Abstract
Patients with ifosfamide-induced renal damage present with Fanconi syndrome. Karyomegalic nephropathy/interstitial nephritis (KNIN) is a rare form of chronic tubulo-interstitial nephritis that was initially considered a type of familial nephropathy. However, several reports of drug-induced KNIN, i.e., KNIN-like nephropathy, have been reported in recent years. We present the case of an 18-year-old man who presented with Fanconi syndrome and progressive renal dysfunction after receiving chemotherapy including ifosfamide and cisplatin for right femoral osteosarcoma. Renal biopsy revealed numerous atrophied tubular epithelial cells with large, polymorphic nuclei, and the definitive diagnosis was KNIN. Most patients with KNIN-like nephropathy who receive ifosfamide are concomitantly treated with cisplatin, indicating that ifosfamide and cisplatin might act synergistically to increase the risk for KNIN-like nephropathy. Further investigation in case series is warranted to reveal potential treatment approaches and to evaluate prognosis.
Keywords: Karyomegalic nephropathy/interstitial nephritis, Ifosfamide, Fanconi syndrome
Introduction
Ifosfamide is an alkylating agent that interferes with DNA replication by forming cross-links and leads to abnormal base pairing between DNA duplexes in tumor cell DNA [1]. Known nephrotoxic effects of ifosfamide include tubular acidosis, enuresis, and Fanconi syndrome. In general, drug-induced Fanconi syndrome tends to be mild and resolves following drug discontinuation in most cases. However, a small number of patients presenting with ifosfamide-induced renal impairment exhibit renal biopsy findings of karyomegalic nephropathy/interstitial nephritis (KNIN). Here, we report a patient who developed Fanconi syndrome and KNIN after treatment with ifosfamide, cisplatin, and adriamycin for osteosarcoma.
KNIN, named by Mihatsch et al. in 1979, is a rare form of chronic tubulo-interstitial nephritis characterized by atypical tubular epithelial cells including large, hyperchromatic nuclei with irregular contours [2, 3]. KNIN was initially assumed to be associated with a family history of recurrent upper respiratory tract infections and progressive renal failure [4]. Recently, cases of drug-induced KNIN (KNIN-like nephropathy), similar to the case presented here, has been reported; however, no treatment has yet been established. Although some studies reported success with steroid treatment, potassium and phosphorus supplementation and urine alkalization resulted in a good course without the use of steroids in the present case.
Case report
An 18-year-old man without medical or family history visited an orthopedic surgeon for right knee pain two years prior to current admission and was diagnosed with osteosarcoma in right distal femur based on bone biopsy. He received preoperative chemotherapy including cisplatin + adriamycin (first dose, 223 mg and 55 mg, respectively) with methotrexate (22.3 g), which were initiated one month after initial admission. However, the patient was switched to combination chemotherapy including cisplatin + adriamycin with ifosfamide due to anaphylactic shock caused by methotrexate. The first dose of ifosfamide was administered at 5 g/day for 5 days and 3.4 g/day each for days 1 and 2 and 3.0 g/day each for days 3, 4, and 5. Due to the presence of renal dysfunction, cisplatin was reduced to 160 mg and adriamycin to 40 mg. After the completion of preoperative chemotherapy, tumor resection and knee replacement was performed 7 months after diagnosis. The resection specimen showed disappearance of tumor cells. Postoperatively, the patient was treated with cisplatin + adriamycin (200 mg + 50 mg in the third session, 175 mg + 45 mg in the fourth and fifth sessions, respectively). Ifosfamide was administered at 3 to 3.4 g/day each for days 1 and 2 and 3.0 g/day each for days 3 and 4. The chemotherapy was completed 12 months after initiation.
