Abstract
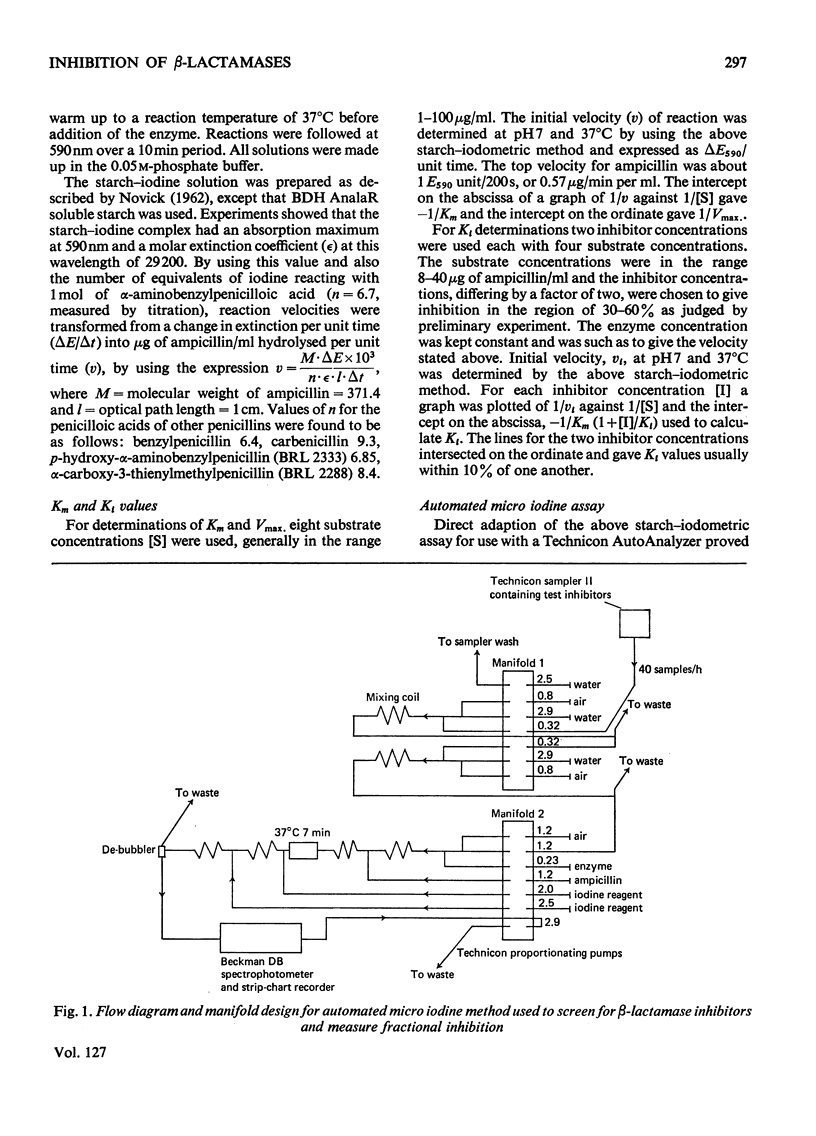
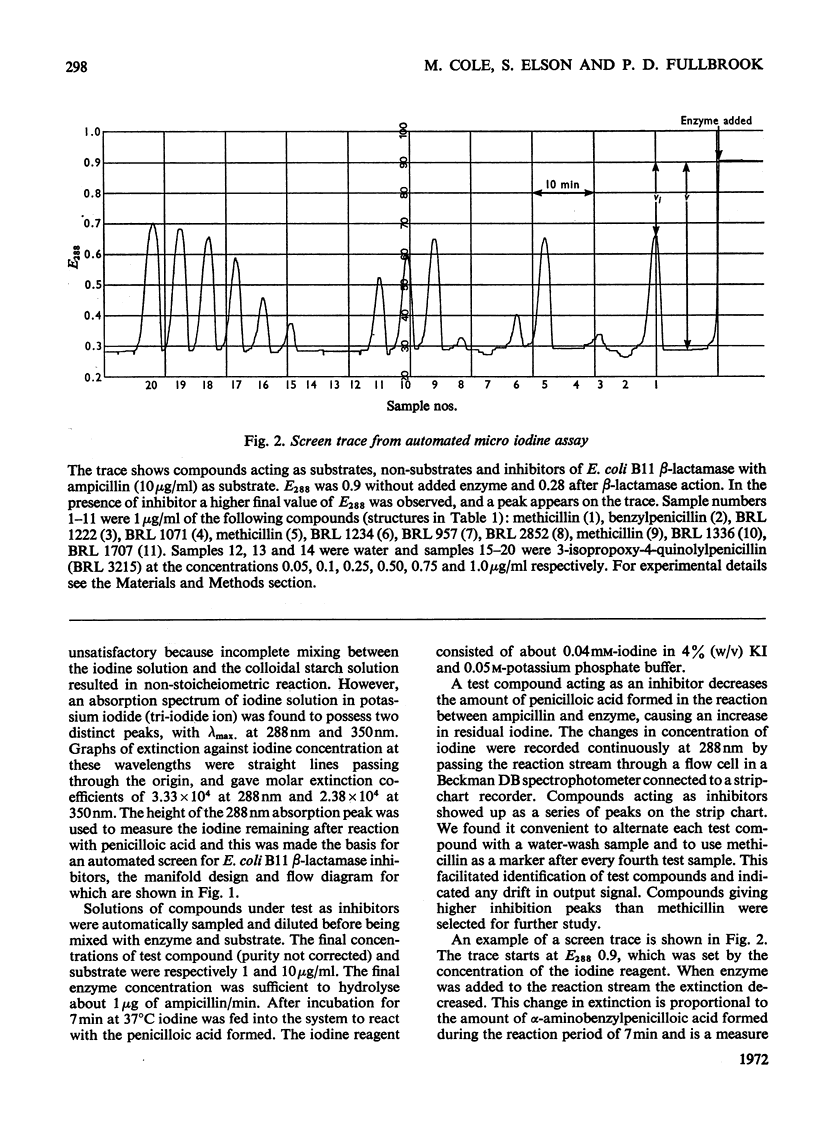
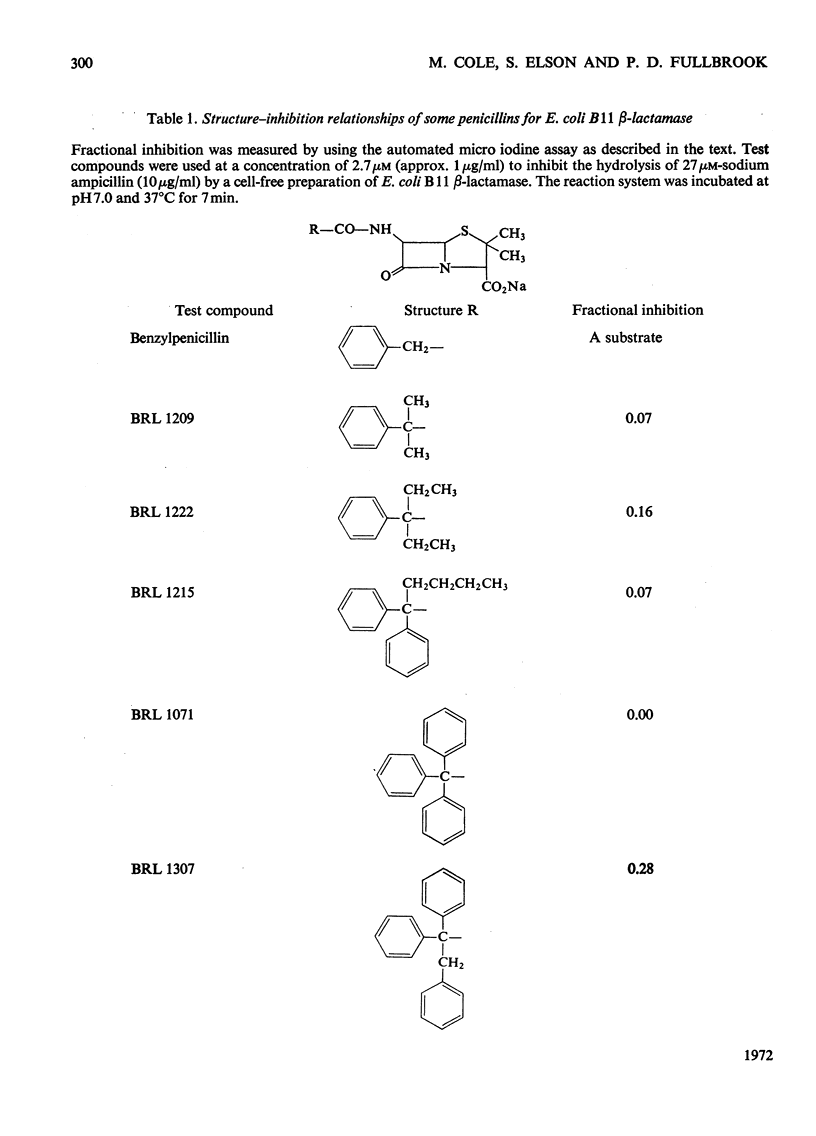
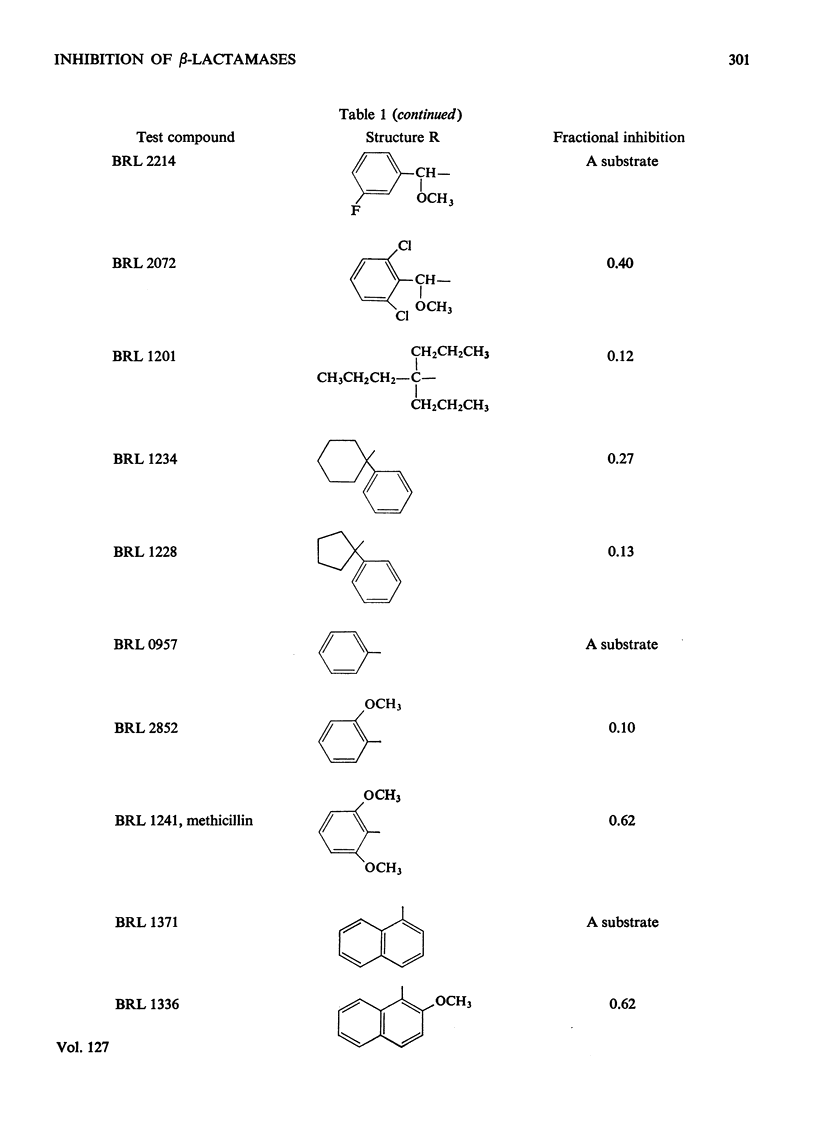
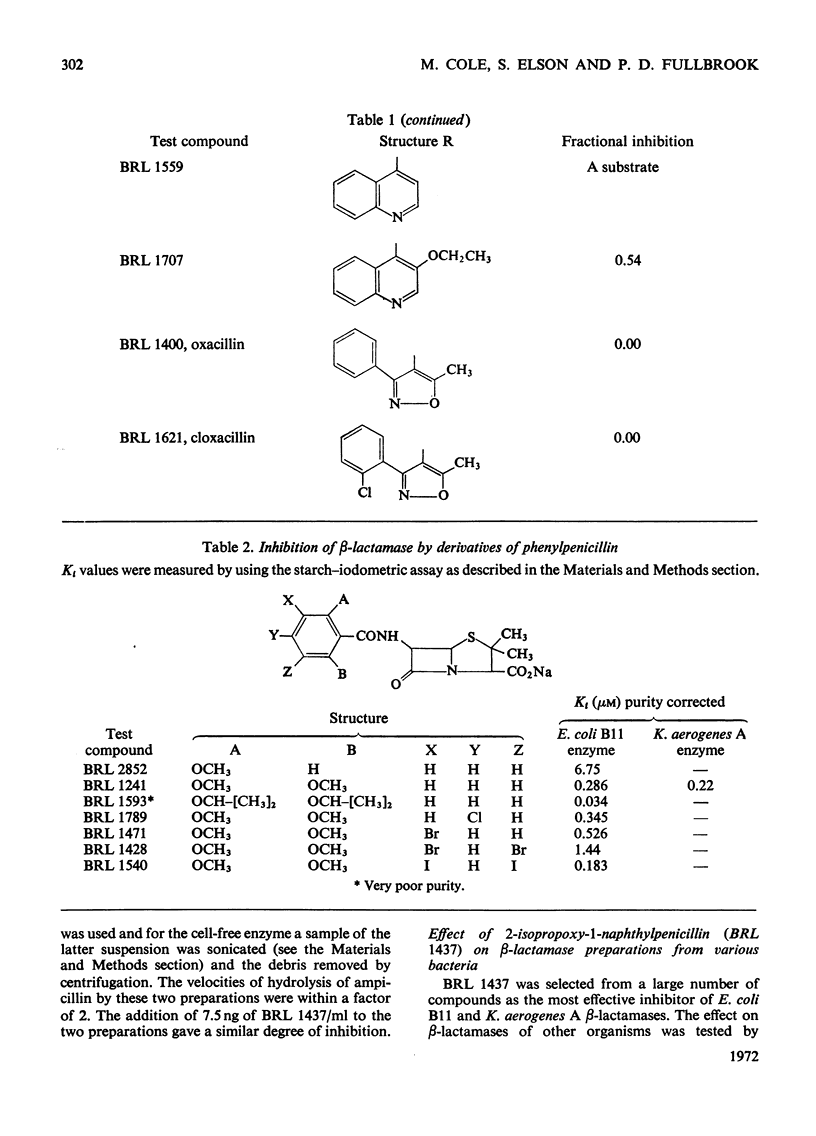
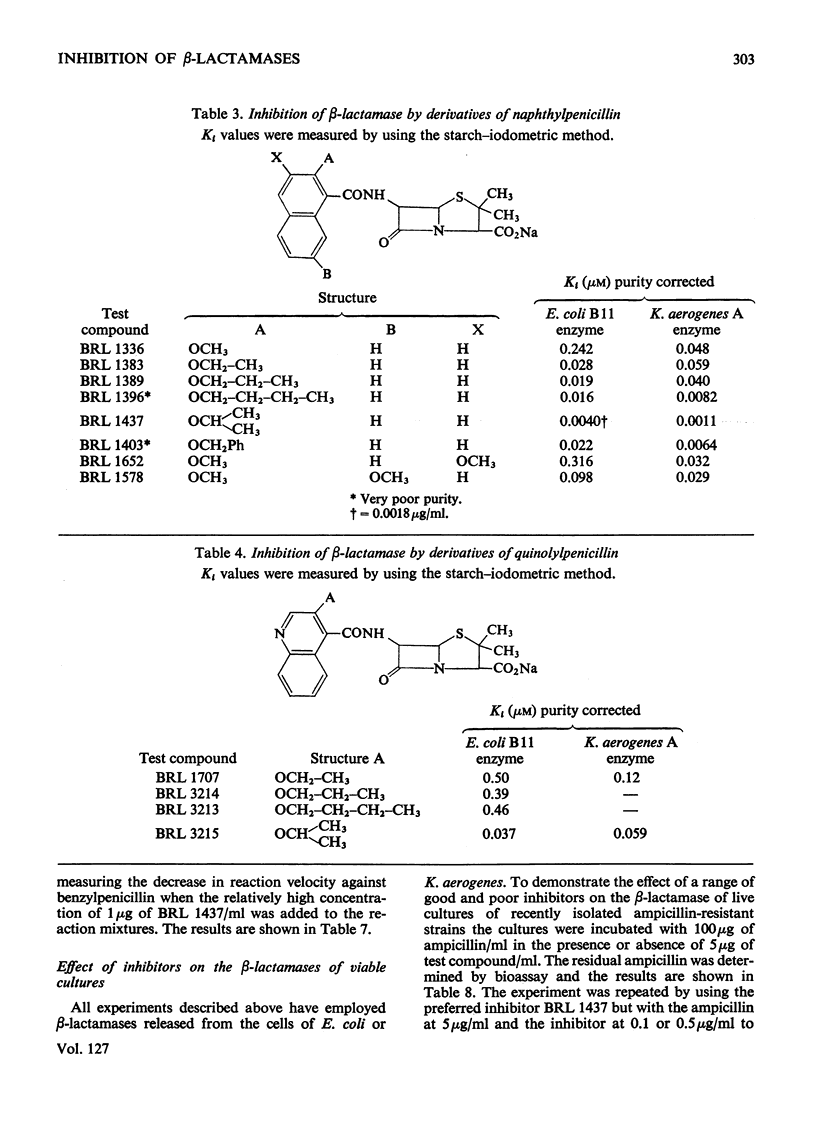
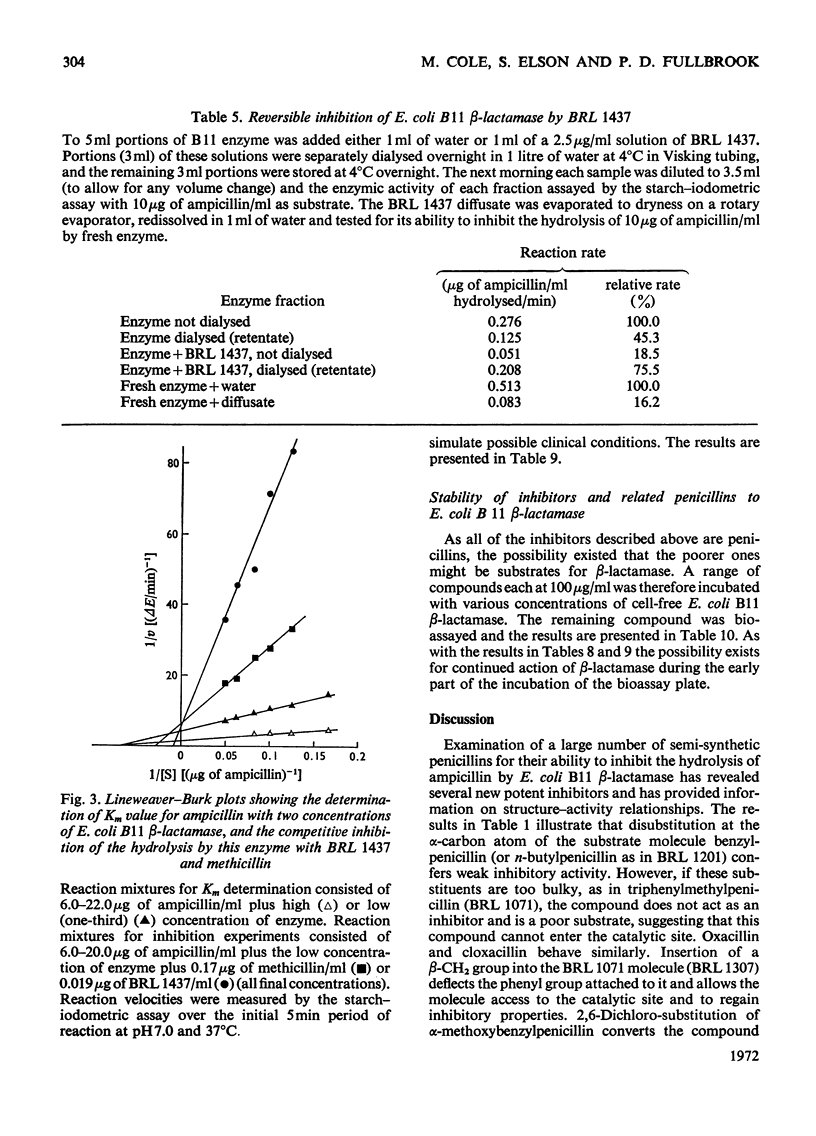
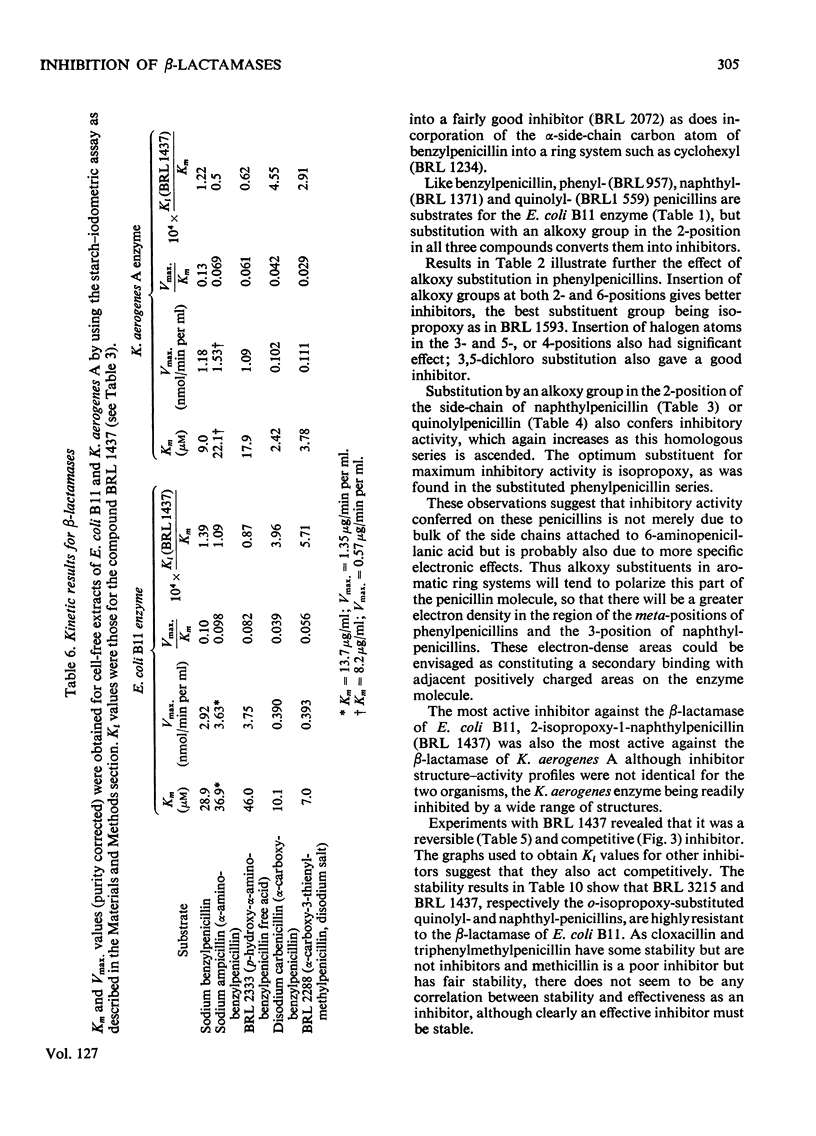
1. A new automated micro-iodometric method is described for screening compounds for inhibitory action against β-lactamase enzymes. 2. Over 1000 semi-synthetic penicillins were tested for inhibitory activity against the β-lactamase of Escherichia coli B11 and 18 showed a fractional inhibition similar to or higher than that of methicillin. 3. The best inhibitors were alkoxy- and halogen-substituted phenyl-, naphthyl- or quinolyl-penicillins. 