 The prevalence across these countries is greatly attributed to the habit of chewing AN. Most strikingly, the rate of transformation into oral cancer in OSF patients is above 4%.29,30 A prospective study over 30 years in mainland of China showed that 32 of 567 (5.6%) OSF patients with habits of chewing AN transformed into oral cancer.31 In Mumbai and Lucknow, above 2.0% and 9.8% of patients with oral squamous cell carcinoma (OSCC), respectively, endured malignant transformation from OSF.32 Another follow-up study of 17 years revealed that 5 of 66 (7.6%) Indian OSF patients had malignant transformation into OSCC.33 These studies support the hypothesis that OSF has malignant transformation potential and suggest that OSF is one of the most important public health problems. However, the current viewpoints of etiology in OSF are multifactorial. The chemical and physical irritation, genetic factors, as well as metabolic disturbance, are acknowledged factors in the onset and development of OSF.
The chemical and physical irritation
The chemical and physical irritation caused by AN is often considered the most significant risk factors in OSF onset. Specifically, the International Agency for Research on Cancer (IARC) has assembled evidence presenting that chemical and physical irritation by chewing AN is the major cause of OSF in their monograph.34 Many studies suggested that the composition of AN can lead to persistent chemical trauma in the oral mucosa. Until now, the identified chemical composition of AN is comprised of alkaloids (such as arecoline, arecaidine, guvacine, and guvacoline), flavonoids, tannins, along with carbohydrates, proteins, fats, crude fiber, and other elements.35 In particular, the arecoline component is confirmed to be the most potent agent which impacts the occurrence of OSF by inducing abnormal deposition of collagen.36
Chronic mechanical irritation (CMI) is important in the onset and development oral mucosal lesions. Some pathological changes such as atrophy, ulceration, keratosis, hyperplasia, and even fibrosis will appear in the oral mucosa under direct contact with a ‘mechanical agent’ (e.g., teeth or denture) and CMI. As a result, OSF is also usually considered to be a result of mechanical trauma of the oral mucosa by the coarse fibers of AN. Pentenero et al. hold the idea that mechanical trauma is a common phenomenon which is caused by AN chewing, and it may initiate negative functions in DNA reparation and cell apoptosis.37 However, the mechanical irritation is a local promotive factor rather than a main direct factor. It has been reported that mechanical trauma by AN coarse fiber can aggravate OSF. Yang et al. found that rubbing arecoline in a particular area significantly promoted the expression of collagen III and TGF-β1 in SD rat model, compared with rubbing alone.38
Genetic susceptibility
Previous studies also provided evidence of genetic susceptibility to OSF from the perspective of gene polymorphisms and cytogenetic damage. These genetic factors contribute to pathogenesis of OSF, especially the changes in gene polymorphisms, (e.g., The increased gene frequency of human leukocyte antigen (HLA), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), glutathione s-transferase (GST), cytochrome P450 (CYP) and matrix metalloproteinase (MMPs)) are main factors in genetic susceptibility to OSF.
A study reported that the frequency of HLA-B76 and the haplotype frequencies of HLA-B48/Cw7, -B51/Cw7, and -B62/Cw7 were significantly higher in OSF patients than in healthy controls. It also suggested that some specific HLA haplotypes may play a more important role in OSF susceptibility than individual HLA phenotypes.39 However, there was a contrary view from South Africa that OSF had no HLA-associated susceptibility.40 Furthermore, other studies have concluded that gene polymorphisms of MHC class I chain-associated gene A (MICA) and the CTLA-4 might confer an increased risk of OSF.41–43 Another comparative study of 90 patients with OSF and 130 healthy controls also reported the GST variants—GSTM1 and GSTT1, increase the risk of OSF progression in the North Indian population.44 Similarly, the expression of MMP-3,45 CYP3A,46 CYP1A1 and CYP2E147 were also shown to have a significant role associated with OSF susceptibility.
In addition, some evidence showed that OSF patients have cytogenetic damage, manifested by elevated indicators such as sister chromatid exchange rates,48,49 micronucleus cell numbers50,51 and degree of DNA damage,52 which can be attributed to the genotoxic effects of AN chewing. These findings suggest that OSF has a genetic background and susceptibility, and that susceptible individuals may have unstable chromosomes with reduced repair capacity and are more susceptible to fibrosis when stimulated by external factors.
Metabolic disturbance
Many studies have found a correlation between metabolic disturbance and susceptibility to OSF by comparing the differences in indicators of trace elements, vitamins, lipids, and proteins between normal subjects and OSF patients.
Many studies have detected trace element levels in OSF and have reported their association with OSF. A meta-analysis containing 34 reports showed that the levels of saliva, serum, or plasma copper were significantly elevated in OSF patients, whereas zinc and iron were significantly decreased.53 Of note, lysyl oxidase (LOX) is a copper-dependent monoamine oxidase that works in collagen cross-linking and deposition. Its activity was found to be upregulated in fibroblasts of OSF tissue.54 Based on this finding, one view suggested that excessive copper intake caused by AN chewing led to the upregulation of LOX activity and the occurrence of fibrosis.55 Furthermore, OSF patients had a significantly decreased lipid profile. One study showed that plasma total cholesterol (TC), high-density lipoprotein (HDL), and Apo-A1 levels were decreased,56 while another study showed that serum TC, HDL, and low-density lipoprotein (LDL) were decreased. Perhaps the lipid profile can be used as an indicator for detecting initial changes in cells of OSF.57 Most importantly, these factors are not isolated, but are interrelated and interact with each other.
Pathogenesis of OSF
OSF is considered to be an excessive repair process that occurs after persisting chronic injury. In this regard, almost all the cell types in the oral mucosa, including epithelial cells, fibroblasts, endothelial cells, as well as the infiltrated immune cells, are involved and participate in some way in the pathogenesis of OSF, which illustrates the immense complexity of this process. In this context, understanding the cellular and molecular mechanism of OSF is paramount and essential, including fibroblast activation, epithelial damage, submucosal vasculature abnormality, immune abnormality, as well as other mechanisms such as oxidative stress, autophagy, and senescence (Fig. 2). All of the above provide a novel insight into pathogenesis of the OSF process.
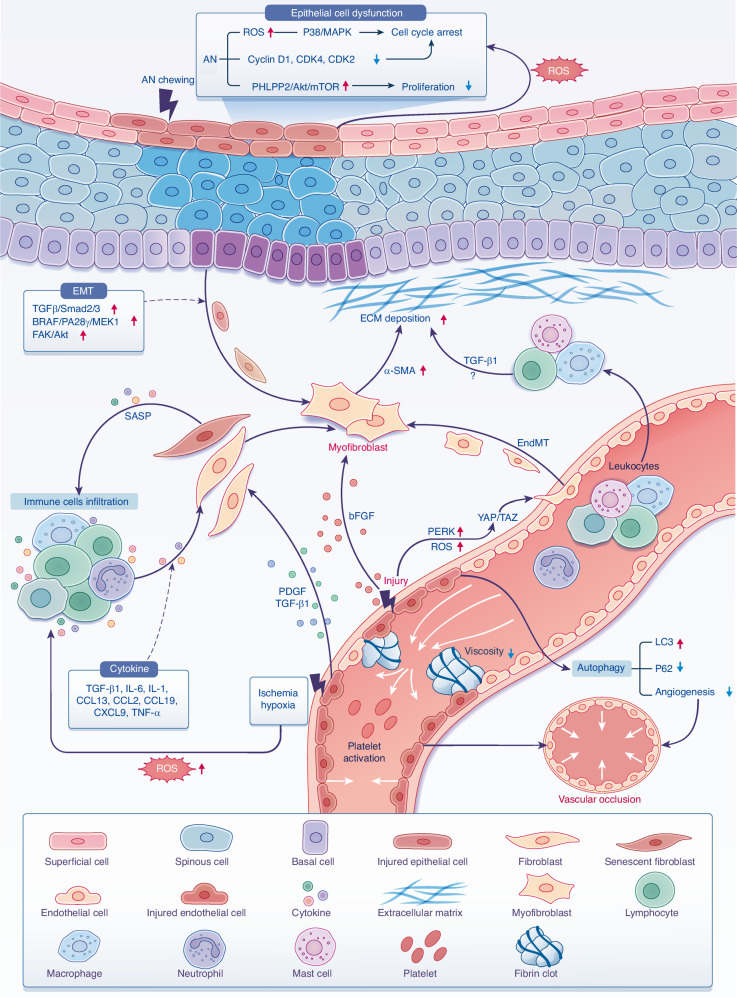
Fig. 2.
The pathogenesis of OSF. The damage of oral epithelial cells through the production of ROS or through the dysfunction of epithelial cells, such as arrested cell cycle and proliferation inhibition work to promote fibrosis. In addition, the injured epithelial cells also promote EMT process by activating EMT-related signaling pathways. The vascular endothelial injury caused by arecoline also leads to the production of ROS, thus promoting EndMT by activating YAP/TAZ and can eventually leads to fibrosis. Infiltrated immune cells secrete cytokines induced by injury, local ischemia, hypoxia, and SASPs, which were derived from fibroblasts in senescence conditions, thus promoting fibrosis. Leukocytes from circulation may be recruited by chemokines to inflamed areas to secrete profibrotic cytokines. Furthermore, secreted mediators such as TGF-β1, PDGF, bFGF cause fibroblasts activation, which produces excessive ECM. Injured endothelial cells also induce autophagy, fibrinolysis system disorder, and abnormal coagulation function, which leads to microvascular occlusion and further accelerates the process of fibrosis
Fibroblast activation
During the development of OSF, persistent and chronic tissue injury inevitably leads to the massive activation of fibroblast, which is arguably an important event in oral mucosa fibrogenesis. These activated fibroblasts are commonly regarded as the principal matrix-producing cells which have skeleton protein phenotype (such as α-SMA) and generate a large amount of mesenchymal matrix components, including fibronectin-1 (FN-1) and collagen I, also known as myofibroblast (MFB).58 MFBs were observed in oral mucosa with OSF patients, and the number of MFBs is significantly correlated with the severity of OSF.59 Further study indicated that buccal mucosa fibroblasts obtained from OSF patients show a significant upexpression of α-SMA, collagen I, FN-1, and collagen gel contraction compared to fibroblasts from healthy subjects.60 Notably, TGF-β is generally regarded as one of the most potent activators known to induce fibroblast activation.61 Canonical and non-canonical signaling pathways involving TGF-β often contribute to fibrosis pathogenesis. For example, the activation of TGF-β induces phosphorylation of Smad2 to promote fibroblast differentiation-specific gene expression in vitro.62 In addition to Smad signaling, TGF-β also activates fibroblasts by induction of non-canonical signaling such as JNK, and p38 MAPK pathways.63,64
Moreover, AN extract is an important pathogenic factor in OSF, and it exerts a crucial role in determining fibroblast activation. In vitro studies confirmed that AN extract markedly induces the high expression level of vimentin of fibroblasts, corresponding to the high expression of vimentin in the fibroblasts from OSF patients.65 Certainly, vimentin is a significant marker for fibroblast activation, as it is expressed de novo specifically in activated, but not quiescent, fibroblasts of adult kidneys.66 Cultured fibroblasts respond to AN extract or arecoline in vitro, and experience differentiation into cells that exhibit features of MFBs including α-SMA expression and collagen gel contraction, and other fibrosis marker expression, such as CTGF.67–70 In addition, buccal mucosa biopsies of subjects with OSF showed increase of SSEA-4 and ZEB-1, as well as non-coding RNAs (such as H19, linc000312 and HOTTIP). These genes were upregulated in fibroblasts treated with AN extract, and promoted the transformation of fibroblasts into MFBs.60,67,71–73 Further research found that AN extract induces the transformation of fibroblasts into MFBs through multiple molecular mechanisms, including activation of PLC/IP3/Ca2+ and Rho kinase regulating cell contraction, as well as direct phosphorylation by NF-κB, JNK, P38 MAPK mediating CTGF production.68,69 Of note, another mechanism that AN may indirectly influence is fibroblastic collagen metabolism by keratinocytes. For example, keratinocytes collected from healthy oral mucosa treated by AN extract induce TIMP-1 released by fibroblasts.74 ECM-related proteins released by fibroblasts have been described in other literature.65,75–77 In addition, AN also directly inhibits fibroblastic collagen degradation. Studies reported that AN caused a dose-dependent inhibition of collagen phagocytosis by fibroblast in vitro. Furthermore, a deficiency in collagen phagocytosis was noted in OSF fibroblasts relative to fibroblasts cultured from healthy buccal mucosa.78 However, more studies will need to be conducted to characterize their contribution to OSF.