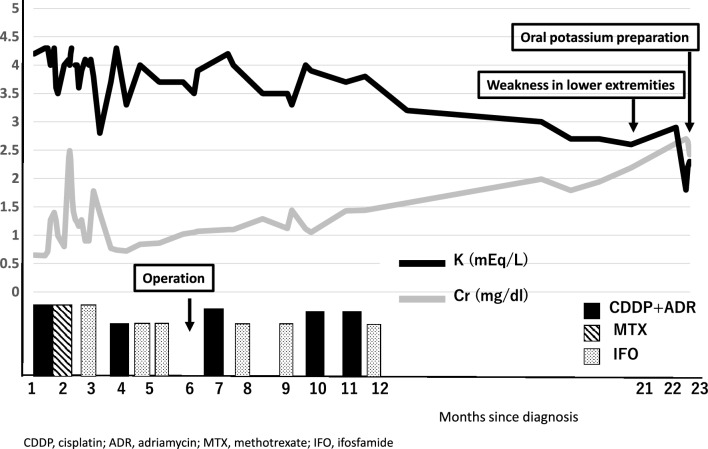
Although the clinical course was initially good after chemotherapy, the patient became aware of weakness in lower extremities 6 months later and developed numbness and weakness within a week 12 months after the discontinuation of chemotherapy. He was initiated on oral potassium L-aspartate tablets for the diagnosis of periodic tetraplegia and referred to our hospital due to progressive renal dysfunction (Fig. 1).
Fig. 1.
Changes in serum creatinine and serum potassium levels following the initiation of chemotherapy. Serum creatinine gradually increased and serum potassium decreased. Lower limb weakness was observed 21 months after the start of treatment and oral potassium preparations were started 23 months later
At admission to our hospital, his vital signs and physical examination revealed no abnormal findings. Chest X-ray showed no cardiac enlargement, and abdominal ultrasound showed mild thinning of the renal parenchyma. Bone mineral density analysis showed decreased bone mass in lumbar spine with a YAM of 74%. Blood tests revealed renal dysfunction (serum creatinine, 2.36 mg/dL), hypokalemia (2.1 mEq/L), hypophosphatemia (1.5 mg/dL), and hypouricemia (2.3 mg/dL) (Table 1). Blood gas analysis showed metabolic acidosis with normal anion gap (pH 7.375; pCO2, 25.3 mmHg; HCO3−, 14.5 mmol/L; Anion Gap 11.5). Urinalysis showed the following: protein, 2.59 g/gCr; glucose, 4 + ; NAG, 8 IU/L; α1-microglobulin, 73.7 mg/L; and increased urinary excretion of potassium, phosphorus and uric acid (fractional excretion of potassium, 63.5%; % tubular reabsorption of phosphate, 18.9%; and fractional excretion of uric acid, 66.6%). Panaminoaciduria was observed, and Fanconi syndrome was considered (Table 1).
Table 1.
Physical and laboratory findings at admission
| Age (years) | 18 |
| Body mass index (kg/m2) | 22.5 |
| Blood pressure (mmHg) | 120/80 |
| Pulse rate (bpm) | 91 |
| Serology | |
| Hemoglobin (g/dL) | 13.9 |
| Total protein (g/dL) | 7.4 |
| Albumin (g/dL) | 4.7 |
| Blood urea nitrogen (mg/dL) | 14.1 |
| Creatinine (mg/dL) | 2.36 |
| e-GFR (mL/min/1.73 m2) | 33.8 |
| Na (mEq/L) | 137 |
| K (mEq/L) | 2.1 |
| Cl (mEq/L) | 111 |
| Ca (mg/dL) | 8.5 |
| P (mg/dL) | 1.5 |
| Mg (mg/dL) | 2 |
| Uric acid (mg/dL) | 2.3 |
| 1α25(OH)2 vit-D (pg/mL) | 15 |
| pH | 7.375 |
| pCO2 (mmHg) | 25.3 |
| HCO3 (mmol/L) | 14.5 |
| Urinalysis | |
| pH | 6.5 |
| Blood | ± |
| Protein | 2 + |
| Glucose | 4 + |
| Red blood cell count (hpf) | 1–4 |
| White blood cell count (hpf) | 1–4 |
|
Urinary protein (g/g creatinine) |
