Abstract
Introduction
Retinol has a long history of treating skin conditions, including photoaging. However, skin irritation with repeated use of retinol is well documented. The present study assessed the effectiveness of a novel topical formulation, referred to as retinol topical formulation (RTF), to improve the quality of skin health. The RTF was composed of a low dose retinol, a synthetic retinoid ester, a pea peptide, and an antioxidant blend.
Methods
In vitro assessment of RTF on human skin co-cultures (human keratinocytes, melanocytes, and dermal fibroblasts) identified gene expression levels and skin biomarkers after 24 h exposure. An 8-week clinical study was conducted to evaluate once-nightly application of the RTF for short-term and long-term benefits in 30 adult subjects between 35 and 70 years of age (21 female, 9 male). Skin evaluations were conducted via bioinstrumentation (for hydration, transepidermal water loss and elasticity) and at 0, 1-, 2-, 4-, and 8-week self-assessment questionnaires and photo-imaging analysis were performed.
Results
RTF treatment of skin in vitro co-cultures upregulated aquaporin-3, PER1, collagen, and elastin, and downregulated expression of MMP1 and the pigmentation genes TYRP1 and MITF. The clinical assessment significantly improved hydration, transepidermal water loss, and elasticity along with incremental but significant increases in nine skin parameters (hydration, clarity, radiance/glow, smoothness, brightness, texture, appearance of pores, dark spots/hyperpigmentation, and skin tone evenness from baseline) with continuous use over 8 weeks compared to baseline values.
Conclusions
The RTF in vitro analysis showed significant positive changes for several skin biomarkers, and the clinical assessment showed RTF significantly improved the visible signs of dermal aging, without irritation.
Keywords: Retinol, Retinoid analogues, Pea peptide, Antioxidant, Formulation, In vitro, Clinical, Skin, Rejuvenation, Antiaging
Key Summary Points
| Antiaging skin therapies have a long history where retinol was used to treat skin conditions, including photoaging (or exposure to the sun). However, skin irritation with repeated use of retinol (retinoic acid) is well documented, which has motived consumers to seek alternative effective skin treatments that are less inflammatory. |
| The present study investigated the effectiveness of a novel topical formulation, referred to as retinol topical formulation (RTF), composed of a low dose of retinol, a synthetic retinoid ester (a retinol analogue), a peptide derived from the pea plant, and an antioxidant blend of plant-derived extracts plus vitamin E (tocopherol) in improving skin health and delaying or reversing the visible signs of dermal aging. |
| This was accomplished by demonstrating significant improvements in (a) in vitro gene expression analysis of skin biomarkers after 24 h of exposure, (b) hydration, transepidermal water loss, and elasticity by bioinstrumentation, (c) nine skin parameters by self-assessment questionnaires, and (d) photo imaging analysis where the benefits of RTF were noticeable in some cases within 1 week of treatment with incremental but significant increases continued up to 8 weeks of RTF treatment. |
| The present results strengthen evidence of the RTF effectiveness via in vitro and clinical investigations, where several skin parameters were shown to improve dermal health and significantly decrease the visible signs of aging. These findings can be incorporated into everyday practice to broaden the approach to antiaging skin therapies. |
Introduction
Skin Renewal with Retinol (History, Benefits, and Challenges)
Retinol (a form of vitamin A) has a long history of treating skin conditions including photoaging [1–3]. Vitamin A cannot be produced by the human body. The only source of vitamin A is obtained through the diet, as lipophilic retinol or carotenoids [2]. Thus, it must be supplied to the body via natural sources, natural derivatives, or by synthetic analogues [4, 5]. For example, retinol along with its analogues (retinal, retinyl palmitate, and retinoic acid) are classified as retinoids, which can be natural or synthetic (derived from vitamin A) [6]. In skin, retinol is metabolized to its active form, retinoic acid, which binds to retinoic acid receptors in cells to transactivate barrier function, anti-inflammation, and extracellular matrix gene expression [2, 5]. In recent years several studies have shown the effectiveness of retinol and analogues of retinoic acid such as retinaldehyde in the treatment of acne, psoriasis, and photoaging [2, 3, 6, 7]. The mechanism of action of retinol and other retinoids primarily involves regulation of cell proliferation, differentiation, and cell turnover [2, 5, 7]. Numerous studies in the literature have shown that the topical application of retinol is effective for treating acne and photodamaged skin [2, 8, 9]. For example, photo-neuro-immuno-endocrinology mechanisms of how ultraviolet radiation regulates the body, brain, and immune systems and neuroendocrine signaling in the skin with epidermal neuropeptides have been reported [10, 11]. However, irritation caused by repeated use of topical retinol includes itching, erythema, and dryness, which has been well documented [2, 3, 12]. As a result, consumers continue to seek effective alternative treatment modalities which are less irritating.
