Abstract
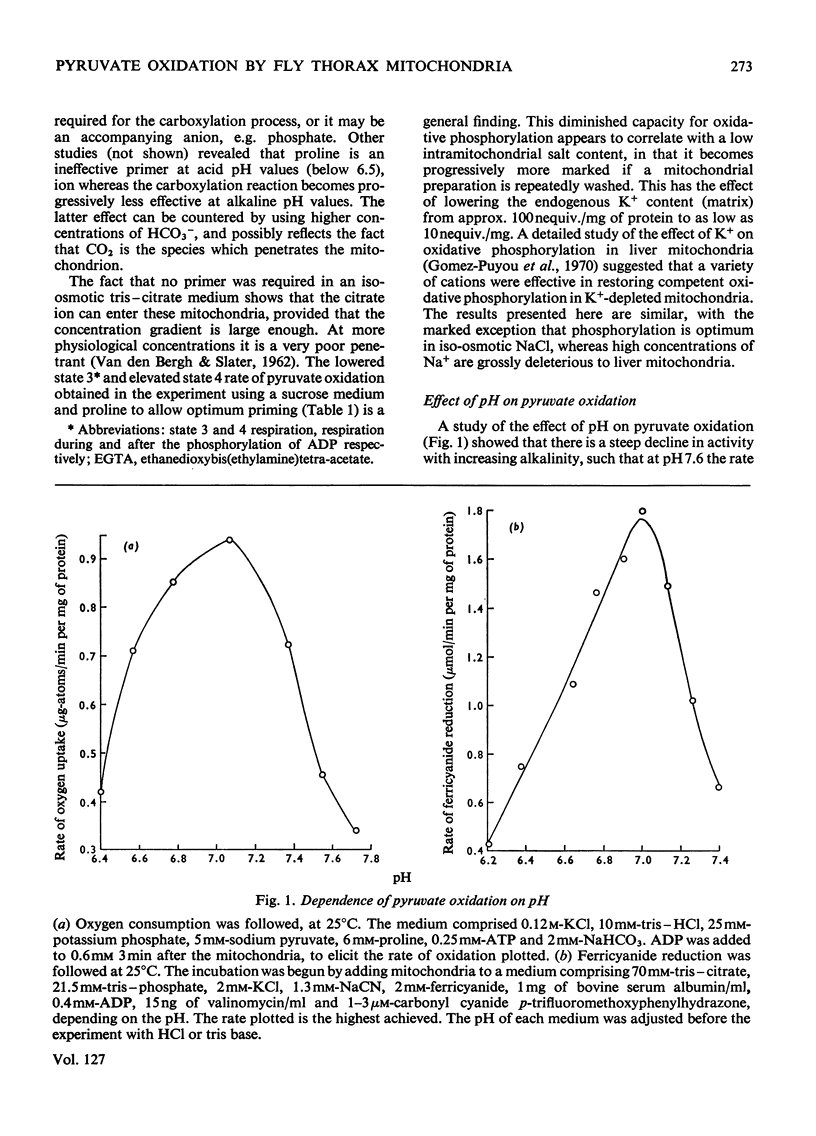
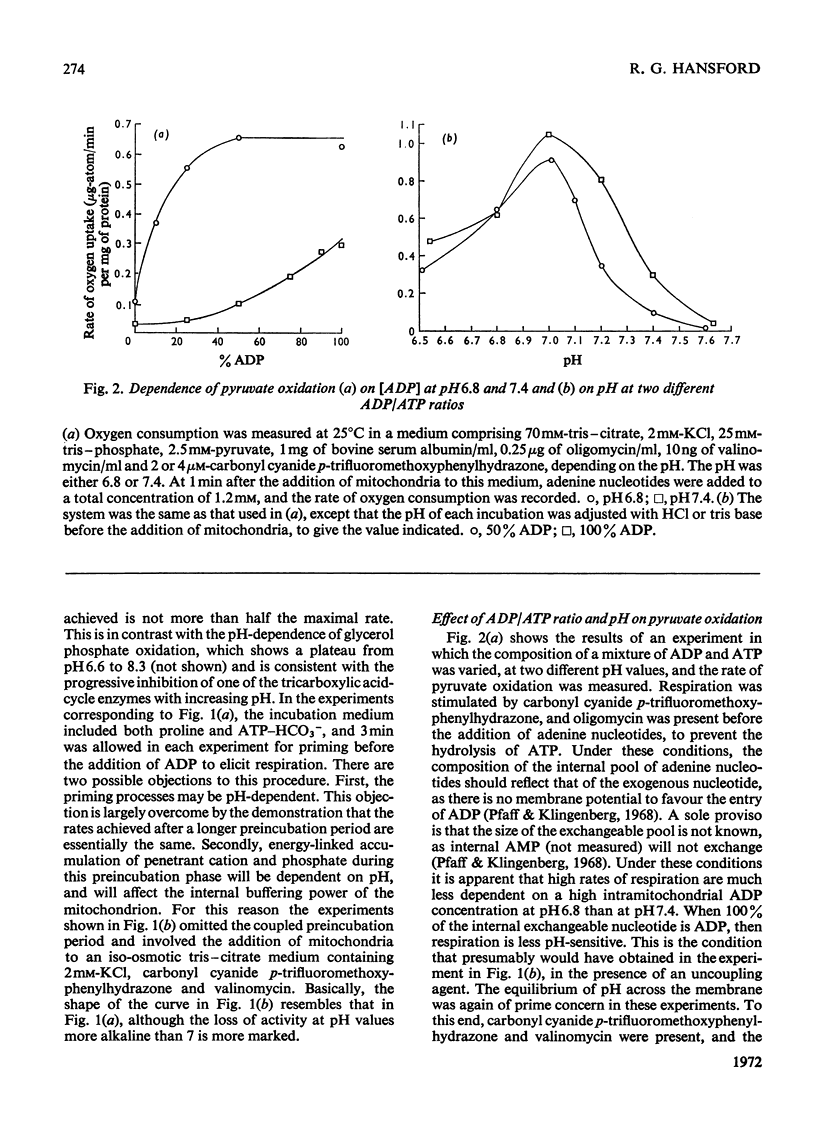
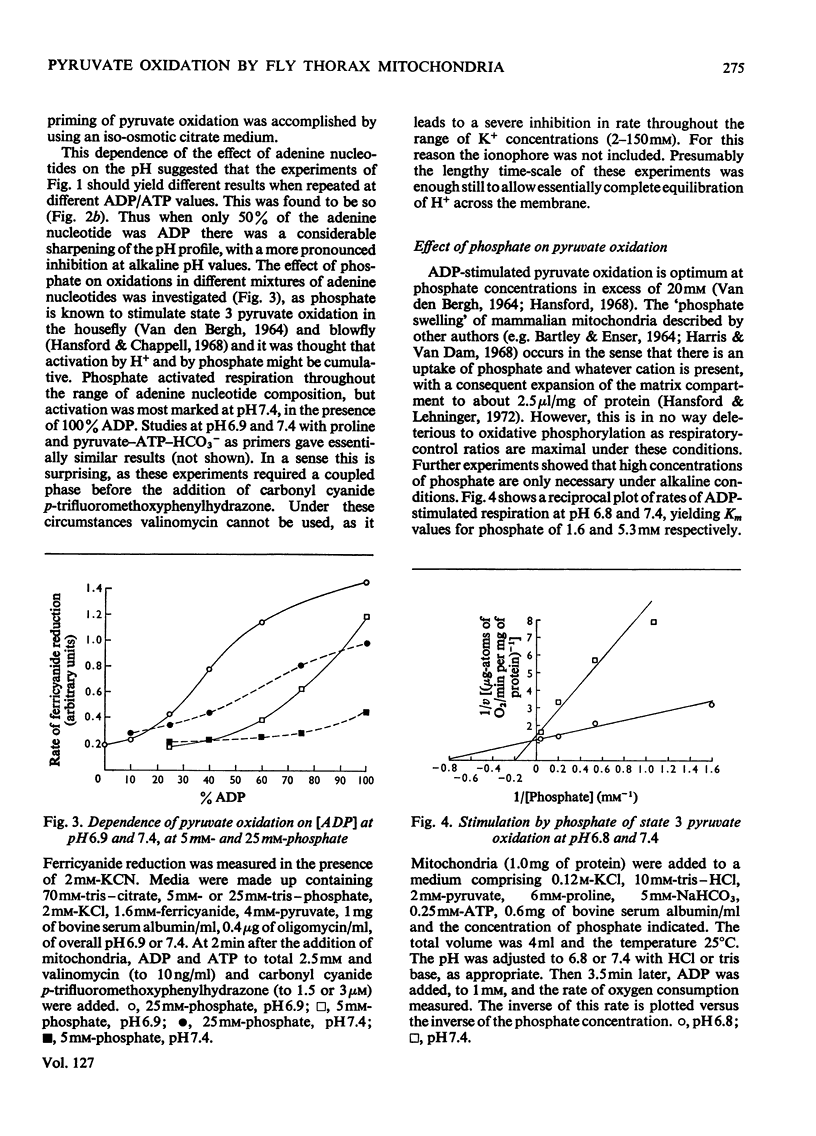
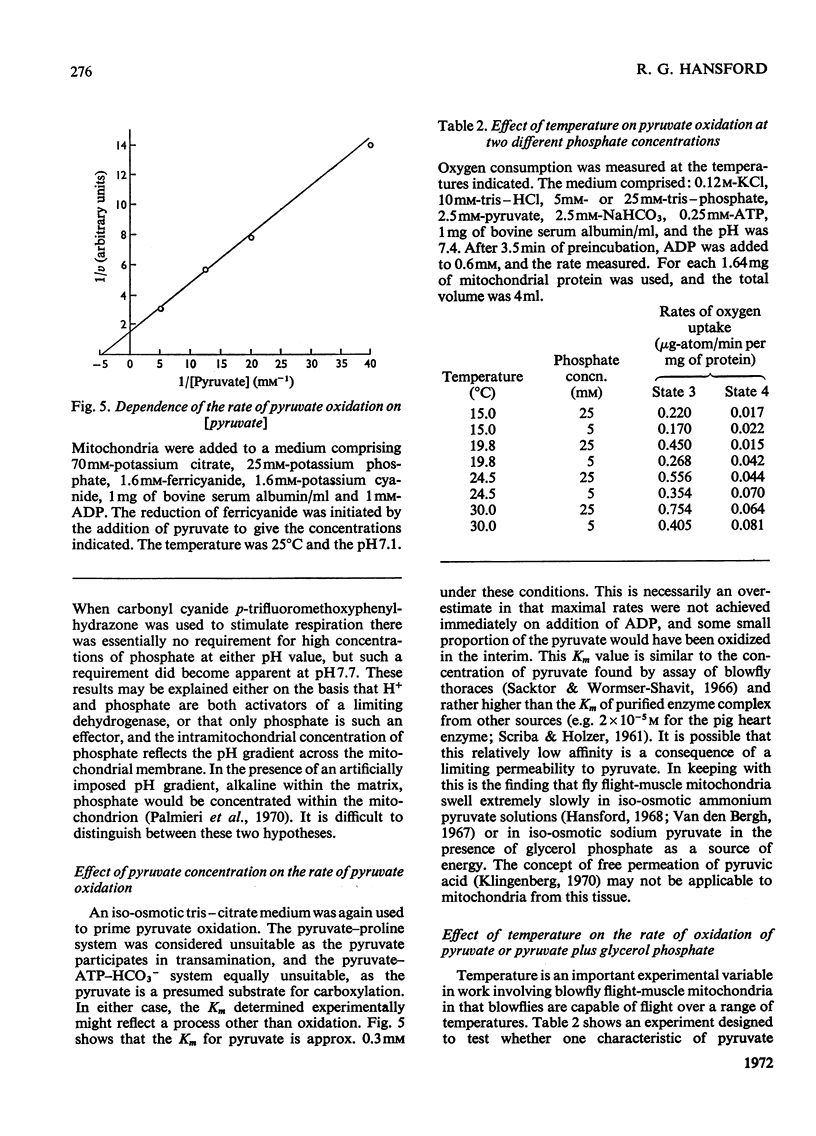
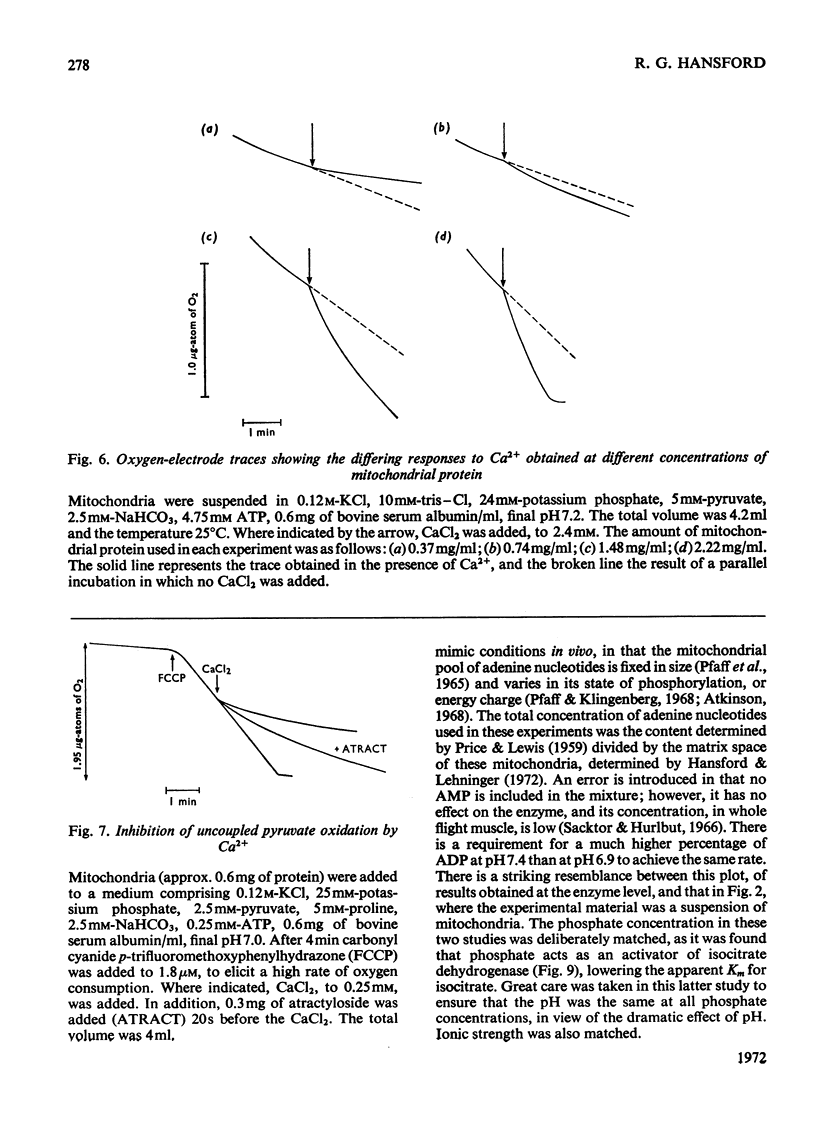
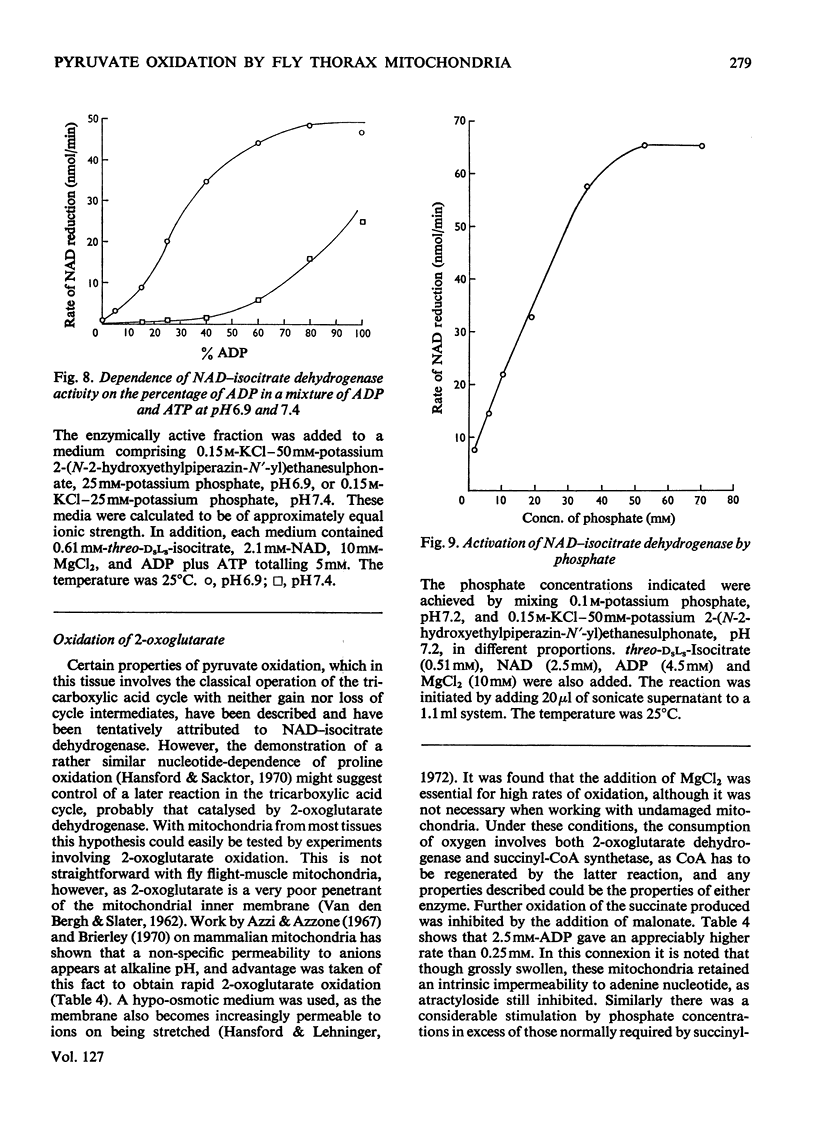
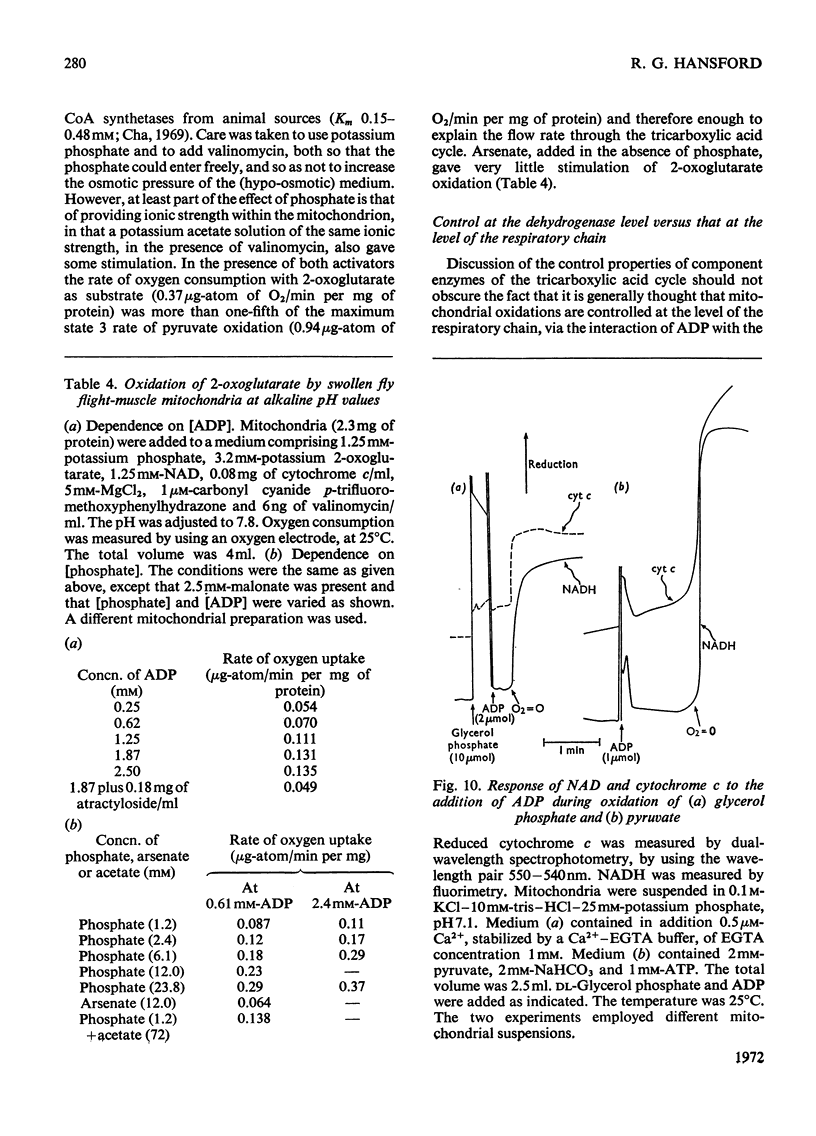
1. High rates of state 3 pyruvate oxidation are dependent on high concentrations of inorganic phosphate and a predominance of ADP in the intramitochondrial pool of adenine nucleotides. The latter requirement is most marked at alkaline pH values, where ATP is profoundly inhibitory. 2. Addition of CaCl2 during state 4, state 3 (Chance & Williams, 1955) or uncoupled pyruvate oxidation causes a marked inhibition in the rate of oxygen uptake when low concentrations of mitochondria are employed, but may lead to an enhancement of state 4 oxygen uptake when very high concentrations of mitochondria are used. 3. These properties are consistent with the kinetics of the NAD-linked isocitrate dehydrogenase (EC 1.1.1.41) from this tissue, which is activated by isocitrate, citrate, ADP, phosphate and H+ ions, and inhibited by ATP, NADH and Ca2+. 