2.59 |
| NAG (IU/L) | 8 |
| α1-Microglobulin (mg/L) | 73.7 |
| Na (mEq/L) | 36 |
| K (mEq/L) | 24 |
| P (mEq/L) | 21.9 |
| Uric acid (mg/dL) | 27.6 |
| FEK (%) | 63.5 |
| %TRP (%) | 18.9 |
| FEUA (%) | 66.6 |
| Panaminuria | + |
bpm beats per minute, e-GFR estimated glomerular filtration rate, hpf high power field, FEK fractional excretion of potassium, %TRP % tubular reabsorption of phosphate, FEUA fractional excretion of uric acid
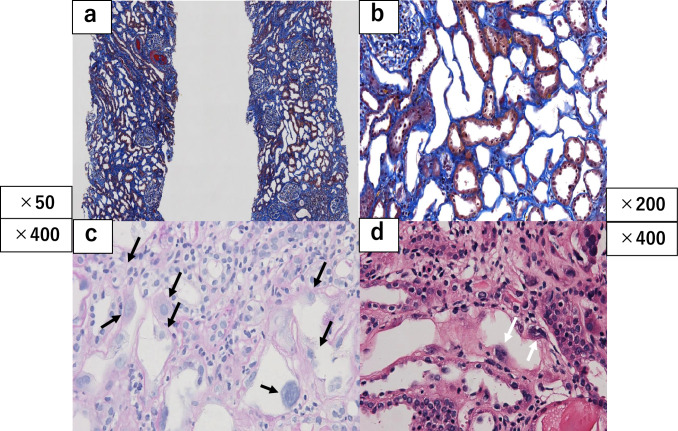
The patient underwent renal biopsy on day 7 after referral to our hospital, and 46 glomeruli were sampled. Histopathologic examination revealed no glomerulosclerosis. Diffuse tubular atrophy and fibrosis of the interstitium were observed, with inflammatory cell infiltration, predominantly mononuclear cells, such as lymphocytes and histiocytes. A large number of atrophied proximal tubular epithelial cells had large, polymorphic nuclei. Arteriosclerosis in the interlobular arteries or arteriolosclerosis was not observed. Immunostaining with the enzyme/antibody method was negative for IgG, IgA, IgM, C1q, and C3c. Electron microscopic findings included mild thickening of the glomerular basement membrane, mild foot process effacement, and moderate villous changes in epithelial cells. There were no notable findings in endothelial cells or mesangium, and immune complex deposits were not observed. A small number of tubular epithelial cells were present, but no abnormalities were found in the area. The pathological diagnosis was KNIN-like nephropathy (Fig. 2).
Fig. 2.
Renal biopsy findings. Low (a) and high (b) magnification of renal biopsy specimen s Masson’s trichrome stain showing atrophy of proximal tubular cells. (Arrow) Periodic acid–Schiff (c) and PAM (d) staining revealing nuclear enlargement. (Arrrow)
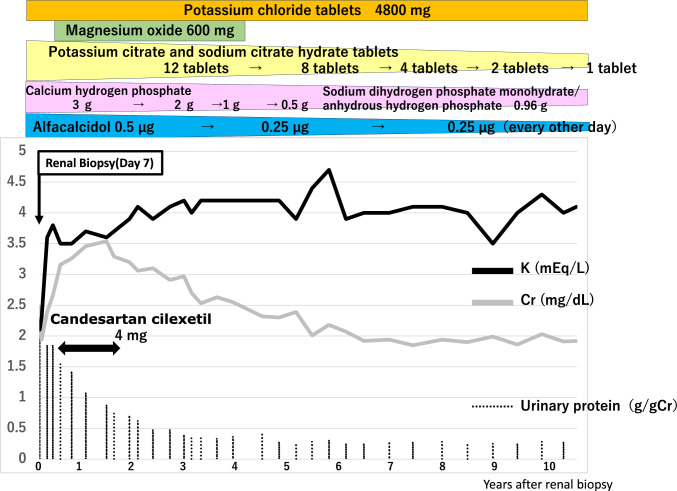
Following diagnosis, the patient was initiated on oral potassium replacement with potassium chloride tablets, phosphorus replacement with sodium dihydrogen phosphate monohydrate and anhydrous disodium hydrogen phosphate granules, and urine alkalization with potassium citrate and sodium citrate hydrate tablets. During the long-term follow-up of 10 years, the patient did not experience deterioration of renal function, with a serum creatinine of around 2.0 mg/dL and an estimated glomerular filtration rate ranging between 35 and 40 mL/min/1.73 m2. In addition, potassium, phosphorus, uric acid, and HCO3− levels were within normal ranges despite reductions in the doses of the various replacement drugs (Fig. 3).