Epithelial damage
Epithelial cells derived from oral mucosa, which include basal cells, spinous cells, and superficial cells, are essential cells in maintaining the integrity of the epithelial wall and tissue homeostasis in oral submucosa.79 During the lesion of OSF development, the stimulation of chewing AN will result in the damage of epithelial cells, thus destroying epithelial structure, and promoting dysfunctional repair. A series of studies showed that AN extract has significantly toxic effect to the proliferation of keratinocytes and induces cell apoptosis in a concentration-dependent manner.80,81 In addition, the treatment of AN extract also caused the cell cycle of injured epithelial cells to be arrested at the G1/G0 phase and suppressed epithelial cell viability by activation of important signaling pathways.82,83 For example, the arecoline suppressed epithelial cell viability and proliferation by activating TGF-β/Smad or PHLPP2/Akt/mTOR signaling pathway to promote OSF development.84,85
Furthermore, AN can damage oral epithelial cells leading to epithelial-mesenchymal transition (EMT), which is considered to be an important process in the development of OSF. The EMT process is characterized by substantial change of normal epithelial structure, that epithelial cells lose contact and adhesion markers (such as E-cadherin), and obtain mesenchymal markers (such as α-SMA, vimentin, and N-cadherin) through drastic changes in the shape of cytoskeleton.58 These cell-intrinsic changes in epithelial cells have also been shown to promote the occurrence of OSF by maintaining the activation of some key profibrotic pathways.86,87 For instance, Xie et al. found that arecoline, as the pathogenic component of OSF, can upregulate the levels of PA28γ, BRAF, and phosphorylated MEK1 protein, which interact with each other to form a protein complex, thus activating MEK1/ERK signaling pathway and promoting EMT in oral epithelial cells.19 Other studies also revealed that over-expression of DEC1 in the oral epithelium of OSF mice, along with synchronized activation of FAK/Akt signaling pathways, which caused EMT in epithelial cells, and promoted fibrosis development.88
In addition, damaged epithelial cells secrete high levels of cytokines, such as TGF-β, which stimulates the proliferation of fibroblasts and increases the deposition of ECM, driving the development of OSF.16 The expression levels of TGF-β are elevated in the oral epithelium, up to the stratum spinosum from OSF patient tissue.89 Concurrently, the TGF-β signaling pathway is the main cascade reaction of fibroblast differentiation. A recent study demonstrated that arecoline enhanced the secretion of TGF-β1 by epithelial cells, which promoted the expression of THBS1 in fibroblasts, thereby contributing to OSF development.90 Notably, Pant et al. reported that the OSF invoking involvement of JNK/ATF2/Jun axis in TGF-β/Smad pathway activation by AN extract treatment leads to a stained TGF-β autocrine loop in epithelial cells which promotes OSF.63
Hence, epithelial damage has an important role through EMT or cooperation of TGF-β related signaling pathways in the initiation and development of the OSF. However, the mechanisms associated with EMT into tissue fibrosis have not been fully identified. Exactly what triggers the formation of the EMT after epithelial injury is an unresolved question. How the EMT cells are initially migrated to mesenchymal area is unknown. Future exploration of the pro-fibrotic role of epithelium should provide further insight into the pathogenesis of OSF.
Submucosal vasculature abnormality
Functional vasculature is significant in the process of tissue repair and fibrosis, and it is essential for maintaining the stability of oral mucosa by transporting oxygen and other nutrients. Previously, observations of OSF reported abnormal vascular changes, including incomplete vascular basement membrane, low microvascular density, and diameter around areas of fibrosis.91 More detailed study of 40 cases of OSF patients later clarified that with the development of OSF, the number of blood vessels gradually narrows and decreases, even completely occluded in the advanced stage, resulting atrophic changes and subsequent fibrosis.92,93 In addition, AN extract has a cytotoxic effect on endothelial cells, and a study has reported that arecoline markedly inhibits the proliferation of vascular endothelial cells, promotes apoptosis, and induces the arrest of cell cycle, thus causing vascular damage and dysfunction and leading to fibrosis.94 However, it is not clear how these vascular abnormalities cause OSF. Several mechanisms have been proposed for abnormal vascular patterns in OSF.
First, the injured endothelial cells are confirmed to promote inflammation and indirectly activate MFB and ECM deposition. Li et al. found high expression of CXCL9 in the endothelial cells and immune cells by observing 56 OSF patients with AN chewing habit.95 It suggests that AN extract changes the adhesive profile of endothelial cells by increasing the expression of the surface proteins that promote the attraction of circulating leukocytes to it, thus, facilitating the initiation or maintenance of vascular inflammation. Other studies revealed that exposing human umbilical vein endothelial cells (HUVECs) to arecoline increases the expression of bFGF and ET-1 while down-regulating interleukin (IL)-1, IL-6, G-CSF, and GM-CSF, and these abnormally secreted cytokines by endothelial cells further regulate the proliferation of fibroblasts and promote the occurrence and development of OSF.96
Secondly, coagulation and fibrinolysis systems also could be modulated by injured endothelial cells, thus promoting the development of OSF. A previous study by Phatak reported that the blood of patients with OSF was hypercoagulable and accompanied by fibrinolysis disorder, which suggested that OSF is a chronic disseminated intravascular coagulation syndrome (DIC) with local coagulopathy.97 Fang et al. reported that there was an extensive microvascular injury in OSF, accompanied by vasodilation and increased permeability.98 The plasma leakage and fibrin deposition caused by these pathological changes further activated the coagulation mechanism, which led to the decrease of blood flow and fibrosis in oral mucosa tissue. Moreover, arecoline can effectively stimulate platelet aggregation and thromboxane production, causing the activation of local blood coagulation mechanism. Another study also demonstrated the fibrosis level was regulated by high expression of PAI-1, a plasminogen inhibitor, which will lead to the decline of plasminogen activity, as well as the degradation of ECM.99
Thirdly, injured endothelial cells may transdifferentiate towards MFBs, which contributes to OSF. A typical example is that AN induces endothelial cell to undergo endothelial‐to‐mesenchymal transition (EndMT) in vitro. In this study. the activation of PERK pathway causes the expression of transcription factors such as Yes-associated protein (YAP), which subsequently initiates EndMT and promotes OSF development.17 Overexpression of nuclear receptor coactivator 7 (NCOA7) reduced AN-induced EndMT. Furthermore, the specific YAP inhibitor, Verteporfin, has also been found to reduce collagen accumulation through inhibition of EndMT, thereby alleviating OSF in mice.100 Comprehensive studies are required and understanding of EndMT processes is pivotal for OSF treatment.
Immune abnormality
Immune abnormalities, including dysregulated immune cells and overproduction of pro-inflammatory or pro-fibrotic molecules, are one of the main features of OSF pathogenesis, which is involved in OSF development.101 Several studies have reported that monocytes/macrophages, mast cells (MCs), and T cells expand in OSF, with increased expression of cytokines, including CCL19, IL-1α, IL-1β, IL-6, TNF-α, and TGF-β1 in tissue and peripheral blood mononuclear cells (PBMCs) of patients with OSF. Both the innate and adaptive immune responses have critical roles in the fibrotic process and promote disease development in patients with OSF.
In the innate immune response, macrophages are commonly regarded as the key immune regulator, which participates in various organ fibrosis. Macrophages have a high plasticity, which results in a broad spectrum of macrophage phenotypes. The monocytes that later transform into inflammatory macrophages seem to be the main immune cells in fibrosis formation.102 Numerous studies have shown that targeted deletion of monocyte-derived macrophages (CCR2+ macrophage) is effective in alleviating fibrosis of lung, liver, and kidney.103–105 Notably, a previous immunohistochemical study showed that the infiltration of CD68+ macrophages in OSF tissue increased, and the level of infiltration was positively correlated with the aggravation of pathological stage.106 Interestingly, the onset of OSF accompanied by the upregulation of CCR2 and CCL2; this phenomenon seems to support the consistent profibrotic role of monocytes/CCR2+ macrophage in OSF as previously reported in other organ fibroses.107–109 However, the specific phenotypes of macrophages in OSF are unproven and need comprehensive investigation. Apart from macrophages, MCs and natural killer (NK) cells are also emerging as innate immune effectors with central roles in the pathogenesis of OSF. Not only are MCs producing a series of profibrotic cytokines such as TGF-β, FGF, and PDGF, but they also are considered to be closely related to the formation of microvasculature in OSF by releasing tryptase and chymotrypsin.110,111 In addition, the activity of NK cells in OSF patients is significantly lower than that in healthy people.112
In the adaptive immune response, CD4+ T cells are also important effector cells in OSF pathogenesis.11 Myriad CD4+ T cell subsets are implicated in OSF development. In a study of 72 patients with OSF and 30 healthy individuals, the researchers identified OSF development associated with enrichment of Th17 cell and IL-17 infiltration occurred in the tissues and PBMCs of patients with OSF.113 Compared with healthy individuals, patients with OSF have an increased Th17 cells to regulatory T cells (Tregs) ratio in PBMCs, the former of which is the main producer of IL-17A in fibrotic diseases such as OSF.113,114 Other potential profibrotic mechanisms includes the activation of mTOR and MEK induction by amphiregulin derived from Th17 cells, thereby promoting intestinal fibrosis.115 The PD-1+ Th17 cells contribute to the production of collagen I in lung fibrosis by regulating transcription factor STAT3.116 Furthermore, some recent data also indicated that aberrantly activated T cells instigate the transition of fibroblasts into MFBs, which are involved in OSF development. Using scRNA-seq from OSF patients, researchers have revealed that epithelia-mediated T cell activation via CD74/CXCR4 can substantially contribute to the development of OSF.117
Other immune cells, such as neutrophils, are one of the main features of early OSF.118 However, whether these cells participate in the OSF development remains unclear. Further mechanistic insights and understanding of these immune cells in OSF might facilitate the finding of novel therapeutic targets for patients with OSF.
Others
Oxidative stress
Currently, the detection of oxidative stress and reduced antioxidant defenses has been found in both in the AN chewer and OSF patients. In particular, the activation of reactive oxygen species (ROS) signaling is a primary indicator in the oxidative stress reaction, and the excessive accumulation of ROS will contribute to the initial development of OSF.119 Evidence has shown that AN extract can activate p38 MAPK, ERK, and NF-κB signaling to enhance the production of ROS.82,120 AN extract can also, through NCOA7(which is an anti-oxidation protective factor) and silencing the expression of miR-155, inhibit ROS levels from fibroblasts of OSF patients.100,121 In addition, ROS bind directly to DNA, lipids, and proteins, and leads to DNA base oxidation, generation of lipid peroxidation, and protein carbonylation, respectively, therefore causing oxidative damage of cells. Serum derived from OSF patients showed increased level of 8-OHdG,122 malondialdehyde (MDA),119,123–125 4-hydroxynonenal (4-HNE)126 and protein carbonyl.122 For example, a study including 65 OSF samples showed that the severity of disease was positively correlated with the level of MDA in serum and saliva.124,125 Meanwhile, increased ROS related oxidative damage and mitochondrial disruption such as 8-oxoG, 4-HNE, and NOX4 were also verified in OSF tissues.120,126,127 Notably, numerous studies in vitro have provided convincing evidence that AN can give rise to oxidative stress, which is mediated through the generation of ROS.68,100 First of all, previous studies have confirmed that AN extract can induce oral fibroblasts to secrete a variety of cytokines (such as GRO-α, IL-6 and IL-8) through ROS activation, thus promoting damage of normal epithelium and giving rise to the development of OSF.120 Secondly, AN-induced ROS generation activated various pathways leading to aberration of cell cycle and cell viability, EMT, and collagen deposition. AN upregulated the expression of the stress-responsive genes, such as heme oxygenase-1 and ferritin light chain, and induced cell cycle arrest and apoptosis.82,128,129 Of note, other evidence also showed that arecoline activates YAP via ROS-induced endoplasmic reticulum stress generation to mediate EndMT, leading to OSF.17,100 In addition to excessive ROS generation, reduced antioxidant defense mechanisms contribute to the fibrosis process. Published evidence clearly indicates that the levels of β-carotene and Vitamin E decreased in the plasma derived from OSF patients, and they are known to act in antioxidant roles by quenching lipid peroxidation.123 Meanwhile, the reduction of activity and content of antioxidant enzyme, including glutathione (GSH) and superoxide dismutase (SOD) have been also demonstrated in OSF patients.125,130 Notably, in vitro studies further indicated that AN extract significantly inhibited GST activity or promoted GSH depletion in human buccal mucosal fibroblasts.131 Collectively, oxidative stress, especially the ROS activation in pathogenesis of OSF, is a direction which should be of deep concern and comprehensive exploration.