4. Studies of the redox state of NAD and cytochrome c show that addition of ADP during pyruvate oxidation causes a slight reduction, whereas addition during glycerol phosphate oxidation causes a `classical' oxidation. Nevertheless, it is concluded that pyruvate oxidation is probably limited by the respiratory chain in state 4 and by the NAD-linked isocitrate dehydrogenase in state 3. 5. The oxidation of 2-oxoglutarate by swollen mitochondria is also stimulated by high concentrations of ADP and phosphate, and is not uncoupled by arsenate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Azzi A., Azzone G. F. Swelling and shrinkage phenomena in liver mitochondria. VI. Metabolism-independent swelling coupled to ion movement. Biochim Biophys Acta. 1967 May 9;131(3):468–478. doi: 10.1016/0005-2728(67)90006-0. [DOI] [PubMed] [Google Scholar]
- Azzi A. Redistribution of the electrical charge of the mitochondrial membrane during energy conservation. Biochem Biophys Res Commun. 1969 Oct 8;37(2):254–260. doi: 10.1016/0006-291x(69)90727-x. [DOI] [PubMed] [Google Scholar]
- Bartley W., Enser M. B. The swelling and contraction of isolated rat-liver mitochondria. Biochem J. 1964 Nov;93(2):322–330. doi: 10.1042/bj0930322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley G. P. Energy-linked alteration of the permeability of heart mitochondria to chloride and other anions. Biochemistry. 1970 Feb 17;9(4):697–707. doi: 10.1021/bi00806a001. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- CHEFURKA W. On the importance of alpha-glycerophosphate dehydrogenase in glycolysing insect muscle. Biochim Biophys Acta. 1958 Jun;28(3):660–661. doi: 10.1016/0006-3002(58)90545-6. [DOI] [PubMed] [Google Scholar]
- CHEN R. F., PLAUT G. W. ACTIVATION AND INHIBITION OF DPN-LINKED ISOCITRATE DEHYDROGENASE OF HEART BY CERTAIN NUCLEOTIDES. Biochemistry. 1963 Sep-Oct;2:1023–1032. doi: 10.1021/bi00905a020. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Hansford R. G., Sackton B., Lehninger A. L. Interaction of Ca2+ with blowfly flight muscle mitochondria. J Biol Chem. 1971 Feb 25;246(4):964–972. [PubMed] [Google Scholar]