Fig. 3.
Changes in serum creatinine, potassium, and urinary protein levels after treatment initiation following the patient’s transfer to our hospital. After the start of treatment, serum creatinine level started to decline with concomitant increase in serum potassium level. Urinary protein level also decreased
Discussion
In the present case, the patient diagnosed with osteosarcoma of the right femur was referred to our hospital due to periodic tetraplegia caused by renal dysfunction and hypokalemia after multi-agent chemotherapy including ifosfamide and cisplatin. Examination at admission revealed hypophosphatemia, hyperuricemia, and metabolic acidosis with normal anion gap in addition to renal dysfunction and hypokalemia. The increased urinary excretion of potassium, phosphorus, and uric acid accompanied by panaminoaciduria led to the diagnosis of Fanconi syndrome (Table 1).
Fanconi syndrome can be congenital or acquired, but in this case there was no evidence of renal dysfunction or hypokalaemia before chemotherapy and no other nephrotoxic drugs were used, suggesting an acquired Fanconi syndrome due to cisplatin or ifosfamide and severe proximal tubular damage. Renal biopsy performed to investigate the cause of progressive renal dysfunction revealed diffuse tubular atrophy, interstitial fibrosis, and mononuclear cell-dominated inflammatory cell infiltration. Atrophic tubules were found to have numerous large nuclei with polymorphism in the epithelial cells. Adenovirus, cytomegalovirus and EB virus infections with nuclear atypia were negative, and a pathological diagnosis of tubulo-interstitial nephrotitis, Karyomegalic-like nephropathy was made. KNIN is characterized by chronic tubulo-interstitial nephritis, including tubular epithelial cells harboring enlarged, atypical nuclei and interstitial fibrosis. In 1974, Burry et al. reported the first case of KNIN-like nephropathy in a 22-year-old woman who died of hepatocellular carcinoma [5]. In 1979, Mihatsch et al. reported a patient with a history of recurrent respiratory infections and progressive renal failure, representing which later became known as karyomegalic interstitial nephritis [3]. The reported prevalence is less than 5% of all kidney biopsies. In 2002, Bhandari et al. postulated that DNA polyploidy was involved as the cause of each variant [6]. In 2012, Zhou et al. reported that the nuclear abnormality observed in karyomegalic interstitial nephritis was due to a DNA repair defect based on a mutation in Fanconi anemia-associated protein 1 (FAN1) [7]. Renal injury induced with cisplatin or UUO was more pronounced in proximal tubular epithelial cells in a model where FAN1 expression was knocked out specifically in these cells. In that model, proximal tubular endothelial cells simultaneously expressed Ki67 and p21 and DNA repair defects due to reduced FAN1 expression occurred via p21. The subsequent development of karyomegaly was considered to lead to age-related shedding of cells and proximal tubular damage [8]. In the present case, Ki67 and P21 should also be searched for to detect abnormalities in the cell cycle, but unfortunately no sections remained and could not be examined.