2-Isopropoxy-1-naphthylpenicillin (BRL 1437) was clearly the best and had a Ki value about 1% of that of methicillin. 4. The inhibition of the β-lactamase of E. coli B11 by BRL 1437 was shown to be reversible and competitive. The Ki was 0.004μm and Ki/Km with ampicillin and p-hydroxyampicillin (BRL 2333) was about 0.0001. The Km and Vmax. values were determined for the β-lactamases of E. coli B11 and Klebsiella aerogenes A against a variety of penicillins. Cell-bound and solubilized enzymes gave similar Ki and Km values. 5. BRL 1437 was superior to cloxacillin and methicillin for inhibition of the β-lactamase of live, fully grown cultures of several strains of E. coli and K. aerogenes. Of a group of inhibitors BRL 1437 was the most stable to the β-lactamase of E. coli B11.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYLIFFE G. A. Ampicillin inactivation and sensitivity of coliform bacilli. J Gen Microbiol. 1963 Feb;30:339–348. doi: 10.1099/00221287-30-2-339. [DOI] [PubMed] [Google Scholar]
- Bach J. A., Buono N., Chisholm D., Price K. E., Pursiano T. A., Gourevitch A. In vitro and in vivo synergism of mixtures of penicillins. Antimicrob Agents Chemother (Bethesda) 1966;6:328–336. [PubMed] [Google Scholar]
- Cole M., Sutherland R. The role of penicillin acylase in the resistance of gram-negative bacteria to penicillins. J Gen Microbiol. 1966 Mar;42(3):345–356. doi: 10.1099/00221287-42-3-345. [DOI] [PubMed] [Google Scholar]
