Abstract
1. Leghaemoglobins were extracted from the root nodules of lupin (Lupinus luteus L.) and serradella (Ornithopus sativus Brot.) plants and fractionated into different leghaemoglobin components on DEAE-cellulose–acetate columns. 2. The first two fractions eluted from columns loaded with either lupin or serradella leghaemoglobins were in the Fe3+ oxidation state. 3. These components have protohaem IX as the prosthetic group and glycine as the N-terminal amino acid. 4. Other properties are: lupin component I, pI5.08, molecular weight 19000; lupin component II, pI5.13, molecular weight 20600; serradella component I, pI5.00, molecular weight 17500; serradella component II, pI5.05, molecular weight 19100. 5. Leghaemoglobins are thus heterogeneous with respect to size and charge.
Full text
PDF

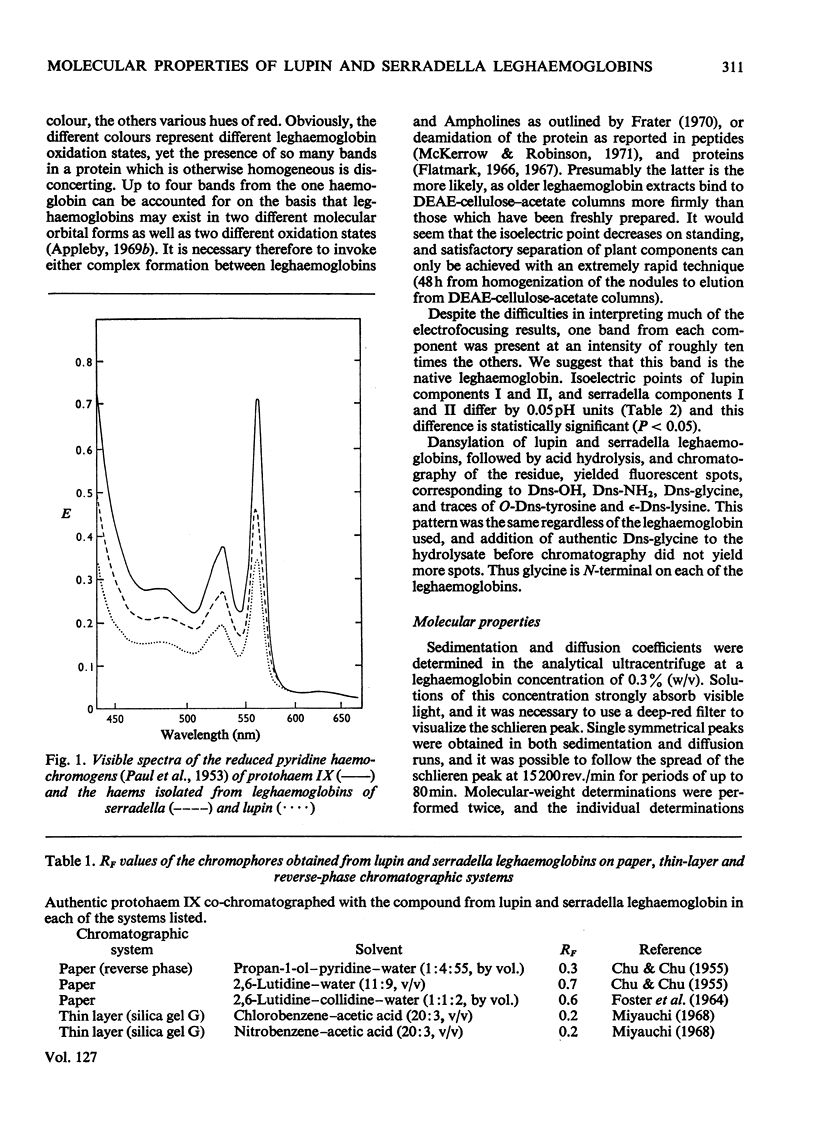
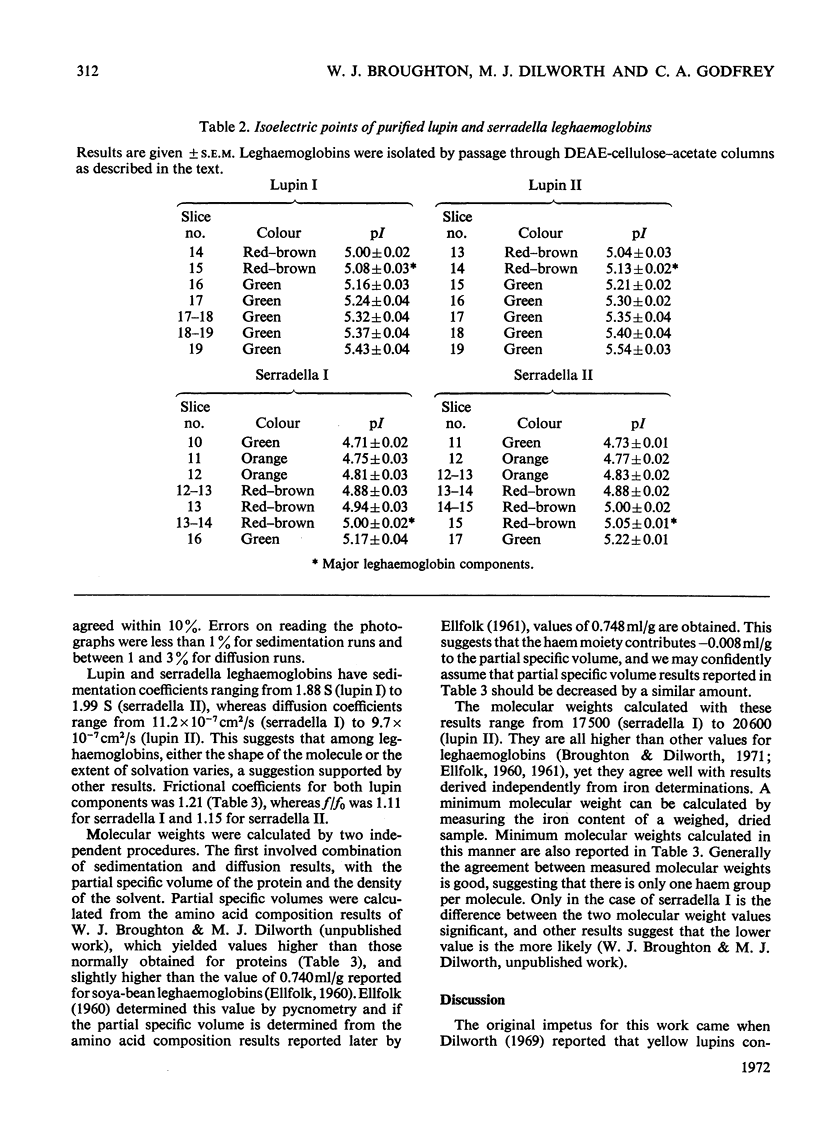


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A. Properties of leghaemoglobin in vivo, and its isolation as ferrous oxyleghaemoglobin. Biochim Biophys Acta. 1969;188(2):222–229. doi: 10.1016/0005-2795(69)90069-5. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. The separation and properties of low-spin (haemochrome) and native, high-spin forms of leghaemoglobin from soybean nodule extracts. Biochim Biophys Acta. 1969 Oct 21;189(2):267–279. doi: 10.1016/0005-2728(69)90053-x. [DOI] [PubMed] [Google Scholar]
- Broughton W. J., Dilworth M. J. Control of leghaemoglobin synthesis in snake beans. Biochem J. 1971 Dec;125(4):1075–1080. doi: 10.1042/bj1251075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU T. C., CHU E. J. H. Paper chromatography of iron complexes of porphyrins. J Biol Chem. 1955 Jan;212(1):1–7. [PubMed] [Google Scholar]
- CONNELLY J. L., MORRISON M., STOTZ E. Hemins of beef heart muscle. J Biol Chem. 1958 Sep;233(3):743–747. [PubMed] [Google Scholar]