Cellular senescence
Senescent fibroblasts were observed in early OSF, and accumulated with the progress of the disease. Reports in tissues and cultured fibroblasts of OSF have documented accumulation of p16INK4A, the formation of senescence associated heterochromatic foci (SAHF) and high levels of SA-βGal activity in association with OSF.132 It is reported that unrepaired DNA double-strand breaks is a major mechanism of senescence. Cultured fibroblasts from OSF tissues showed an increased DNA damaged foci doubly labeled for 53BP1 and γH2A.X accompanied within OSF development.132 In particular, the senescence induced by DNA damage was mainly caused by ROS and independent of the shorter telomeres.133 ROS-induced senescence has been extensively identified in multiple diseases. A senescent model induced by ionizing radiation in vitro indicated that the expression of p16INK4A and SA-βGal increased in senescent oral fibroblast.134 Likewise, the treatment of AN extract also caused cellular senescence phenotypes, as determined by an increased activity of SA-βGal and accumulation of p16INK4A,135 which suggested that the development of OSF caused by AN extract may mainly be owed to the role of cellular senescence. On the contrary, a study reported that the fibroblasts of non-diseased (ND) AN users showed no evidence of the increase of senescent cells compared with the healthy controls group.132 These data indicated that senescence is associated with intrinsic pathology context in OSF and the mechanism that ultimately leads to OSF development may depend on senescence-related secretory phenotypes (SASPs) derived from senescent cells. Current research revealed senescent oral epithelial cells are involved in OSF development by secreting TGF-β.136 Notably, senescent fibroblasts induced by ionizing radiation showed the secretion of TIMP-1 and TIMP-2, and as SASPs, both of them increased in the media of fibroblast cultures from OSF patients where senescent cells are more abundant.133 Additionally, most of the OSF biopsies contained significantly higher levels of mRNAs encoding the SASPs components IL-6, IL-8, GRO-α, and IL-1β, but these cytokines have not been verified in the senescent model.137 However, current studies have confirmed that SASPs-derived senescent cells are significant in the antifibrosis mechanism, which plays a role by increasing the degradation of collagen. Compared with TIMPs, senescent cells could ameliorate fibrosis by increasing the secretion of MMPs. Reducing the fibroblast population of senescent cells in OSF greatly decreased the levels of MMP-1 and MMP-2 in a conditioned medium indicating that there are both non-fibrotic senescent fibroblasts and collagen-deposited fibroblasts in the cell population.132
Overall, the fibrosis-promoting and fibrosis-suppressing functions of senescence seemed to be incompatible, but in fact most senescent cells secrete SASPs that impact fibrosis development, which is a vital mechanism in OSF progression and should be comprehensively explored in the future.
Autophagy
Autophagy is a cellular defense mechanism that can improve cell survival by removing useless intracellular organelles or proteins to counteract damage138 and is increasingly regarded as an important physiological process of tissue remodeling and fibrosis.139 The activation of autophagy promotes α-SMA expression and collagen production in oral mucosa, and it may determine MFBs differentiation.140 A previous study has demonstrated the overexpression of microtubule-associated protein 1 light chain 3 (LC3), a marker of autophagy, in OSF tissues. The study in vitro confirmed autophagy in cultured oral fibroblast can be activated by TGF-β, and the inhibition of autophagy significantly reduces the expression of critical fibrogenic gene, Col1A2, and protects against fibrosis induced by TGF-β.141 In addition, Zhu et al. revealed that autophagy-related proteins LC3 and P62 are also upregulated in OSF epithelium by immunohistochemistry. The study in vitro further found that the apoptosis of HOKs can be attributed to arecoline-induced autophagy, thereby promoting OSF development.142 Similarly, arecoline has been reported to induce autophagy in HUVECs, and activate autophagy while significantly inhibiting angiogenesis and cell viability of endothelial cells. It suggested that the autophagy in endothelial cells may promote OSF by regulating angiogenesis.143
Microbiota
A few studies hold the idea that microbial flora is also a key trigger that promotes the onset and malignant potential of OSF. According to the linear discriminant analysis, the abundance of Oscillospira and Megamonas was significantly increased in the OSF patient, while Fusobacterium and Rothia was decreased, indicating that it may pose a risk for OSF.144 The results of 16S rRNA gene sequencing showed the differences in the bacterial composition at different stages of the progression of AN-associated OSCC.144 More importantly, dysbiosis of oral microbiota is a significant risk factor for oral cancer. Porphyromonas gingivalis (P. gingivalis), Fusobacterium nucleatum (F. nucleatum), Treponema denticola (T. denticola), and Streptococcus anginosus (S. anginosus) have been recognized as being involved in oral carcinogenesis, and several clinical studies detected the relatively significant presence of these bacteria in OSCC sample compared with normal controls.145 Notably, previous studies also showed that AN chewing is one of the main reasons for the disturbance of host microbiota.146 As a result, it can be concluded that the oral microbiota plays an important role in the onset and transformation of OSF into OSCC, and it has broad prospects for further investigation.
The clinical evaluation of disease staging in OSF
For the evaluation of OSF progression, a variety of strategies have been proposed in the past few decades. Nevertheless, in clinical practice, finding out the most effective method for assessing oral mucosa impairment in patients remains a tremendous challenge. This is primarily because prognosis and effective therapy are dependent on the assessment of the severity and the extent of OSF patients. Historically, all these parameters were provided by oral mucosa biopsy. Oral mucosa biopsy is among the traditional, effective, and most accurate assessment methods for evaluating oral mucosa histology and the development of OSF. However, in recent years, with the emergence of high-throughput sequencing technology, some biomarkers from the body fluids of OSF patients have also been put forward with the potential for diagnostic value.
Functional evaluation for OSF development
The estimate of the degree of mouth opening is the most intuitive and convenient way to diagnose OSF in clinical practice. This is because the limited mouth opening is attributed to the submucosal collagen deposition and it is a typical characteristic of OSF.147 Of note, the latest guideline was proposed by the Chinese Stomatological Association (CSA) in 2022 and revised the diagnosis and clinical management of OSF. It divided the clinical manifestation of OSF into 4 stages. The characteristic of the first stage is the distance of mouth opening ≥30 mm with partial blanching of oral mucosa, but the texture and elasticity of mucosa have no significant difference. As the lesion develops, the distance of mouth opening is 20–30 mm in the second stage, accompanied by a blanching sheet or stripe in the mucosa. At this point, the texture becomes hard and the elasticity declines. In the third stage, the distance of mouth opening is 10–20 mm, with extensive blanching areas around oral mucosa as well as fibrous bands having been formed. The mucosa shows as a slab or leather change with poor elasticity. In the fourth stage, the distance of mouth opening ≤10 mm, and at this stage, malignant transformation is easily induced, especially into OSCC.
Oral mucosa biopsy for the evaluation of fibrosis development
In addition to functional evaluation, it is vitally significant to identify that an accurate diagnosis of OSF can only be made when the clinical evaluation and biopsy confer information. A biopsy is the gold standard of diagnosis that is widely used to evaluate and differentiate histological stages of OSF. The pathological change of OSF is mainly collagen deposition in connective tissue, which is characterized by the obvious increase in type I and III collagen. In particular, in histological staging, the number and distribution of fibroblasts, collagen fibers, inflammatory cells, and blood vessels are used to determine OSF grades.148 At the earliest stage, there is marked edema of the collagen network, vasodilation, and congestion accompanied by quantities of fibroblasts. The inflammatory cells are mainly composed of polymorphonuclear leukocytes with a small number of eosinophils and the epithelium is essentially normal in this period. In the early stage of OSF, the main characteristics are subepithelial hyalinization, collagen thickening, vasodilation, and congestion, accompanied by a moderate number of fibroblasts. The infiltration of chronic inflammatory cells is dominated by lymphocytes, eosinophils, and a few plasma cells. With the variety of degrees of keratinization, the epithelial nails become shorter. In the middle stage, the epithelium is hyalinized, with thickened collagen bundles and mostly constricted blood vessels. Mature fibroblasts with sparse cytoplasm and spindle-shaped nuclei are obvious. The epithelium is markedly atrophic with a complete loss of reticular nails. Muscle fibers and scattered dense collagen fibers are seen. In some areas, muscle fibers begin to degenerate and show irregular striations. In the advanced stage, extensive fibrosis makes mucosal vessels disappear. Fibroblasts are absent within the area of hyalinization, and occasional elongated cells are seen along the fiber bundles. Epithelial mesh plugs disappear completely, and a few patients show extensive myofiber degeneration.
Biomarker-based diagnosis of OSF
Although biopsy can effectively assist in clinical diagnosis and observe lesion development of OSF, however, due to the invasive and low acceptance of patients, it is often not an option. The identification of more accessible and less costly biomarkers, such as serum and saliva biomarkers, would increase their use in clinical practice. Various biological biomarkers of OSF have been discovered and characterized in the recent past. The change of trace elements is a typical example. The level of vitamin-c, iron, and zinc was significantly decreased in the serum of OSF patients, accompanied by the increase of copper, and it may indicate serum zinc, copper, and iron levels have potential diagnostic value in OSF.149–151 Besides, another study revealed that the serum copper and zinc levels and the Cu/Zn ratio of OSF patients can be regarded as potential markers in the evaluation of the susceptibility towards OSCC.152 Bale et al. reported that with the progress of OSF, serum MDA levels were increased while the level of SOD was reduced.125 Other potential serum or saliva biomarkers such as lactate dehydrogenase,153 and β-carotene,154 which may also play an important role in evaluating the development of OSF.
Non-invasive tools for the identification of OSF
Most strikingly, some novel adjunctive methods are also emerging for the evaluation of OSF. Advances in the understanding of the pattern of epithelial thickness and collagen distribution have enabled the development and validation of numerous computer-assisted techniques for the non-invasive detection of OSF grade. The assessment of collagen by Computer-Aided Diagnosis (CAD) is helpful in identifying OSF grade. For example, Paul et al. reported a new collagen fiber transmission electron microscope image, CAD technology based on Artificial Neural Network (ANN) to identify the progress of OSF.155 Additionally, autofluorescence spectroscopy has shown the potential to be a promising technique that can successfully differentiate oral malignancy and OSF.156–158 Wang et al. have indicated that the PLS-ANN classification algorithm can effectively differentiate OSF from oral malignant lesions based on autofluorescence spectroscopy at 330-nm excitation.159 Vedeswari et al. found that the fluorescence intensity will change due to the fact that there is a difference between the normal tissue and OSF mucosa in the fluorescence emission spectrum after therapy, and it is a useful strategy for the evaluation in the development of OSF.160 Kumar et al. further reported a non-invasive strategy by combining in fluorescence spectroscopy with principal component analysis (PCA) and partial least square discriminate analysis (PLS-DA) algorithm to evaluate the development of OSF.161 Furthermore, a study reported assessment of the epithelium thickness of OSF by a swept-source optical coherence tomography (OCT) technique.162 Rai et al. showed the possibility of FTIR spectroscopy coupled with chemometric techniques to screen OSF patients.163 Manjunath et al. reported a novel ultrasonographic technique, which has great superiority for identifying the area and depth of OSF by different submucosal echogenicity between the submucosa and muscle layer.164 In addition, micronucleus detection in exfoliated oral epithelial cells is also a non-invasive method used to assess the risk of malignant transformation.51,165 As a result, these adjunctive techniques act as a non-invasive strategy that has great potential and should be further investigated in the future.
Pharmacological treatment of OSF
Due to the complexity of pathogenesis, there is no effective modality that provides precise therapy for OSF. The main purpose of treatment is to alleviate the symptoms of patients to the maximum extent, prevent the further progress of the disease and improve the patient’s quality of life. Our present knowledge of the current treatment strategies for OSF are mainly based on etiology, pathogenesis, disease progression and symptoms of patients. The treatment strategies are divided into three categories: medical treatment, functional physical exercise, as well as elective surgery. In particular, medical treatment is the main therapy strategy due to the fact that functional physical exercise and elective surgery are usually suitable for patients with severe OSF. Different drugs that target the potential mechanisms to hinder the development of OSF is necessary. This section briefly recapitulates current drug strategies based on potential mechanisms of OSF, with a focus on possibility that may be considered for future clinical application (Fig. 3).
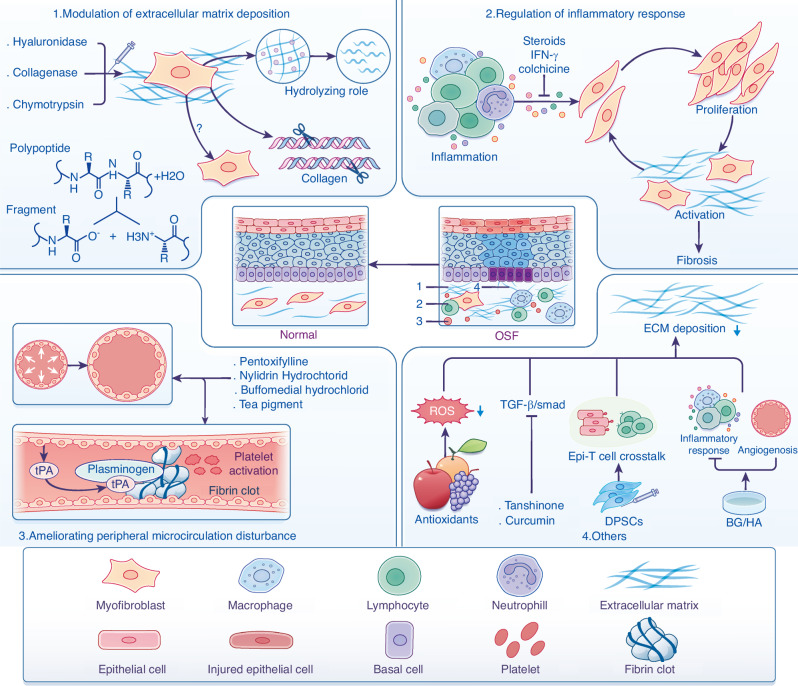
Fig. 3.