- Croydon E. A., Sutherland R. -amino-p-hydroxybenzylpenicillin (BRL 2333), a new semisynthetic penicillin: absorption and excretion in man. Antimicrob Agents Chemother (Bethesda) 1970;10:427–430. [PubMed] [Google Scholar]
- HAMILTON-MILLER J. M. Penicillinase from Klebsiella aerogenes. A comparison with penicillinases from gram-positive species. Biochem J. 1963 Apr;87:209–214. doi: 10.1042/bj0870209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON-MILLER J. M., SMITH J. T. INHIBITION OF PENICILLINASES FROM GRAM-POSITIVE AND GRAM-NEGATIVE BACTERIA BY SUBSTRATE ANALOGUES. Nature. 1964 Mar 7;201:999–1001. doi: 10.1038/201999a0. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M., Smith J. T., Knox R. Interaction of cephaloridine with penicillinase-producing gram-negative bacteria. Nature. 1965 Oct 16;208(5007):235–237. doi: 10.1038/208235a0. [DOI] [PubMed] [Google Scholar]
- JAGO M., MIGLIACCI A., ABRAHAM E. P. PRODUCTION OF A CEPHALOSPORINASE BY PSEUDOMONAS PYOCYANEA. Nature. 1963 Jul 27;199:375–375. doi: 10.1038/199375a0. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Muggleton P. W., Kirby S. M., Ryan D. M. Inhibition of beta-lactamase decomposition of cephaloridine and cephalothin by other cephalosporins. Antimicrob Agents Chemother (Bethesda) 1966;6:337–343. [PubMed] [Google Scholar]
- PERCIVAL A., BRUMFITT W., DE LOUVOIS J. THE ROLE OF PENICILLINASE IN DETERMINING NATURAL AND ACQUIRED RESISTANCE OF GRAM-NEGATIVE BACTERIA TO PENICILLINS. J Gen Microbiol. 