- Childress C. C., Sacktor B. Pyruvate oxidation and the permeability of mitochondria from blowfly flight muscle. Science. 1966 Oct 14;154(3746):268–270. doi: 10.1126/science.154.3746.268. [DOI] [PubMed] [Google Scholar]
- Gómez-Puyou A., Sandoval F., Chávez E., Tuena M. On the role of K+ on oxidative phosphorylation. J Biol Chem. 1970 Oct 25;245(20):5239–5247. [PubMed] [Google Scholar]
- Hansford R. G., Chappell J. B. The effect of Ca2+ on the oxidation of glycerol phosphate by blowfly flight-muscle mitochondria. Biochem Biophys Res Commun. 1967 Jun 23;27(6):686–692. doi: 10.1016/s0006-291x(67)80090-1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G., Lehninger A. L. The effect of the coupled oxidation of substrate on the permeability of blowfly flight-muscle mitochondria to potassium and other cations. Biochem J. 1972 Feb;126(3):689–700. doi: 10.1042/bj1260689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Sacktor B. The control of the oxidation of proline by isolated flight muscle mitochondria. J Biol Chem. 1970 Mar 10;245(5):991–994. [PubMed] [Google Scholar]
- Harris E. J., van Dam K. Changes of total water and sucrose space accompanying induced ion uptake or phosphate swelling of rat liver mitochondria. Biochem J. 1968 Feb;106(3):759–766. doi: 10.1042/bj1060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Klingenberg M., Palmieri F., Quagliariello E. Quantitative correlation between the distribution of anions and the pH difference across the mitochondrial membrane. Eur J Biochem. 1970 Dec;17(2):230–238. doi: 10.1111/j.1432-1033.1970.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- PRICE G. M., LEWIS S. E. Distribution of phosphorus compounds in blowfly thoracic muscle. Biochem J. 1959 Jan;71(1):176–185. doi: 10.1042/bj0710176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M. Adenine nucleotide translocation of mitochondria. 1. Specificity and control. Eur J Biochem. 1968 Oct 17;6(1):66–79. doi: 10.1111/j.1432-1033.1968.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M., Heldt H. W. Unspecific permeation and specific exchange of adenine nucleotides in liver mitochondria. Biochim Biophys Acta. 1965 Jun 15;104(1):312–315. doi: 10.1016/0304-4165(65)90258-8. [DOI] [PubMed] [Google Scholar]