Pharmacological strategies may lead to OSF regression. (1) Modulation of extracellular matrix deposition that control OSF development is an antifibrotic approach: Local injection of hyaluronidase, collagenase, and chymotrypsin is used to reduce the levels of ECM deposition by the hydrolysis of hyaluronic acid, esters, and peptide bonds. (2) Regulation of inflammatory response. Inhibition of inflammation and prevention of activation of MFB by the injection of immunomodulators such as steroids, or anti-fibrotic cytokines. (3) Ameliorating peripheral microcirculation disturbance with established drugs to improve vascular occlusion and fibrinolysis system disorders will attenuate OSF progression, with multiple optimized strategies under development. (4) Other therapeutic strategies are under development to inhibit the collagen aggregation, including antioxidants and natural medicine such as tanshinone and curcumin that act on TGF-β/Smad signaling. BG/HA have been used to inhibit inflammatory responses and promote angiogenesis. DPSCs might have beneficial therapeutic effects via inhibiting crosstalk between epithelial cells and T cells, although further studies are needed
Modulation of extracellular matrix deposition
Excessive deposition of ECM in the lamina propria of oral mucosa is an important feature of OSF.166 According to previous studies, enzymes such as hyaluronidase, collagenase, and chymotrypsin were applied in the inhibition of ECM deposition and, for many years, it has been regarded as an effective strategy for the treatment of OSF. Hyaluronidase is a naturally occurring enzyme that can hydrolyze hyaluronic acid and reduce collagen formation in extracellular matrix, thus reducing the viscosity of intercellular substances, and hyaluronidase is widely reported to play an important therapeutic role in improve ECM deposition of OSF.167 Early evidence demonstrated that OSF patients who experienced local injection of hyaluronidase alone had rapid alleviation in burning sensation.168 In addition, collagenase is a type of proteolytic enzyme that was approved by Food and Drug Administration (FDA) and European Medicines Agency (EMA) in therapy of fibrotic diseases such as palmar fibromatosis and Peyronie’s disease.169,170 It has a similar effect as hyaluronidase in ameliorating symptoms (e.g., such as trismus, burning sensation) of OSF patients by efficiently cleaving collagen proteins into fragments. Study found that the development of OSF is accompanied by persistent collagen accumulation, and the lack of collagenase significantly contributed to lesion progression. Lin et al. reported that OSF patient, who were injected collagenase significantly improved mouth opening and eating function in a 2-year follow-up study. The study in vivo and histology of biopsy specimens further showed this effect is probably due to the dissolution of submucosal fibrous tissue which led to the increase of vascular circulation and epithelial regeneration.171 Of note, chymotrypsin is an endopeptidase that has also been reported in alleviating rational symptoms of OSF patients by hydrolyzing esters and peptide bonds.167 However, the effects of these enzymes seem to be transitory due to the labile nature, which will lose their activity completely in a short period of time. Although recently, some studies focus on drug delivery and aim at protecting the activity of enzyme and keeping a sustained controllable release of enzyme for a longer period.172 Some studies have also advocated that injection of hyaluronidase combined with corticoids, such as triamcinolone acetonide and dexamethasone, can improve the symptoms of OSF quickly as well as lengthen the effect.173,174 The evidence-based medicine shows that these enzymes have insufficient evidence of curative effects, and compelling study of efficiency in therapy was deficient, so they have not been widely popularized in clinical application at present.
Given that MFBs drive collagen formation and ECM deposition, and MFBs are the main source of ECM, it is exciting direction on whether targeted MFB activation will be an effective strategy for OSF treatment in the future.
Regulation of inflammatory response
OSF has long been considered an outcome of chronic inflammation perpetuating the production of cytokines by tissue-resident cells such as epithelial and mesenchymal cells, as well as infiltrated immune cells such as monocytes, macrophages, and lymphocytes.118,175 Currently, therapeutic approaches for OSF have focused on the anti-inflammation role by steroids and corticosteroids. However, these drugs are relatively ineffective in reversing the abnormal deposition of collagen and restoring elasticity of the oral mucosa. They are also usually accompanied by a series of side effects and rare complications such as local swelling and central serous chorioretinopathy.173,176 Therefore, it is now commonly accepted that the corticosteroid should be given in combination with other drugs.167 CSA recommends the local injection of steroids, especially the triamcinolone acetonide, should be combined with lidocaine or tanshinone, and is effective for alleviating the limitation of mouth opening, oral burning sensation, as well as the area of lesions. This outcome is similar but more effective than that in the clinical studies by Haque et al. who showed that local injection of IFN-γ (50 μg of IFN-γ twice a week for 8 weeks) improved the mouth opening limitation of 29 patients (100%). Of note, the burning sensation and ability to taste also improved, which accounted for 57% and 61%, respectively. The study also showed a reduction of inflammatory cells and levels of cytokines in the lesion sections, and a study in vitro further demonstrated that IFN-γ inhibits collagen synthesis associated with the anti-fibrotic response to arecoline.177
Other regimens including tablet colchicine and immunized milk are considered as anti-inflammation agents. Colchicine is a natural alkaloid with anti-inflammatory properties, which inhibits collagen secretion by neutralizing profibrotic cytokines such as IL-4 and TGF-β, thus achieving an anti-fibrotic effect. A clinical comparative study done by Krishnamoorthy et al. on OSF was implemented using 0.05 mg tablets of colchicine twice daily for 12 weeks and found a significant effect in improving symptoms of 50 patients with OSF, including mouth opening limitation and burning sensation (almost 99.8%). The histopathological findings also showed a marked reduction in the inflammatory cells and density of collagen fibrils.178 The immune milk produced by immunizing cows with human intestinal bacteria contains anti-inflammatory ingredients, which can inhibit the inflammatory response and regulate the production of cytokines. A clinical study by Tai et al. showed that oral administration of immune milk can improve symptoms and signs in OSF patients. Approximately 69.2% of those who received immune milk (45 mg of immune milk powder twice a day for three months) showed a significant remission in maximum mouth opening with more than 3 mm.179 However, the clinical application of immunomodulators is limited to some extent because of their serious side effects and short-term efficacy on OSF.
Ameliorating peripheral microcirculation disturbance
Some histopathologic studies have demonstrated the phenomenon that there is a progressive loss of vascularity in the local lesion of OSF and is accompanied by hemodynamic disorder. It is suggested that the underlying cause of OSF pathogenesis are due to progressive vascular occlusion and fibrinolysis system disorders. Therefore, some vasodilators such as buflomedial hydrochloride,180 nylidrin hydrochloride,181 and isoxsuprine182 were used to treat OSF by relaxing peripheral blood vessels, restoring blood supply to ischemic tissues, and reducing local tissue hardness. For instance, 10-year clinical research with 150 OSF cases reported that buflomedial hydrochloride combined with vitamin B-complex and topical triamcinolone acetonide 0.1% can effectively improve maximal interincisal distance of OSF patients. Another study also showed that ~62.07% patients with OSF obtained satisfactory improvement in the suppleness of oral mucosa by using nylidrin hydrochloride. In addition, a clinical study in 40 OSF patients revealed that isoxsuprine 10 mg four times per day also showed a desirable effect in improving mouth opening and burning sensation. Of note, a clinical trial by Rajendran et al. showed pentoxifylline, was effective in alleviating burning sensation and mouth opening of OSF patients. Another meta-analysis further deciphered the therapeutic efficacy of pentoxifylline in improving mouth opening limitation over time.183,184 Currently, a phase 4 study was carried out to evaluate the efficacy of triamcinolone with pentoxifylline and vitamin E in patients with stage II and III OSF (NCT05660694). Moreover, tea pigment taken orally was also regarded as an effective strategy for OSF treatment due to its ability to reduce blood hyper-viscosity, and improve ischemia, and microcirculation of local mucosa. A previous clinical study in 39 OSF patients demonstrated that oral administration of tea pigment (250 mg twice per day) significantly increased hemorheology in OSF patients and above 58.3% of these patients improved mouth opening by average 7.9 mm.185
However, these drugs have certain limitations, and the use of pentoxifylline is usually accompanied by side effects to the gastrointestinal tract and central nervous system, which may limit its clinical popularization. In the long run, other vasodilators are only effective in symptomatic relief, and the long-term effect on OSF is unsatisfactory.
Others
In addition to the above drugs, some natural compounds with antioxidant effects have also been used in OSF treatment, mainly including lycopene,186,187 spirulina,188 and aloe.189,190 A study on the clinical efficacy of lycopene showed that 21 OSF patients were treated with 16 mg/d lycopene for 2 months, and their mouth opening was obviously improved, with an average of 2.2–4.6 mm.186 In addition, some other natural compounds, such as tanshinone and curcumin, which have the characteristics of diverse structures, wide sources, and low toxicity, have attracted much attention in the multi-target therapy of OSF. Tanshinone is an extract from Salvia miltiorrhiza with anti-inflammatory and wound-healing properties, which has been recommended in OSF treatment by guideline of CSA. A study has confirmed the inhibitory effects of tanshinone on OSF development by suppressing arecoline-induced EMT by epigenetically reactivating the p53 pathway.191 Tanshinone also effectively inhibited the activation of fibroblasts and collagen aggregation stimulated by arecoline by acting on TGF-β/Smad pathway.192 Similarly, curcumin is also a diketone compound extracted from plant roots, which has good anti-inflammatory and anti-tumorigenic properties. A randomized clinical trial of 15 patients with OSF evaluated the therapeutic effect of curcumin, and the results showed that curcumin could effectively improve the burning sensation and mouth opening of OSF patients, without any side effects.193
Although above-mentioned clinical studies are certainly limited, available data using AN-induced OSF model suggest that some novel therapies, including sodium hyaluronate/bioglass composite hydrogel (BG/HA) and mesenchymal stem cells (MSCs) are proven to be effective in the treatment of OSF. A typical example that Guo et al. demonstrated the submucosal administration of BG/HA ameliorates the effects of AN by inhibiting the production of pro-inflammatory cytokines, promoting angiogenesis, and reducing collagen deposition.194 In addition, recent studies in AN-induced animal models found that the submucosal injection of dental pulp stem cells (DPSCs) can limit the progression of OSF by intervening the crosstalk between epithelial cells and T cells.117 Moreover, in vitro study showed that adipose stem cell-derived extracellular vesicles significantly suppressed proliferation, migration, invasion, and expression level of fibrosis marker via the miR-375/FOXF1 axis in fibrotic buccal mucosal fibroblasts.195 However, these findings warrant further investigation to explore potential clinical applications. Notably, recent advancements in anti-fibrotic treatments have been directed towards targeting the activation of MFBs, focusing on factors that play in a role in the progression of fibrosis. Pirfenidone (PFD), luspatercept, and AVID-200 are anti-fibrotic drugs which were approved by FDA for pulmonary fibrosis and myelofibrosis, and all of them act as an antagonist for the deposition of ECM by inhibiting the activation of TGF-β.196 Tocilizumab, an IL-6 inhibitor, showed effectiveness in the treatment of systemic sclerosis and pulmonary fibrosis,197,198 which indicates that the strategies of targeting MFB and immunity have great potential in the treatment of OSF. Above studies indicate that these therapeutic agents have beneficial effects in fibrosis disease, but to date, none of them have been approved for OSF. Further investigation of their role is certainly warranted in OSF.
Malignant transformation of OSF
The potential of OSF in the malignant transformation was first described by Paymaster in 1956, and it has been regarded as an oral precancerous condition for decades and the WHO redefined OSF as OPMDs in 2005.199 However, given that the specific pathogenesis of OSF is not clear at present time, the comprehensive research on the mechanism of its malignant transformation has been greatly hindered. A large number of studies reported that the process of OSF transformation into OSCC is multifactorial, and accompanied by a series of complex biological behavioral changes, which consists of genetic alterations and dynamics of the microenvironments.
Genetic alteration during OSF carcinogenesis
Multiple studies have reported chromosomal alterations and genetic characteristics of the link between OSF and OSCC. Telomerase reactivation in the chromosome plays a major role in the process of malignant transformation. A typical example is hTERT expression, which shows a strong correlation with telomerase activity. The consistent increase in hTERT levels from normal mucosa to OSF to OSCC tissue samples was also observed by immunohistochemical evaluation.200 Moreover, chromosomal alterations, such as copy number variation, have been described in malignant transformation of OSF. The chromosomal 1q21 region harboring S100A14 was found to be deleted during OSF carcinogenesis.201 Another cytogenetic study demonstrated the genomic damage caused by AN consumption. Of note, the frequencies of sister chromatid exchanges and chromosome aberrations both increased in peripheral blood lymphocytes of OSF and OSCC patients.202 Lin et al. evaluated the polymorphisms of COX-2-765G/G genotype in OSF and OSCC patients by PCR-RFLP methods, and the result revealed COX-2-765C allele vs. -765G/G genotype was a risk factor for malignant transformation of OSF (OR = 3.20, 95%CI = 1.32–8.94).203 A previous study evaluated the genetic signatures for carcinogenesis in OSF from 15 blood genomic DNA samples of OSF patients, and the loss of heterozygosity (LOH) loci was observed in over 50% of the OSF tissues. Many of these LOH loci were consistent when compared to OSCC and have been shown to be associated with malignant transformation.204 In addition, methylation has also been described in the malignant transformation. Wnt inhibitory factor-1 (WIF1) is regarded as a significant molecule in the progression of OSF malignancy, and the promoter methylation of WIF1 is tumor-specific; it serves as a potential epigenetic biomarker for the OSF carcinogenesis.205 Other studies even confirmed noncoding RNAs such as LncADAMTS9-AS2206 and circEPSTI1207 were also involved in the process of OSF carcinogenesis.