In this study we describe a novel topical formulation, henceforth referred to as retinol topical formulation (RTF), composed of the following: (1) retinol; (2) a pea peptide, which was identified from deep learning and artificial intelligence analysis of Pisum sativum (pea) proteome and genome [13]; (3) hydroxypinacolone retinoate (HPR), a novel synthetic retinoid ester [14]; and (4) an antioxidant blend composed of Oryza sativa (rice) bran extract [15], rosemary leaf extract [16], sunflower extract [17], and tocopherol—vitamin E [18] that are known to improve several aspects of skin health.
Methods
Objective
The objective of this study was to investigate the beneficial effects of RTF via in vitro skin cell co-cultures for antiaging gene expression through assessment of epidermal and dermal-matrix biomarkers and to conduct an 8-week clinical study to evaluate the effectiveness of RTF for improvement of skin attributes such as hydration, firmness, brightening (radiance), texture, and skin tone evenness.
This clinical study was approved by the Allendale Institutional Review Board on March 21, 2024, with the assigned study number (NSE-18–24). The study was conducted in accordance with the ethical principles of the 1975 Declaration of Helsinki and its subsequent amendments. Prior to the performance of any study-specific procedures, subjects were provided with an explanation of the nature of the study, including the purpose, procedures, expected duration, and potential risk. Prospective subjects were informed of their right to withdraw from the study at any time without being obliged to give a reason. If consent was obtained, the subject signed and dated the informed consent form. Specific written consent to use the patient’s facial photos was obtained.
Retinol Topical Formulation
RTF Sample Preparation for Co-culture Study
The following four components were combined to produce the sample for in vitro testing. The final concentration of retinol in the sample was 0.2%. Ingredient names are mentioned using the International Nomenclature of Cosmetic Ingredients (INCI) nomenclature.
- Retinol (0.1% w/v):
- Composed of allyl methacrylate cross-polymer (0.2% w/v), (as a texture enhancer) and polysorbate 20 (0.1% w/v) (as an emulsifier to help stabilize the formula).
Pep_RTE62G (0.0035% w/v): peptide isolated from Pisum sativum, shown to stimulate synthesis of collagen and elastin.
Hydroxypinacolone retinoate (HPR) (0.1% w/v), a synthetic retinoid ester.
Antioxidant blend (0.01% w/v): key components are INCI (composed of Oryza sativa (rice) bran extract, Rosmarinus officinalis (rosemary) leaf extract, Helianthus annuus (sunflower) extract) and tocopherol 0.1% w/v.
The co-culture RTF formula differed from the in vivo RTF formula to be compatible with in vitro medium conditions. Whereas the RTF formula for the clinical study below was prepared as a topical application, for the active ingredients to penetrate the skin.
The RTF blend was soluble in 0.01% dimethyl sulfoxide (DMSO)/water, while the controls contained only 0.01% DMSO/water, which were added to the co-cultures (below) at 100 µl.
RTF Formulation for the Clinical Study was Developed by Combining the Following
- Retinol 0.1% w/v,
- Allyl methacrylates cross-polymer 0.2% w/v (to stabilized emulsion/enhance texture).
Pep_RTE62G 0.0035% w/v.
Hydroxypinacolone retinoate (HPR) 0.1% w/v.
Dimethyl isosorbide 0.9% w/v (to enhance texture and help active ingredients to penetrate the skin).
Polysorbate 0.1% w/v (emulsifier and stabilizer).
The antioxidant blend 0.01% w/v (composed of Oryza sativa (rice) bran extract, Rosmarinus officinalis (rosemary) leaf extract, Helianthus annuus (sunflower extract), and tocopherol 0.1% w/v.