In a review of 42 recently reported KNIN cases, the mean age of onset was 36.5 years, 19 patients (46%) had family history, and extrarenal involvement included respiratory infection and liver dysfunction in 19 (46%) and 21 (50%) patients, respectively, with dialysis required in 13 (30%) patients [4]. On the other hand, the abnormal nuclear morphology observed in tubular epithelial cells in KNIN renal biopsy specimens has also been reported in drug-induced renal injury, which has been termed KNIN-like nephropathy. No study to date has reported adriamycin-induced KNIN, and ifosfamide was considered as the main causative agent in the present case. Chloroacetaldehyde, a metabolite of ifosfamide, induces oxidative stress and damages tubular cell mitochondria, resulting in the inhibition of DNA synthesis [9]. Ifosfamide is a diacidic alkylating agent that damages DNA by forming DNA cross-links, the repair of which requires FAN1-mediated repair [7]. Although no FAN1 gene abnormalities were identified in this case study, it is possible that there is some underlying FAN1 functional abnormality that prevents DNA repair and results in KNIN-like nephropathy. Studies have also reported that proximal tubular epithelial cell damage and KNIN caused by ifosfamide are more likely to occur in patients receiving concomitant cisplatin treatment, in those with one kidney, and in those receiving higher total doses of > 60 g/m2 [10–12]. In the present case, the total ifosfamide dose was slightly lower (57 g/m2); however, the combination treatment with ifosfamide and cisplatin was considered as the cause of KNIN-like nephropathy and severe tubulo-interstitial nephritis resulting in drug-induced Fanconi syndrome. In this case, the loss of tubular epithelial cells was more severe than tubular atrophy. This has resulted in severe proximal tubular damage to the extent that Fanconi’s syndrome is present, but it is not known whether this is due to KNIN-like nephropathy or not. The reported long-term prognosis of ifosfamide-induced renal damage is relatively good in patients with only tubulo-interstitial nephritis, who exhibit normal or mildly compromised in renal function in the first decade following the completion of treatment [13]. However, there are few reports on the long-term prognosis of patients with KNIN-like nephropathy caused by ifosfamide [11, 14, 15]. Nivolumab, an immune checkpoint inhibitor, has also been reported to be associated with KNIN-like nephropathy [16]; however, the prognosis and treatment vary among patients. There have been reports of good response to steroid treatment with ifosfamide for KNIN-like nephropathy in patients with acute interstitial nephritis but poor response to treatment in patients with chronic interstitial nephritis [17] and steroid treatment for delayed renal damage [11]; however, the association with long-term renal outcome is unclear. In a report of two patients who developed KNIN-like nephropathy after nivolumab treatment [16], one patient experienced partial recovery from acute kidney injury after nivolumab discontinuation and the other patient fully recovered with additional corticosteroid treatment. Steroid therapy was not used in this case because about one year had passed since the end of chemotherapy, some time had passed since the appearance of renal dysfunction, there was no evidence of acute active changes such as inflammatory cell infiltration, and there was a possibility of improvement with discontinuation of the causative drug. During approximately 10 years since the end of fosfamide and cisplatin, renal function has remained unchanged although the treatment of electrolyte abnormalities and acidosis in Fanconi’s syndrome has also been tapered off. Furthermore, in the present case, there was moderate urinary protein and focal segmental loss of foot processes on electron microscopy, suggesting epithelial cell damage of some cause, but the urinary protein almost normalized with natural history.
In summary, in this report, we presented a patient who developed Fanconi syndrome caused by the synergistic effect of ifosfamide and cisplatin, which led to a diagnosis of KNIN-like nephropathy by renal biopsy. There are few reports of drug-induced KNIN (KNIN-like nephropathy), and most reports to date have used steroids in most cases [11.17], but in the present case, the renal function of the patient had not deteriorated with only correction of electrolytes and metabolic acidosis. In this respect, KNIN (especially secondary KNIN) is not necessarily a disease that progressively worsens. Future case series should clarify the need for therapeutic intervention and prognosis.
Declarations
Conflict of interest
All authors have declared no competing interest.
Ethical approval
All procedures performed in this research involving human participants are in accordance with the 1964 Declaration of Helsinki and its subsequent amendments or equivalent ethical standards.
Informed consent
Informed consent was obtained from the patient for this case report and for the release of potentially identifying information.