Genomic instability denotes abnormal changes of cell proliferation, apoptosis, as well as cell cycles, thus promoting the process of malignant transformation. Zhou et al. reported that expression of surviving Thr34 phosphorylation, cyclin B1, and p34cdc2 are increased in malignant OSF, and survivin, cyclin B1, and p34cdc2 are key molecules contributing to the malignant transformation of OSF by regulating cell apoptosis and cell cycle.208,209 In addition, the Bcl-2 family members, anti-apoptotic Mcl-1 protein,210 cyclin D1,211 and p63212,213 are also involved in the process of malignant transformation.
Dynamics of the microenvironments during OSF carcinogenesis
As one of the most important features of carcinogenesis, the role of hypoxia and immune balance in shaping the microenvironment of malignant transformation from OSF into OSCC cannot be ignored. The local hypoxia of OSF caused by microvascular lesions may contribute the expression of hypoxia-inducible factor, which is widely regarded as the key molecule to regulate the process of malignant transformation in OSF.214–216
Of note, accumulating evidence has also supported the concept of immune surveillance as a key barrier for OSF carcinogenesis. Indeed, the infiltration patterns of dendritic cells (DCs), Th17, and Tregs were demonstrated to be markedly changed in the normal-OSF-malignant transformation. During the malignant transformation of OSF, the increased infiltration of Tregs and decreased expression of DCs are consistent with the formation of a suppressive immune microenvironment. On the one hand, the Tregs expression is statistically higher in OSCC than in OSF, meanwhile Th17/Treg ratio is skewed to favor Tregs. Further analysis showed that Th17/Treg imbalance can be used to indicate a risk factor for malignant transformation of OSF.113 On the other hand, the expression of DCs, especially CD1a+ and CD207+ DCs, is higher in OSCC than OSF.217 These results suggest that Tregs and DCs participate in the formation of an immunosuppressive microenvironment and the carcinogenesis of OSF, to a certain extent. Furthermore, previous studies also showed that the MCs may be involved in OSF carcinogenesis.111
Most importantly, the abnormal changes of stromal cells, increased matrix stiffness, and senescence also are striking characteristics during the malignant transformation of OSF. The increase of Snail1 and Twist1 were expressed by epithelial cells in OSF during the development of OSF, accompanied by the deletion of E-cadherin, suggesting that OSF has a malignant transformation to OSCC by the EMT process.218 Moreover, the abnormal hyperplasia and increased nuclear-cytoplasmic ratio of the basal cell layer are high-risk features for OSF, and these characteristics are closely associated with carcinogenesis.219 Another compelling piece of evidence is the basal stem cell in OSF with abnormal self-renewal capacity, which contributes to the malignant transformation of OSF.220 In addition, the progression of OSF is accompanied by the increase of matrix hardness, which can promote the migration phenotype by mediating the nuclear transfer of YAP/TAZ.221 The increased matrix stiffness was caused by excessive deposition of ECM, which is closely associated with activation of MFBs. Punnya et al. confirmed that as the OSF was malignantly transformed, it was accompanied by epithelial cell dysplasia, as well as the increase of α-SMA+ MFBs, thus enhancing local tissue stiffness.59,222 Furthermore, the senescence of fibroblasts and epithelial cells will lead to malignant transformation by secreting SASPs.137,223 Altogether, stromal cells, matrix stiffness, and senescence have been shown to play a role in the crosstalk between OSF progression and oncogene activation.
Perspectives and conclusions
At present time, it is undeniable that OSF is a disease with a malignant tendency, and the specific pathogenesis of its occurrence and malignant transformation is not clear yet. In spite of enormous efforts, non-invasive, effective diagnostic approaches, precise therapy strategies, and available preventive methods are the ultimate aims to vanquish this disease. In addition, a favorable animal model is an urgent requirement for the comprehensive study of OSF. Existing in vivo models of OSF are mainly mice or rats induced by arecoline or bleomycin.38,194,224,225 Both of them only replicate similar pathological conditions, which may not accurately simulate the pathogenesis of OSF, leading to the research on the treatment of OSF being greatly limited. Thus, the research focus should be on the construction of in vivo models, and on this basis, research in the field of OSF administration should be carried out.226 Unfortunately, some intractable questions and challenges remain.
First, most studies reported that organ fibrosis (heart, liver, kidney, lung, etc.) is usually mediated by the type II immune response, which is a chronic inflammatory response, but it has not been identified in OSF. The immunological research of OSF is limited to the phenotypic changes of immune cells and related cytokines, but the key immune cells or cytokines have not been figured out in vivo. Secondly, with regard to the pathogenesis of OSF, the EMT of epithelial cells has currently received a lot of attention. However, the importance of basal stem cells is usually ignored, and the integrity of basal stem cells is crucial for epithelial homeostasis. Most importantly, a recent study has reported reduced stem cell activity in OSF.220 As a result, the interaction between basal cells and submucosal cells should be considered for further investigation. Third, the role of human microbiota in organ fibrosis is one of the hot research topics currently. Dysbiosis of oral microbiota, which could damage the epithelial barrier, activate immune responses, and stimulate submucosal cells, may be related to the progression and malignant transformation of OSF. According to the present viewpoint, microbiota biogeography plays a vital role in disease progression.227 The field of oral microbiota may recognize the importance of spatially resolved information in understanding the complexity of the oral ecosystem and its potential role in mucosal diseases such as OSF. However, it is challenging to prove the causal relationship between microbiota and oral mucosal diseases, and most findings only exhibit correlation rather than causal evidence so far. Whether the occurrence and development of OSF can be attributed to a single pathogen remains controversial, and it’s even harder to reach a consensus on major pathogenic components of the complex oral microbiome. There are still many questions about the interaction between the microbiota and host cells in a certain ecological niche of the oral mucosa that have yet to be unraveled. Finally, although we have made impressive progress in understanding the pathogenesis of OSF in the past few years, we still need to overcome multiple challenges to turn this information into effective anti-fibrosis therapies. At present, there have been pharmacological therapies aimed at improving local blood circulation and targeting inflammatory response. Surprisingly, few of the medical therapies currently proposed are directed at MFBs, which is the core of the pathogenesis of OSF. Therefore, the new direction aims to explore strategies of drugs that target MFBs of OSF.
Taken together, chewing AN is the main factor for OSF. Although it is known that a variety of pathogeneses of OSF exists, events that contribute to the onset and development of OSF are still not fully understood. Deciphering the biology that underlies these events will enable the devising and testing of novel and effective antifibrotic therapies. Additionally, there is also a demand for improving diagnostic approaches that can early, accurately, and noninvasively evaluate fibrosis, which will allow for the prevention of malignant transformation of OSF and improve the quality of individuals’ life.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2022YFC2402900); National Natural Science Foundation of China (82470989, 52103327); The Joint Funds of the Hunan Provincial Natural Science Foundation (2023JJ60509); The Science and Technology Talent Support Project of the Hunan Provincial Science Popularization Special Project (2023TJ-Z08); Hunan Provincial Innovation Foundation for Postgraduate (2023ZZTS0218); The Postgraduate Independent Exploration Innovation Fund of the Central South University (2023ZZTS0987).
Author contributions
Conceptualization, O.L. and X.D.; Investigation, J.T.; Visualization, J.T., J.L.; Supervision, J.J.; Validation, Z.Z.; Funding acquisition, J.T., Z.Z., X.D. and O.L.; Project administration, H.T. and X.C.; Writing-Original Draft Preparation, J.T. and B.C.; Writing—Review & Editing, J.T., J.L. All authors have read and agreed to the published version of the manuscript
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Jianfei Tang, Junjie Liu.
Contributor Information
Xiaohan Dai, Email: daixiaohan@csu.edu.cn.
Ousheng Liu, Email: liuousheng@csu.edu.cn.
References
- 1.Warnakulasuriya, S. et al. Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral. Dis.27, 1862–1880 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya, S. et al. Areca nut and oral cancer: evidence from studies conducted in humans. J. Dent. Res101, 1139–1146 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sirsat, S. M. et al. Submucous fibrosis of the palate in diet-preconditioned Wistar rats. Induction by local painting of capsaicin-an optical and electron microscopic study. Arch. Pathol.70, 171–179 (1960). [PubMed] [Google Scholar]
- 4.Pindborg, J. J. et al. Frequency of oral carcinoma, leukoplakia, leukokeratosis, leukoedema, submucous fibrosis, and lichen planus in 10,000 Indians in Lucknow, Uttar Pradesh, India; preliminary report. J. Dent. Res.44, 61 (1965). [DOI] [PubMed] [Google Scholar]
- 5.Wahi, P. N. et al. Submucous fibrosis of the oral cavity. 2. Studies on epidemiology. Bull. World Health Organ.35, 793–799 (1966). [PMC free article] [PubMed] [Google Scholar]
- 6.Shiau, Y. Y. et al. Submucous fibrosis in Taiwan. Oral. Surg. Oral. Med. Oral. Pathol.47, 453–457 (1979). [DOI] [PubMed] [Google Scholar]
- 7.Canniff, J. P. et al. The aetiology of oral submucous fibrosis: the stimulation of collagen synthesis by extracts of areca nut. Int. J. Oral. Surg.10, 163–167 (1981). [PubMed] [Google Scholar]
- 8.Harvey, W. et al. Stimulation of human buccal mucosa fibroblasts in vitro by betel-nut alkaloids. Arch. Oral. Biol.31, 45–49 (1986). [DOI] [PubMed] [Google Scholar]
- 9.Murti, P. R. et al. Effect on the incidence of oral submucous fibrosis of intervention in the areca nut chewing habit. J. Oral. Pathol. Med.19, 99–100 (1990). [DOI] [PubMed] [Google Scholar]
- 10.Misra, S. P. et al. Oesophageal subepithelial fibrosis: an extension of oral submucosal fibrosis. Postgrad. Med. J.74, 733–736 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang, C. P. et al. Quantitative analysis of immunocompetent cells in oral submucous fibrosis in Taiwan. Oral. Oncol.38, 56–63 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Chiu, C. J. et al. Interaction of collagen-related genes and susceptibility to betel quid-induced oral submucous fibrosis. Cancer Epidemiol. Biomark. Prev.11, 646–653 (2002). [PubMed] [Google Scholar]
- 13.Li, X. et al. Effect of arecoline on the differentiation of myofibroblasts of oral mucosa. Chin. J. Stomatol.42, 423–427 (2007). [PubMed] [Google Scholar]
- 14.Moutasim, K. A. et al. Betel-derived alkaloid up-regulates keratinocyte alphavbeta6 integrin expression and promotes oral submucous fibrosis. J. Pathol.223, 366–377 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Khan, I. et al. Role of TGF-β and BMP7 in the pathogenesis of oral submucous fibrosis. Growth Factors29, 119–127 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Pant, I. et al. Role of areca nut induced TGF-β and epithelial-mesenchymal interaction in the pathogenesis of oral submucous fibrosis. PLoS One10, e0129252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, J. et al. YAP-induced endothelial-mesenchymal transition in oral submucous fibrosis. J. Dent. Res.98, 920–929 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Yao, M. et al. Role of the arecoline/YAP1/BMP4 pathway in promoting endothelial-mesenchymal transition in oral submucous fibrosis. J. Oral. Pathol. Med.49, 305–310 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Xie, C. et al. Identification of a BRAF/PA28γ/MEK1 signaling axis and its role in epithelial-mesenchymal transition in oral submucous fibrosis. Cell Death Dis.13, 701 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou, M. Y. et al. MiR-424/TGIF2-mediated pro-fibrogenic responses in oral submucous fibrosis. Int. J. Mol. Sci.24, 5811 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu, C. H. et al. XIST/let-7i/HMGA1 axis maintains myofibroblasts activities in oral submucous fibrosis. Int. J. Biol. Macromol.232, 123400 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Gupta, P. C. et al. Global epidemiology of areca nut usage. Addict. Biol.7, 77–83 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Yuwanati, M. et al. Prevalence of oral submucous fibrosis among areca nut chewers: a systematic review and meta-analysis. Oral. Dis.29, 1920–1926 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Cai, X. et al. Oral submucous fibrosis: a clinicopathological study of 674 cases in China. J. Oral. Pathol. Med.48, 321–325 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh, A. K. et al. Prevalence and risk factors for oral potentially malignant disorders in Indian population. J. Pharm. Bioallied Sci.13, S398–s401 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reichart, P. et al. Betel quid chewing, oral cancer and other oral mucosal diseases in Vietnam: a review. J. Oral. Pathol. Med.37, 511–514 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Zhang, S. et al. Betel-quid and oral submucous fibrosis: a cross-sectional study in Hunan province, China. J. Oral. Pathol. Med.41, 748–754 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Gupta, S. et al. Oral submucous fibrosis: an overview of a challenging entity. Indian J. Dermatol. Venereol. Leprol.87, 768–777 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Kujan, O. et al. Malignant transformation of oral submucous fibrosis: a systematic review and meta-analysis. Oral. Dis.27, 1936–1946 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Murthy, V. et al. Malignant transformation rate of oral submucous fibrosis: a systematic review and meta-analysis. J. Clin. Med.11, 1793 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xinchun, J. et al. Oral submucous fibrosis transforming into squamous cell carcinoma: a prospective study over 31 years in mainland China. Clin. Oral. Investig.25, 2249–2256 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Pindborg, J. J. Frequency of oral submucous fibrosis in North India. Bull. World Health Organ.32, 748–750 (1965). [PMC free article] [PubMed] [Google Scholar]
- 33.Murti, P. R. et al. Malignant transformation rate in oral submucous fibrosis over a 17-year period. Community Dent. Oral. Epidemiol.13, 340–341 (1985). [DOI] [PubMed] [Google Scholar]
- 34.International Agency for Research on Cancer. IARC monographs on the Identification of Carcinogenic Hazards to Humans. Vol. 85, 1–334 (International Agency for Research on Cancer, 2004).