- Catsimpoolas N. Micro isoelectric focusing in polyacrylamide gel columns. Anal Biochem. 1968 Dec;26(3):480–482. doi: 10.1016/0003-2697(68)90219-4. [DOI] [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The biogenesis of leghemoglobin. The determinant in the Rhizobium-legume symbiosis for leghemoglobin specificity. Biochim Biophys Acta. 1971 Jan 19;229(1):58–62. [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The site of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1969 Dec 30;192(3):486–493. doi: 10.1016/0304-4165(69)90398-5. [DOI] [PubMed] [Google Scholar]
- Deyl Z., Rosmus J. Thin layer chromatography of Dansyl amino acid derivatives. J Chromatogr. 1965 Dec;20(3):514–520. doi: 10.1016/s0021-9673(01)97453-9. [DOI] [PubMed] [Google Scholar]
- Dilworth M. J. The plant as the genetic determinant of leghaemoglobin production in the legume root nodule. Biochim Biophys Acta. 1969 Jul 30;184(2):432–441. doi: 10.1016/0304-4165(69)90047-6. [DOI] [PubMed] [Google Scholar]
- FOSTER M. A., DILWORTH M. J., WOODS D. D. COBALAMIN AND THE SYNTHESIS OF METHIONINE BY ESCHERICHIA COLI. Nature. 1964 Jan 4;201:39–42. doi: 10.1038/201039a0. [DOI] [PubMed] [Google Scholar]
- Flatmark T. Multiple molecular forms of bovine heart cytochrome c. V. A comparative study of their physicochemical properties and their reactions in biological systems. J Biol Chem. 1967 May 25;242(10):2454–2459. [PubMed] [Google Scholar]
- Flatmark T. On the heterogeneity of beef heart cytochrome c. 3. A kinetic study of the non-enzymic deamidation of the main subfractions (Cy I-Cy 3). Acta Chem Scand. 1966;20(6):1487–1496. doi: 10.3891/acta.chem.scand.20-1487. [DOI] [PubMed] [Google Scholar]
- Jackson E. K., Evans H. J. Propionate in heme biosynthesis in soybean nodules. Plant Physiol. 1966 Dec;41(10):1673–1680. doi: 10.1104/pp.41.10.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerrow J. H., Robinson A. B. Deamidation of asparaginyl residues as a hazard in experimental protein and peptide procedures. Anal Biochem. 1971 Aug;42(2):565–568. doi: 10.1016/0003-2697(71)90074-1. [DOI] [PubMed] [Google Scholar]
- Morse D., Horecker B. L. Thin-layer chromatographic separation of DNS-amino acids. Anal Biochem. 1966 Mar;14(3):429–433. doi: 10.1016/0003-2697(66)90285-5. [DOI] [PubMed] [Google Scholar]