1963 Jul;32:77–89. doi: 10.1099/00221287-32-1-77. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTHERLAND R., BATCHELOR F. R. SYNERGISTIC ACTIVITY OF PENICILLINS AGAINST PENICILLINASE-PRODUCING GRAM-NEGATIVE BACILLI. Nature. 1964 Feb 29;201:868–869. doi: 10.1038/201868a0. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND R. THE NATURE OF THE INSENSITIVITY OF GRAM-NEGATIVE BACTERIA TOWARDS PENICILLINS. J Gen Microbiol. 1964 Jan;34:85–98. doi: 10.1099/00221287-34-1-85. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Finland M. Resistance of penicillins and cephalosporins to beta-lactamases from Gram-negative bacilli: some correlations with antibacterial activity. Ann N Y Acad Sci. 1967 Sep 27;145(2):237–247. doi: 10.1111/j.1749-6632.1967.tb50222.x. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., McCall C. E., Steigbigel N. H., Finland M. Synergistic penicillin combinations for treatment of human urinary-tract infections. Antimicrob Agents Chemother (Bethesda) 1966;6:149–155. [PubMed] [Google Scholar]
- Sutherland R., Burnett J., Rolinson G. N. -carboxy-3-thienylmethylpenicillin (BRL 2288), a new semisynthetic penicillin: in vitro evaluation. Antimicrob Agents Chemother (Bethesda) 1970;10:390–395. [PubMed] [Google Scholar]
- Sutherland R., Rolinson G. N. -amino-p-hydroxybenzylpenicillin (BRL 2333), a new semisynthetic penicillin: in vitro evaluation. Antimicrob Agents Chemother (Bethesda) 1970;10:411–415. doi: 10.1128/AAC.10.3.411. [DOI] [PubMed] [Google Scholar]