- 35.Cirillo, N. et al. Are there betel quid mixtures less harmful than others? A scoping review of the association between different betel quid ingredients and the risk of oral submucous fibrosis. Biomolecules12, 664 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, Y. J. et al. The pharmacology, toxicology and potential applications of arecoline: a review. Pharm. Biol.54, 2753–2760 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Pentenero, M. et al. Chronic mechanical trauma/irritation and oral carcinoma: a systematic review showing low evidence to support an association. Oral. Dis.28, 2110–2118 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang, B. et al. Rat model with oral submucous fibrosis induced by arecoline and mechanical stimulation. West Chin. J. Stomatol.37, 260–264 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen, H. M. et al. HLA typing in Taiwanese patients with oral submucous fibrosis. J. Oral. Pathol. Med.33, 191–199 (2004). [DOI] [PubMed] [Google Scholar]
- 40.CW, V. W. et al. HLA-antigens in oral submucous fibrosis. J. Oral. Pathol. Med23, 23–27 (1994). [DOI] [PubMed] [Google Scholar]
- 41.Liu, C. J. et al. Polymorphism of the MICA gene and risk for oral submucous fibrosis. J. Oral. Pathol. Med.33, 1–6 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Jeevankumar, S. et al. Association of MICA gene Exon-5 polymorphism in oral submucous fibrosis. Oral. Surg. Oral. Med., Oral. Pathol. Oral. Radiol.135, 110–116 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Shin, Y. N. et al. Association of CTLA-4 gene polymorphism with oral submucous fibrosis in Taiwan. J. Oral. Pathol. Med.33, 200–203 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Agrawal, D. et al. Role of GSTM1 and GSTT1 polymorphism: susceptibility to oral submucous fibrosis in the North Indian population. Oncology79, 181–186 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Chaudhary, A. K. et al. Synergistic effect of stromelysin-1 (matrix metalloproteinase-3) promoter (-1171 5A->6A) polymorphism in oral submucous fibrosis and head and neck lesions. BMC Cancer10, 369 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li, N. et al. Novel genetic biomarkers for susceptibility to oral submucous fibrosis: cytochrome P450 3A. Med. Hypotheses77, 834–836 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Chaudhuri, S. R. et al. CYP1AI and CYP2E1 gene polymorphisms may increase susceptibility to oral submucous fibrosis among betel quid chewers of eastern India. Gene513, 268–271 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Adhvaryu, S. G. et al. SCE frequencies in lymphocytes of tobacco/betel nut chewers and patients with oral submucous fibrosis. Br. J. Cancer53, 141–143 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeyapradha, D. et al. Comparison of the frequency of sister chromatid exchange in pan chewers and oral submucous fibrosis patients. J. Oral. Maxillofac. Pathol.15, 278–282 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desai, S. S. et al. Cytogenetic damage in exfoliated oral mucosal cells and circulating lymphocytes of patients suffering from precancerous oral lesions. Cancer Lett.109, 9–14 (1996). [DOI] [PubMed] [Google Scholar]
- 51.Joshi, M. S. et al. Cytogenetic alterations in buccal mucosa cells of chewers of areca nut and tobacco. Arch. Oral. Biol.56, 63–67 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Paulose, S. et al. Estimation of serum malondialdehyde and assessment of DNA damage using comet assay in patients with oral submucous fibrosis. J. Investig. Clin. Dent.7, 286–293 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Sachdev, P. K. et al. Zinc, copper, and iron in oral submucous fibrosis: a meta-analysis. Int. J. Dent.2018, 3472087 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma, R. H. et al. Increased lysyl oxidase activity in fibroblasts cultured from oral submucous fibrosis associated with betel nut chewing in Taiwan. J. Oral. Pathol. Med.24, 407–412 (1995). [DOI] [PubMed] [Google Scholar]
- 55.Trivedy, C. et al. Copper content in Areca catechu (betel nut) products and oral submucous fibrosis. Lancet349, 1447 (1997). [DOI] [PubMed] [Google Scholar]
- 56.Mehrotra, R. et al. Lipid profile in oral submucous fibrosis. Lipids Health Dis.8, 29 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanthem, R. K. et al. Serum lipid profile in oral submucous fibrosis: a clinico pathological study. J. Oral. Maxillofac. Pathol.19, 139–144 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Younesi, F. et al. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. (2024). [DOI] [PubMed]
- 59.Angadi, P. et al. Evaluation of myofibroblasts in oral submucous fibrosis: correlation with disease severity. J. Oral. Pathol. Med.40, 208–213 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Yu, C. H. et al. LINC00312/YBX1 axis regulates myofibroblast activities in oral submucous fibrosis. Int. J. Mol. Sci.21 (2020). [DOI] [PMC free article] [PubMed]
- 61.Meng, X. M. et al. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol.12, 325–338 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Khan, I. et al. Activation of TGF-β pathway by areca nut constituents: a possible cause of oral submucous fibrosis. PLoS One7, e51806 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pant, I. et al. Role of areca nut induced JNK/ATF2/Jun axis in the activation of TGF-β pathway in precancerous oral submucous fibrosis. Sci. Rep.6, 34314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang, J. Z. et al. EGCG blocks TGFβ1-induced CCN2 by suppressing JNK and p38 in buccal fibroblasts. Clin. Oral. Investig.17, 455–461 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Chang, Y. C. et al. Elevated vimentin expression in buccal mucosal fibroblasts by arecoline in vitro as a possible pathogenesis for oral submucous fibrosis. Oral. Oncol.38, 425–430 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol.7, 684–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang, Y. C. et al. Arecoline-induced myofibroblast transdifferentiation from human buccal mucosal fibroblasts is mediated by ZEB1. J. Cell Mol. Med.18, 698–708 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang, M. C. et al. Areca nut-induced buccal mucosa fibroblast contraction and its signaling: a potential role in oral submucous fibrosis-a precancer condition. Carcinogenesis34, 1096–1104 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Deng, Y. T. et al. Arecoline-stimulated connective tissue growth factor production in human buccal mucosal fibroblasts: Modulation by curcumin. Oral. Oncol.45, e99–e105 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Yeh, M. C. et al. Low-power laser irradiation inhibits arecoline-induced fibrosis: an in vitro study. Int J. Oral. Sci.9, 38–42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu, C. C. et al. Aberrant SSEA-4 upregulation mediates myofibroblast activity to promote pre-cancerous oral submucous fibrosis. Sci. Rep.6, 37004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu, C. C. et al. Targeting lncRNA H19/miR-29b/COL1A1 axis impedes myofibroblast activities of precancerous oral submucous fibrosis. Int. J. Mol. Sci.22, 2216 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee, Y. H. et al. Inhibition of lncRNA HOTTIP ameliorated myofibroblast activities and inflammatory cytokines in oral submucous fibrosis. J. Formos. Med. Assoc.120, 1188–1193 (2021). [DOI] [PubMed] [Google Scholar]
- 74.Xia, L. et al. Arecoline and oral keratinocytes may affect the collagen metabolism of fibroblasts. J. Oral. Pathol. Med.38, 422–426 (2009). [DOI] [PubMed] [Google Scholar]
- 75.Tsai, C. H. et al. Raised keratinocyte growth factor-1 expression in oral submucous fibrosis in vivo and upregulated by arecoline in human buccal mucosal fibroblasts in vitro. J. Oral. Pathol. Med.34, 100–105 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Bishen, K. A. et al. The role of basic fibroblast growth factor in oral submucous fibrosis pathogenesis. J. Oral. Pathol. Med.37, 402–411 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Lee, Y. H. et al. Elevation of Twist expression by arecoline contributes to the pathogenesis of oral submucous fibrosis. J. Formos. Med. Assoc.115, 311–317 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Tsai, C. C. et al. Deficiency in collagen and fibronectin phagocytosis by human buccal mucosa fibroblasts in vitro as a possible mechanism for oral submucous fibrosis. J. Oral. Pathol. Med.28, 59–63 (1999). [DOI] [PubMed] [Google Scholar]
- 79.Groeger, S. et al. Oral mucosal epithelial cells. Front Immunol.10, 208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng, Y. et al. Research on inhibition of areca nut extract on human buccal keratinocytes. West Chin. J. Stomatol17, 233–235 (1999). [PubMed] [Google Scholar]
- 81.Zhou, Z. S. et al. Arecoline suppresses HaCaT cell proliferation through cell cycle regulatory molecules. Oncol. Rep.29, 2438–2444 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Thangjam, G. S. et al. Regulation of oxidative-stress responsive genes by arecoline in human keratinocytes. J. Periodontal Res.44, 673–682 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Chang, M. C. et al. The induction of prostaglandin E2 production, interleukin-6 production, cell cycle arrest, and cytotoxicity in primary oral keratinocytes and KB cancer cells by areca nut ingredients is differentially regulated by MEK/ERK activation. J. Biol. Chem.279, 50676–50683 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Li, L. et al. Arecoline suppresses epithelial cell viability by upregulating tropomyosin-1 through the transforming growth factor-β/Smad pathway. Pharm. Biol.58, 1244–1251 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gu, L. et al. Arecoline suppresses epithelial cell viability through the Akt/mTOR signaling pathway via upregulation of PHLPP2. Toxicology419, 32–39 (2019). [DOI] [PubMed] [Google Scholar]
- 86.Shetty, S. S. et al. Signaling pathways promoting epithelial mesenchymal transition in oral submucous fibrosis and oral squamous cell carcinoma. Jpn. Dent. Sci. Rev.56, 97–108 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das, R. K. et al. Epithelio-mesenchymal transitional attributes in oral sub-mucous fibrosis. Exp. Mol. Pathol.95, 259–269 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Hu, X. et al. Overexpression of DEC1 in the epithelium of OSF promotes mesenchymal transition via activating FAK/Akt signal axis. J. Oral. Pathol. Med.51, 780–790 (2022). [DOI] [PubMed] [Google Scholar]
- 89.Gao, Y. et al. Expression of transforming growth factor beta 1 in keratinocytes of oral submucous fibrosis tissue. Chin. J. Stomatol.32, 239–241 (1997). [PubMed] [Google Scholar]
- 90.Yang, X. et al. Stromal thrombospondin 1 suppresses angiogenesis in oral submucous fibrosis. Int. J. Oral. Sci.16, 17 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li, L. J. & P.X, X. The OSF ultrastructure study of danshen root prior-treatment and post-treatment. J. Clin. Stomatol.24, 751–752 (2008). [Google Scholar]
- 92.Thakkannavar, S. S. et al. Histochemical and immunohistochemical analysis of collagen fibers and microvascular density in various grades of oral submucous fibrosis. Iran. J. Pathol.14, 127–134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tekade, S. A. et al. Early stage oral submucous fibrosis is characterized by increased vascularity as opposed to advanced stages. J. Clin. Diagn. Res.11, Zc92–zc96 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tseng, S. K. et al. Arecoline induced cell cycle arrest, apoptosis, and cytotoxicity to human endothelial cells. Clin. Oral. Investig.16, 1267–1273 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Li, N. et al. Cys-X-Cys ligand 9 might be an immunological factor in the pathogenesis of oral submucous fibrosis and its concomitant oral lichenoid lesion. Clin. Oral. Investig.17, 1251–1258 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Ullah, M. et al. Arecoline increases basic fibroblast growth factor but reduces expression of IL-1, IL-6, G-CSF and GM-CSF in human umbilical vein endothelium. J. Oral. Pathol. Med.44, 591–601 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Phatak, A. G. Oral submucous fibrosis-a chronic disseminated intravascular coagulation syndrome with local coagulopathy. Gut34, 713 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fang, C. et al. The pathological damage of microvessels in oral submucosal fibrosis. Chin. J. Mod. Med.6, 72–73 (1996). [Google Scholar]
- 99.Yang, S. F. et al. The upregulation of type I plasminogen activator inhibitor in oral submucous fibrosis. Oral. Oncol.39, 367–372 (2003). [DOI] [PubMed] [Google Scholar]