- Randle P. J., England P. J., Denton R. M. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 1970 May;117(4):677–695. doi: 10.1042/bj1170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCRIBA P., HOLZER H. [Production of alpha-hydroxyethyl-2-thiamine pyrophosphate with pyruvate oxidase from pig heart muscle]. Biochem Z. 1961;334:473–486. [PubMed] [Google Scholar]
- SLATER E. C. Mechanism of phosphorylation in the respiratory chain. Nature. 1953 Nov 28;172(4387):975–978. doi: 10.1038/172975a0. [DOI] [PubMed] [Google Scholar]
- Sacktor B., Hurlbut E. C. Regulation of metabolism in working muscle in vivo. II. Concentrations of adenine nucleotides, arginine phosphate, and inorganic phosphate in insect flight muscle during flight. J Biol Chem. 1966 Feb 10;241(3):632–634. [PubMed] [Google Scholar]
- Sacktor B., Wormser-Shavit E. Regulation of metabolism in working muscle in vivo. I. Concentrations of some glycolytic, tricarboxylic acid cycle, and amino acid intermediates in insect flight muscle during flight. J Biol Chem. 1966 Feb 10;241(3):624–631. [PubMed] [Google Scholar]
- Vaughan H., Newsholme E. A. The effects of Ca(2+) and ADP on the activity of NAD-linked isocitrate dehydrogenase of muscle. FEBS Lett. 1969 Oct 21;5(2):124–126. doi: 10.1016/0014-5793(69)80311-x. [DOI] [PubMed] [Google Scholar]
- Wieland O., Siess E. Interconversion of phospho- and dephospho- forms of pig heart pyruvate dehydrogenase. Proc Natl Acad Sci U S A. 1970 Apr;65(4):947–954. doi: 10.1073/pnas.65.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEBE E. C., MCSHAN W. H. Lactic and alpha-glycerophosphate dehydrogenases in insects. J Gen Physiol. 1957 May 20;40(5):779–790. doi: 10.1085/jgp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den BERGH S., SLATER E. C. The respiratory activity and permeability of housefly sarcosomes. Biochem J. 1962 Feb;82:362–371. doi: 10.1042/bj0820362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bergh S. G. Pyruvate oxidation and the permeability of housefly sarcosomes. Biochem J. 1964 Oct;93(1):128–136. doi: 10.1042/bj0930128. [DOI] [PMC free article] [PubMed] [Google Scholar]