Footnotes
The original online version of this article was revised: In the original publication the address and city name of the affiliation 1, 2 and 3 has been appeared incorrectly as below: 1 Department of Nephrology and Hypertension, Department of Internal Medicine, St. Marianna University School of Medicine, Yokohama, Japan. 2 Department of Pathology, St. Marianna University School of Medicine, Yokohama, Japan. 3 Department of Nephrology, Shin-Yurigaoka General Hospital, Kanagawa, Japan. The corrected affiliation information should read as: 1 Division of Nephrology and Hypertension, Department of Internal Medicine, St. Marianna University Yokohama City Seibu Hospital, Yokohama, Japan. 2 Department of Pathology, St. Marianna University School of Medicine, Kawasaki, Japan. 3 Department of Nephrology, Shin-Yurigaoka General Hospital, Kawasaki, Japan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/12/2024
A Correction to this paper has been published: 10.1007/s13730-024-00916-9
References
- 1.Buttemer S, Pai M, Lau KK. Ifosfamide induced fanconi syndrome. BMJ Case Rep. 2011;2011(dec17 1):bcr1020114950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monga G, Banfi G, Salvadore M, Amatruda O, Bozzola C, Mazzucco G. Karyomegalic interstitial nephritis: report of 3 new cases and review of the literature. Clin Nephrol. 2006;65:349–55. [DOI] [PubMed] [Google Scholar]
- 3.Mihatsch MJ, Gudat F, Zollinger HU, Heierli C, Thölen H, Reutter FW. Systemic karyomegaly associated with chronic interstitial nephritis. A new disease entity? Clin Nephrol. 1979;12:54–62. [PubMed] [Google Scholar]
- 4.Isnard P, Rabant M, Labaye J, Antignac C, Knebelmann B, Zaidan M. Karyomegalic interstitial nephritis. Medicine (Baltim). 2016;95(20):e3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burry AF. Extreme dysplasia in renal epithelium of a young woman dying from hepatocarcinoma. J Pathol. 1974;113:147–50. [DOI] [PubMed] [Google Scholar]
- 6.Bhandari S, Kalowski S, Collett P, Cooke BE, Kerr P, Newland R, et al. Karyomegalic nephropathy: an uncommon cause of progressive renal failure. Nephrol Dial Transpl. 2002;17:1914–20. [DOI] [PubMed] [Google Scholar]
- 7.Zhou W, Otto EA, Cluckey A, Airik R, Hurd TW, Chaki M, et al. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat Genet. 2012;44:910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Airik M, Phua YL, Huynh AB, McCourt BT, Rush BM, Tan RJ, et al. Persistent DNA damage underlies tubular cell polyploidization and progression to chronic kidney disease in kidneys deficient in the DNA repair protein FAN1. Kidney Int. 2022;102:1042–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanly L, Chen N, Rieder M, Koren G. Ifosfamide nephrotoxicity in children: a mechanistic base for pharmacological prevention. Expert Opin Drug Saf. 2009;8:155–68. [DOI] [PubMed] [Google Scholar]
- 10.Hall AM, Bass P, Unwin RJ. Drug-induced renal fanconi syndrome. QJM An Int J Med. 2014;107:261–9. [DOI] [PubMed] [Google Scholar]
- 11.Matsuura T, Wakino S, Yoshifuji A, Nakamura T, Tokuyama H, Hashiguchi A, et al. Improvement in karyomegalic interstitial nephritis three years after ifosfamide and cisplatin therapy by corticosteroid. CEN Case Rep. 2014;3:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi R, Gödde A, Kleinebrand A, Riepenhausen M, Boos J, Ritter J, et al. Unilateral nephrectomy and cisplatin as risk factors of ifosfamide-induced nephrotoxicity: analysis of 120 patients. J Clin Oncol. 1994;12:159–65. [DOI] [PubMed] [Google Scholar]
- 13.Oberlin O, Fawaz O, Rey A, Niaudet P, Ridola V, Orbach D, et al. Long-term evaluation of Ifosfamide-related nephrotoxicity in children. J Clin Oncol. 2009;27:5350–5. [DOI] [PubMed] [Google Scholar]
- 14.McCulloch T, Prayle A, Lunn A, Watson AR. Karyomegalic-like nephropathy, ewing’s sarcoma and ifosfamide therapy. Pediatr Nephrol. 2011;26:1163–6. [DOI] [PubMed] [Google Scholar]
- 15.Jayasurya R, Srinivas BH, Ponraj M, Haridasan S, Parameswaran S, Priyamvada PS. Karyomegalic interstitial nephropathy following ifosfamide therapy. Indian J Nephrol. 2016;26:294–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryuzaki M, Tokuyama H, Uchiyama K, Nakaya H, Hasegawa K, Miyashita K, et al. Acute interstitial nephritis with karyomegalic epithelial cells after nivolumab treatment—two case reports. Clin Med Insights Case Rep. 2019;12:1179547619853647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Airy M, Raghavan R, Truong LD, Eknoyan G. Tubulointerstitial nephritis and cancer chemotherapy: update on a neglected clinical entity. Nephrol Dial Transpl. 2013;28:2502–9. [DOI] [PubMed] [Google Scholar]