- 100.Wang, Y. et al. Oxidative-protective effect of nuclear receptor coactivator 7 on arecoline-induced endothelial-to-mesenchymal transition. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radio.130, 565–573 (2020). [DOI] [PubMed] [Google Scholar]
- 101.Liping, W. et al. Immunopathogenesis of oral submucous fibrosis by chewing the areca nut. J. Leukoc. Biol.111, 469–476 (2021). [DOI] [PubMed] [Google Scholar]
- 102.Matthias, M. Inflammation and fibrosis. Matrix Biol.68-69, 106–121 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Misharin, A. V. et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med.214, 2387–2404 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krenkel, O. et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology67, 1270–1283 (2018). [DOI] [PubMed] [Google Scholar]
- 105.Xu, L. et al. Tubular GM-CSF promotes late MCP-1/CCR2-mediated fibrosis and inflammation after ischemia/reperfusion injury. J. Am. Soc. Nephrol.30, 1825–1840 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pereira, T. et al. Quantitative evaluation of macrophage expression using CD68 in oral submucous fibrosis: an immunohistochemical study. Ann. Med. Health Sci. Res.5, 435–441 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brody, S. L. et al. Chemokine receptor 2-targeted molecular imaging in pulmonary fibrosis. a clinical trial. Am. J. Respir. Crit. Care Med.203, 78–89 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sarode, G. et al. Myofibroblasts could be recruited in a chemokine (C-C motif) ligand 2-dependent manner in pathogenesis of oral submucous fibrosis. J. Oral. Pathol. Med.46, 443–447 (2017). [DOI] [PubMed] [Google Scholar]
- 109.H, X. et al. Expression of chemokines CCR 2 and CXCL 9 in oral submucous fibrosis. J. Clin. Stomatol.28, 657–659 (2012). [Google Scholar]
- 110.Sabarinath, B. et al. Immunohistochemical evaluation of mast cells and vascular endothelial proliferation in oral submucous fibrosis. Indian J. Dent. Res.22, 116–121 (2011). [DOI] [PubMed] [Google Scholar]
- 111.Yadav, A. et al. Altered immunohistochemical expression of mast cell tryptase and chymase in the pathogenesis of oral submucous fibrosis and malignant transformation of the overlying epithelium. PLoS One9, e98719 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pillai, M. R. et al. Interferon activation of latent natural killer cells and alteration in kinetics of target cell lysis: clinical implications for oral precancerous lesions. Oral. Surg. Oral. Med. Oral. Pathol.70, 458–461 (1990). [DOI] [PubMed] [Google Scholar]
- 113.Liu, S. et al. Skewed Th17/Treg balance during progression and malignant transformation of oral submucous fibrosis. Oral. Dis.28, 2119–2130 (2022). [DOI] [PubMed] [Google Scholar]
- 114.Wang, L. et al. Cytokines secreted by arecoline activate fibroblasts that affect the balance of TH17 and Treg. J. Oral. Pathol. Med.49, 156–163 (2020). [DOI] [PubMed] [Google Scholar]
- 115.Zhao, X. et al. Th17 cell-derived amphiregulin promotes colitis-associated intestinal fibrosis through activation of mTOR and MEK in intestinal myofibroblasts. Gastroenterology164, 89–102 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Celada, L. J. et al. PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci. Transl. Med.10, eaar8356 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.S Y, W. et al. DPSCs regulate epithelial-T cell interactions in oral submucous fibrosis. Stem Cell Res. Ther.15, 113 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Auluck, A. et al. Oral submucous fibrosis, a clinically benign but potentially malignant disease: report of 3 cases and review of the literature. J. Can. Dent. Assoc.74, 735–740 (2008). [PubMed] [Google Scholar]
- 119.Shakunthala, G. K. et al. Role of oxidative stress in the pathogenesis of oral submucous fibrosis: a preliminary prospective study. Contemp. Clin. Dent.6, S172–S174 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Illeperuma, R. P. et al. Areca nut exposure increases secretion of tumor-promoting cytokines in gingival fibroblasts that trigger DNA damage in oral keratinocytes. Int. J. Cancer137, 2545–2557 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chou, M. Y. et al. Depletion of miR-155 hinders the myofibroblast activities and reactive oxygen species generation in oral submucous fibrosis. J. Formos. Med. Assoc.121, 467–472 (2022). [DOI] [PubMed] [Google Scholar]
- 122.Rai, V. et al. Evaluation of oxidative stress and the microenvironment in oral submucous fibrosis. Heliyon5, e01502 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gupta, S. et al. Role of oxidative stress and antioxidants in aetiopathogenesis and management of oral submucous fibrosis. Indian J. Clin. Biochem.19, 138–141 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shetty, S. R. et al. Malondialdehyde levels in oral sub mucous fibrosis: a clinicopathological and biochemical study. N. Am. J. Med. Sci.4, 125–128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bale, R. et al. Oral submucous fibrosis: A quantitative assessment of serum malondialdehyde, superoxide dismutase and correlation with clinical staging. J. Oral. Maxillofac. Pathol.21, 41–45 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chatterjee, R. et al. Pathophysiological relationship between hypoxia associated oxidative stress, Epithelial-mesenchymal transition, stemness acquisition and alteration of Shh/ Gli-1 axis during oral sub-mucous fibrosis and oral squamous cell carcinoma. Eur. J. Cell Biol.100, 151146 (2021). [DOI] [PubMed] [Google Scholar]
- 127.You, Y. et al. Angiotensin (1-7) inhibits arecoline-induced migration and collagen synthesis in human oral myofibroblasts via inhibiting NLRP3 inflammasome activation. J. Cell Physiol.234, 4668–4680 (2019). [DOI] [PubMed] [Google Scholar]
- 128.Yen, C. Y. et al. Arecoline-mediated inhibition of AMP-activated protein kinase through reactive oxygen species is required for apoptosis induction. Oral. Oncol.47, 345–351 (2011). [DOI] [PubMed] [Google Scholar]
- 129.Hung, T. C. et al. Hemeoxygenase-1 expression in response to arecoline-induced oxidative stress in human umbilical vein endothelial cells. Int J. Cardiol.151, 187–194 (2011). [DOI] [PubMed] [Google Scholar]
- 130.Wong, D. Y. et al. Glutathione concentration in oral cancer tissues. Cancer Lett.81, 111–116 (1994). [DOI] [PubMed] [Google Scholar]
- 131.Chang, Y. C. et al. Synergistic effects of nicotine on arecoline-induced cytotoxicity in human buccal mucosal fibroblasts. J. Oral. Pathol. Med30, 458–464 (2001). [DOI] [PubMed] [Google Scholar]
- 132.Pitiyage, G. N. et al. Senescent mesenchymal cells accumulate in human fibrosis by a telomere-independent mechanism and ameliorate fibrosis through matrix metalloproteinases. J. Pathol.223, 604–617 (2011). [DOI] [PubMed] [Google Scholar]
- 133.Pitiyage, G. N. et al. Increased secretion of tissue inhibitors of metalloproteinases 1 and 2 (TIMPs -1 and -2) in fibroblasts are early indicators of oral sub-mucous fibrosis and ageing. J. Oral. Pathol. Med.41, 454–462 (2012). [DOI] [PubMed] [Google Scholar]
- 134.James, E. L. et al. Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. J. Proteome Res.14, 1854–1871 (2015). [DOI] [PubMed] [Google Scholar]
- 135.Rehman, A. et al. Areca nut alkaloids induce irreparable DNA damage and senescence in fibroblasts and may create a favourable environment for tumour progression. J. Oral. Pathol. Med.45, 365–372 (2016). [DOI] [PubMed] [Google Scholar]
- 136.Wang, Z. et al. Senescent epithelial cells remodel the microenvironment for the progression of oral submucous fibrosis through secreting TGF-β1. PeerJ11, e15158 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sharma, M. et al. Emerging role of cellular senescence in the pathogenesis of oral submucous fibrosis and its malignant transformation. Head. Neck43, 3153–3164 (2021). [DOI] [PubMed] [Google Scholar]
- 138.Aguilera, M. O. et al. Chronic infections: a possible scenario for autophagy and senescence cross-talk. Cells7, 162 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Migneault, F. et al. Autophagy, tissue repair, and fibrosis: a delicate balance. Matrix Biol.100-101, 182–196 (2021). [DOI] [PubMed] [Google Scholar]
- 140.Vescarelli, E. et al. Autophagy activation is required for myofibroblast differentiation during healing of oral mucosa. J. Clin. Periodontol.44, 1039–1050 (2017). [DOI] [PubMed] [Google Scholar]
- 141.Li, J. et al. Autophagy mediates oral submucous fibrosis. Exp. Ther. Med.11, 1859–1864 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhu, B. et al. Role of autophagy and apoptosis in atrophic epithelium in oral submucous fibrosis. J. Oral. Sci.62, 184–188 (2020). [DOI] [PubMed] [Google Scholar]
- 143.Dai, Z. et al. Role of autophagy induced by arecoline in angiogenesis of oral submucous fibrosis. Arch. Oral. Biol.102, 7–15 (2019). [DOI] [PubMed] [Google Scholar]
- 144.Zhong, X. et al. Oral microbiota alteration associated with oral cancer and areca chewing. Oral. Dis.27, 226–239 (2021). [DOI] [PubMed] [Google Scholar]
- 145.Zhang, W. L. et al. Who is who in oral cancer? Exp. Cell Res.384, 111634 (2019). [DOI] [PubMed] [Google Scholar]
- 146.Chen, L. et al. Potential role of host microbiome in areca nut-associated carcinogenesis and addiction. Molecules27, 8171 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sharma, M. et al. Limited mouth opening in oral submucous fibrosis: reasons, ramifications, and remedies. J. Oral. Pathol. Med.46, 424–430 (2017). [DOI] [PubMed] [Google Scholar]
- 148.Shih, Y. H. et al. Oral submucous fibrosis: a review on etiopathogenesis, diagnosis, and therapy. Int J. Mol. Sci.20, 2940 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guruprasad, R. et al. Serum vitamin C and iron levels in oral submucous fibrosis. Indian J. Dent.5, 81–85 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yadav, A. et al. Estimation of serum zinc, copper, and iron in the patients of oral submucous fibrosis. Natl J. Maxillofac. Surg.6, 190–193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jani, Y. V. et al. Evaluation of role of trace elements in oral submucous fibrosis patients: a study on Gujarati population. J. Oral. Maxillofac. Pathol.21, 455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kode, M. A. et al. Estimation of the serum and the salivary trace elements in OSMF patients. J. Clin. Diagn. Res.7, 1215–1218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mishra, S. et al. Estimation of salivary and serum lactate dehydrogenase in oral submucous fibrosis. J. Int Soc. Prev. Community Dent.8, 289–295 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aggarwal, A. et al. Estimation of serum beta carotene levels in patients with oral submucous fibrosis in India. J. Oral. Sci.53, 427–431 (2011). [DOI] [PubMed] [Google Scholar]
- 155.Paul, R. R. et al. A novel wavelet neural network based pathological stage detection technique for an oral precancerous condition. J. Clin. Pathol.58, 932–938 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Haris, P. S. et al. Autofluorescence spectroscopy for the in vivo evaluation of oral submucous fibrosis. Photomed. Laser Surg.27, 757–761 (2009). [DOI] [PubMed] [Google Scholar]
- 157.Chaturvedi, P. et al. Fluorescence spectroscopy for noninvasive early diagnosis of oral mucosal malignant and potentially malignant lesions. J. Cancer Res. Ther.6, 497–502 (2010). [DOI] [PubMed] [Google Scholar]
- 158.Sivabalan, S. et al. In vivo native fluorescence spectroscopy and nicotinamide adinine dinucleotide/flavin adenine dinucleotide reduction and oxidation states of oral submucous fibrosis for chemopreventive drug monitoring. J. Biomed. Opt.15, 017010 (2010). [DOI] [PubMed] [Google Scholar]
- 159.Wang, C. Y. et al. PLS-ANN-based classification model for oral submucous fibrosis and oral carcinogenesis. Lasers Surg. Med.32, 318–326 (2003). [DOI] [PubMed] [Google Scholar]
- 160.Vedeswari, C. P. et al. In vivo autofluorescence characteristics of pre- and post-treated oral submucous fibrosis: a pilot study. Indian J. Dent. Res.20, 261–267 (2009). [DOI] [PubMed] [Google Scholar]
- 161.Kumar, K. et al. Discrimination of oral submucous fibrosis (OSF) affected oral tissues from healthy oral tissues using multivariate analysis of in vivo fluorescence spectroscopic data: a simple and fast procedure for OSF diagnosis. Anal. Methods5, 3482–3489 (2013). [Google Scholar]
- 162.Lee, C. K. et al. Diagnosis of oral submucous fibrosis with optical coherence tomography. J. Biomed. Opt.14, 054008 (2009). [DOI] [PubMed] [Google Scholar]
- 163.Rai, V. et al. Serum-based diagnostic prediction of oral submucous fibrosis using FTIR spectrometry. Spectrochim. Acta A Mol. Biomol. Spectrosc.189, 322–329 (2018). [DOI] [PubMed] [Google Scholar]
- 164.Manjunath, K. et al. Evaluation of oral submucous fibrosis using ultrasonographic technique: a new diagnostic tool. Indian J. Dent. Res22, 530–536 (2011). [DOI] [PubMed] [Google Scholar]
- 165.Grover, S. et al. A comparative study for selectivity of micronuclei in oral exfoliated epithelial cells. J. Cytol.29, 230–235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rajalalitha, P. et al. Molecular pathogenesis of oral submucous fibrosis-a collagen metabolic disorder. J. Oral. Pathol. Med.34, 321–328 (2005). [DOI] [PubMed] [Google Scholar]
- 167.Gupta, D. et al. Oral submucous fibrosis-a new treatment regimen. J. Oral. Maxillofac. Surg.46, 830–833 (1988). [DOI] [PubMed] [Google Scholar]
- 168.Kakar, P. K. et al. Oral submucous fibrosis-treatment with hyalase. J. Laryngol. Otol.99, 57–59 (1985). [DOI] [PubMed] [Google Scholar]
- 169.Bae-Harboe, Y. S. et al. Collagenase followed by compression for the treatment of earlobe keloids. Dermatol Surg.40, 519–524 (2014). [DOI] [PubMed] [Google Scholar]
- 170.Akerman, J. et al. Treatment of Peyronie’s disease via preoperative intralesional collagenase clostridium histolyticum followed by placement of an inflatable penile prosthesis: the new standard of care? Transl. Androl. Urol.6, S822–s823 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lin, H. J. et al. Treatment of oral submucous fibrosis by collagenase: effects on oral opening and eating function. Oral. Dis.13, 407–413 (2007). [DOI] [PubMed] [Google Scholar]
- 172.Villegas, M. R. et al. Collagenase nanocapsules: an approach to fibrosis treatment. Acta Biomater.74, 430–438 (2018). [DOI] [PubMed] [Google Scholar]
- 173.Borle, R. M. et al. Management of oral submucous fibrosis: a conservative approach. J. Oral. Maxillofac. Surg.49, 788–791 (1991). [DOI] [PubMed] [Google Scholar]
- 174.Singh, M. et al. Efficacy of hydrocortisone acetate/hyaluronidase vs triamcinolone acetonide/hyaluronidase in the treatment of oral submucous fibrosis. Indian J. Med Res.131, 665–669 (2010). [PubMed] [Google Scholar]
- 175.Sirsat, S. M. et al. Subepithelial changes in oral submucous fibrosis. Acta Pathol. Microbiol. Scand.70, 161–173 (1967). [DOI] [PubMed] [Google Scholar]
- 176.Kar, I. B. et al. A rare ocular complication following treatment of oral submucous fibrosis with steroids. Natl J. Maxillofac. Surg.2, 93–95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Haque, M. F. et al. Interferon gamma (IFN-gamma) may reverse oral submucous fibrosis. J. Oral. Pathol. Med.30, 12–21 (2001). [DOI] [PubMed] [Google Scholar]
- 178.Krishnamoorthy, B. et al. Management of oral submucous fibrosis by two different drug regimens: a comparative study. Dent. Res J.10, 527–532 (2013). [PMC free article] [PubMed] [Google Scholar]
- 179.Tai, Y. S. et al. Oral administration of milk from cows immunized with human intestinal bacteria leads to significant improvements of symptoms and signs in patients with oral submucous fibrosis. J. Oral. Pathol. Med.30, 618–625 (2001). [DOI] [PubMed] [Google Scholar]
- 180.Lai, D. R. et al. Clinical evaluation of different treatment methods for oral submucous fibrosis. A 10-year experience with 150 cases. J. Oral. Pathol. Med.24, 402–406 (1995). [DOI] [PubMed] [Google Scholar]
- 181.Sharma, J. K. et al. Clinical experience with the use of peripheral vasodilator in oral disorders. Int. J. Oral. Maxillofac. Surg.16, 695–699 (1987). [DOI] [PubMed] [Google Scholar]
- 182.Bhadage, C. J. et al. Vasodilator isoxsuprine alleviates symptoms of oral submucous fibrosis. Clin. Oral. Investig.17, 1375–1382 (2013). [DOI] [PubMed] [Google Scholar]
- 183.Rajendran, R. et al. Pentoxifylline therapy: a new adjunct in the treatment of oral submucous fibrosis. Indian J. Dent. Res.17, 190–198 (2006). [DOI] [PubMed] [Google Scholar]
- 184.Liu, J. et al. Evaluating the efficacy of pentoxifylline in the treatment of oral submucous fibrosis: a meta-analysis. Oral. Dis.24, 706–716 (2018). [DOI] [PubMed] [Google Scholar]
- 185.Li, X. et al. Clinical treatment observation of tea pigment for oral submucous fibrosis. West Chin. J. Stomatol16, 50–52 (1998). [PubMed] [Google Scholar]
- 186.Kumar, A. et al. Efficacy of lycopene in the management of oral submucous fibrosis. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radio. Endod.103, 207–213 (2007). [DOI] [PubMed] [Google Scholar]
- 187.Guo, J. et al. Efficacy of lycopene in the treatment of oral submucous fibrosis: a meta-analysis of randomized controlled trials. J. Evid. Based Dent. Pract.20, 101471 (2020). [DOI] [PubMed] [Google Scholar]
- 188.Mulk, B. S. et al. Spirulina and pentoxyfilline - a novel approach for treatment of oral submucous fibrosis. J. Clin. Diagn. Res.7, 3048–3050 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Alam, S. et al. Efficacy of aloe vera gel as an adjuvant treatment of oral submucous fibrosis. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radio.116, 717–724 (2013). [DOI] [PubMed] [Google Scholar]
- 190.Sudarshan, R. et al. Aloe vera in the treatment for oral submucous fibrosis - a preliminary study. J. Oral. Pathol. Med.41, 755–761 (2012). [DOI] [PubMed] [Google Scholar]
- 191.Zheng, L. et al. Tanshinone suppresses arecoline-induced epithelial-mesenchymal transition in oral submucous fibrosis by epigenetically reactivating the p53 pathway. Oncol. Res.26, 483–494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dai, J. P. et al. Inhibition of tanshinone IIA, salvianolic acid A and salvianolic acid B on Areca nut extract-induced oral submucous fibrosis in vitro. Molecules20, 6794–6807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vinay, K. H. et al. Efficacy of curcumin in the treatment for oral submucous fibrosis—a randomized clinical trial. J. Oral. Maxillofac. Pathol.19, 145–152 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guo, Z. X. et al. A biomaterial-based therapy using a sodium hyaluronate/bioglass composite hydrogel for the treatment of oral submucous fibrosis. Acta Biomater.157, 639–654 (2023). [DOI] [PubMed] [Google Scholar]
- 195.Han, B. et al. Adipose-derived stem cell-derived extracellular vesicles inhibit the fibrosis of fibrotic buccal mucosal fibroblasts via the microRNA-375/FOXF1 Axis. Stem Cells Int2021, 9964159 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Neighbors, M. et al. Prognostic and predictive biomarkers for patients with idiopathic pulmonary fibrosis treated with pirfenidone: post-hoc assessment of the CAPACITY and ASCEND trials. Lancet Respir. Med.6, 615–626 (2018). [DOI] [PubMed] [Google Scholar]
- 197.Volkmann, E. R. et al. Treatment of systemic sclerosis-related interstitial lung disease: a review of existing and emerging therapies. Ann. Am. Thorac. Soc.13, 2045–2056 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cardoneanu, A. et al. Targeting systemic sclerosis from pathogenic mechanisms to clinical manifestations: why IL-6? Biomedicines10, 318 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Warnakulasuriya, S. et al. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral. Pathol. Med.36, 575–580 (2007). [DOI] [PubMed] [Google Scholar]
- 200.Raju, K. L. et al. Expression of hTERT in oral submucous fibrosis and oral squamous cell carcinoma—an immunohistochemical analysis. Pathol. Oncol. Res.26, 1573–1582 (2020). [DOI] [PubMed] [Google Scholar]
- 201.Lunde, M. L. et al. Profiling of chromosomal changes in potentially malignant and malignant oral mucosal lesions from South and South-East Asia using array-comparative genomic hybridization. Cancer Genom. Proteom.11, 127–140 (2014). [PubMed] [Google Scholar]
- 202.Dave, B. J. et al. Role of areca nut consumption in the cause of oral cancers. A cytogenetic assessment. Cancer70, 1017–1023 (1992). [DOI] [PubMed] [Google Scholar]
- 203.Lin, Y. C. et al. Polymorphisms of COX-2 -765G>C and p53 codon 72 and risks of oral squamous cell carcinoma in a Taiwan population. Oral. Oncol.44, 798–804 (2008). [DOI] [PubMed] [Google Scholar]
- 204.Teh, M. T. et al. Fingerprinting genomic instability in oral submucous fibrosis. J. Oral. Pathol. Med.37, 430–436 (2008). [DOI] [PubMed] [Google Scholar]
- 205.Zhou, S. et al. Expression and promoter methylation of Wnt inhibitory factor-1 in the development of oral submucous fibrosis. Oncol. Rep.34, 2636–2642 (2015). [DOI] [PubMed] [Google Scholar]
- 206.Zhou, S. et al. Exosome-derived long non-coding RNA ADAMTS9-AS2 suppresses progression of oral submucous fibrosis via AKT signalling pathway. J. Cell Mol. Med.25, 2262–2273 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang, J. et al. The circEPSTI1/mir-942-5p/LTBP2 axis regulates the progression of OSCC in the background of OSF via EMT and the PI3K/Akt/mTOR pathway. Cell Death Dis.11, 682 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhou, S. et al. The phosphorylation of survivin Thr34 by p34cdc2 in carcinogenesis of oral submucous fibrosis. Oncol. Rep.20, 1085–1091 (2008). [PubMed] [Google Scholar]
- 209.Zhou, S. H. et al. Molecules of G(2)/M phase and the phosphorylation of survivin in the carcinogenesis of oral submucosal fibrosis. Chin. J. Stomatol.43, 709–712 (2008). [PubMed] [Google Scholar]
- 210.Mallick, S. et al. Human oral cancers have altered expression of Bcl-2 family members and increased expression of the anti-apoptotic splice variant of Mcl-1. J. Pathol.217, 398–407 (2009). [DOI] [PubMed] [Google Scholar]
- 211.Mishra, R. et al. Cyclin D1 expression and its possible regulation in chewing tobacco mediated oral squamous cell carcinoma progression. Arch. Oral. Biol.54, 917–923 (2009). [DOI] [PubMed] [Google Scholar]
- 212.Haniffa, A. M. et al. Expression pattern of p63 in oral epithelial lesions and submucous fibrosis associated with betel-quid chewing in Sri Lanka. Med. Mol. Morphol.40, 203–207 (2007). [DOI] [PubMed] [Google Scholar]
- 213.Bag, S. et al. Computational analysis of p63(+) nuclei distribution pattern by graph theoretic approach in an oral pre-cancer (sub-mucous fibrosis). J. Pathol. Inf.4, 35 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pereira, T. et al. Qualitative expression of hypoxia-inducible factor-1α in malignant transformation of oral submucous fibrosis: An immunohistochemical study. J. Oral. Maxillofac. Pathol.24, 106–112 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chaudhary, M. et al. The domino effect: Role of hypoxia in malignant transformation of oral submucous fibrosis. J. Oral. Maxillofac. Pathol.19, 122–127 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tilakaratne, W. M. et al. Upregulation of HIF-1alpha in malignant transformation of oral submucous fibrosis. J. Oral. Pathol. Med.37, 372–377 (2008). [DOI] [PubMed] [Google Scholar]
- 217.Silva, L. C. et al. CD1a+ and CD207+ cells are reduced in oral submucous fibrosis and oral squamous cell carcinoma. Med Oral. Patol. Oral. Cir. Bucal25, e49–e55 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hosur, M. B. et al. Evaluation of immunohistochemical expression of epithelial- mesenchymal transition markers E-cadherin, Twist and Snail in oral submucous fibrosis and their possible association with malignant transformation. J. Oral. Maxillofac. Pathol.25, 97–104 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sarode, S. C. et al. Dysplastic features relevant to malignant transformation in atrophic epithelium of oral submucous fibrosis: a preliminary study. J. Oral. Pathol. Med47, 410–416 (2018). [DOI] [PubMed] [Google Scholar]
- 220.Sharma, M. et al. Loss of oral mucosal stem cell markers in oral submucous fibrosis and their reactivation in malignant transformation. Int. J. Oral. Sci.12, 23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sharma, M. et al. Role of Yes-associated protein and transcriptional coactivator with PDZ-binding motif in the malignant transformation of oral submucous fibrosis. Arch. Oral. Biol.128, 105164 (2021). [DOI] [PubMed] [Google Scholar]
- 222.Gandhi, P. et al. Evaluation of myofibroblasts in oral submucous fibrosis and oral squamous cell carcinoma: The pathogenesis and correlation. Dent. Res. J.14, 314–320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang, P. et al. Molecular mechanisms of malignant transformation of oral submucous fibrosis by different betel quid constituents-does fibroblast senescence play a role? Int. J. Mol. Sci.23, 1637 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shan-Shan, Z. et al. A rat model of oral submucous fibrosis induced by bleomycin. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radio.122, 216–223 (2016). [DOI] [PubMed] [Google Scholar]
- 225.Qi-Tao, W. et al. Development of a mouse model of arecoline-induced oral mucosal fibrosis. Asian Pac. J. Trop. Med.10, 1177–1184 (2017). [DOI] [PubMed] [Google Scholar]
- 226.Pal, S. et al. Plant leads for mitigation of oral submucous fibrosis: current scenario and future prospect. Oral Dis. 30, 80–99 (2022). [DOI] [PubMed] [Google Scholar]
- 227.McCallum, G. et al. The gut microbiota and its biogeography. Nat. Rev. Microbiol.22, 105–118 (2023). [DOI] [PubMed] [Google Scholar]