In Vitro Assessment of RTF on Human Skin Co-culture Model Composed of Keratinocytes, Melanocytes, and Dermal Fibroblasts
RTF samples were added (100 µl per well in triplicate by treatments) to the HaCaT keratinocytes/B16 melanoma cells/human dermal fibroblasts (HDF) co-culture model in dermal cell basal medium (ATCC, Manassas, Virgina, USA) through a phospholipid-coated polyvinylidene difluoride (PVDF) membrane (to approximate passage through stratum corneum, mimicking skin epidermal-dermal structures) in a 96-well plate format that was incubated for 24 h at 37 °C (at 5% CO2 with saturating humidity). In brief, all three cell lines were grown in cell culture in DMEM culture medium (Thermo Fisher, Waltham, MA, USA) with 5% fetal bovine serum (FBS) (Thermo Fisher, Waltham, MA, USA), incubator at 37 °C and passaged for 8–10 doublings. The cells were set in tri-culture at a 1:1:1 ratio in a 96-well plate. Once set in the plate tri-cultures, incubations were for 24 h at 37 °C, and the next day the tri-cultures were treated with the test samples and incubated for 24 h. RNA was then extracted with RNeasy Plus Mini kit cat #74134 from Qiagen (Qiagen, Germantown, MD, USA), using QiaCube Connect robotic station (Qiagen Germantown, MD, USA). Purified total RNA was assessed at 260 nm and 280 nm via NanoDrop Lite (Thermo Fisher Scientific, Waltham, MA, USA), to quantify RNA content. For PCR reactions, cDNA was prepared using AzuraQuant cDNA kit (Azura Genomics, Raynham, MA, USA) and the expression of the genes of interest. These included MMP1 (matrix metalloproteinase 1), COL1A1 (collagen type 1 alpha-1), AQP3 (aquaporin-3), ELN (Elastin), RXR-α (retinoid X receptor alpha), RXR-y (retinoid X receptor gamma), PER1 (period circadian regulator 1) were measured by real-time quantitative PCR with BioRad iCycler iQ Detection System using PCR primers from Realtime primers (Elkins Park, PA, USA) and Fast Green qPCR Master Mix-Fluor (Azura Genomics, Raynham, MA, USA). This was accomplished by a prepared mix of cDNA with primers of genes of interest. The expression of the genes of interest was measured by real-time quantitative PCR. Efficiency ΔΔCt method was used for quantification of gene expression, after the normalization of gene expression to five housekeeping genes (GAPDH, HPRT1, PPIA, RPL13A, and RPS18). Genes were considered differentially expressed if the level of expression was high (fewer than 30 cycles to detect), p value, as determined by the two-tailed t test, was p ≤ 0.05, and the expression was greater than twofold.
Clinical Assessment of the Topical RTF on Skin Attributes
An 8-week clinical study was conducted to evaluate RTF for short- and long-term skin benefits in Nu Skin’s clinical laboratory from March 27 to May 22, 2024. IRB approval was granted from the Allendale Investigational Review Board on March 21, 2024 with the study number NSE-18–24. Thirty subjects (21 female, 9 male) between 35 and 70 years of age, with varying degrees of skin photoaging and with Fitzpatrick skin types I–VI completed the study. Subjects used RTF once nightly after cleansing their facial skin. Skin evaluations were completed via bioinstrumentation (Corneometer, Tewameter-Courage + Khazaka electronic GmbH, Koln, Germany; and Cortex DermaLab, Aalborg, Denmark for elasticity/firmness), self-assessment questionnaires, and imaging at weeks 0, 1, 2, 4, and 8 for skin attributes (on a clean facial region with no make-up). Subjects were excluded in cases of known dermatological disorders, previous hypersensitivity reactions to any of the active ingredients, pregnancy, use of indoor tanning booth, cigarette smokers, or other medical disorders. The full list of inclusion and exclusion criteria can be found in the online supplement section.
Statistical Analysis
For the gene expression data, genes were considered statistically significant if the level of expression was twofold or greater compared to control levels as determined by two-tailed t test (p < 0.05). For the analysis of the clinical data, the mean values comparing instrumental and self-perception assessments at baseline (week 0) to weeks 1, 2, 4, and 8 were considered significant (p < 0.05) by a within-subject paired t test.
Results
Effect of RTF on Skin Rejuvenation and Renewal: In Vitro Evidence
RTF activated the expression of retinoic acid receptor pathway genes, RXR-γ and RXR-α, indicating its specificity related to targeting of the retinoic acid pathway. Further, RTF upregulated expression of aquaporin (AQP), a marker for skin hydration, and PER1 (period circadian regulator 1, a clock gene), a marker for circadian rhythm. RTF showed antiaging activity by upregulating expression of extracellular matrix genes, collagen (COL1A1), and elastin (ELN), while simultaneously downregulating the matrix metalloproteinase 1 (MMP1) gene expression in skin. Further, RTF downregulated TYRP1 (tyrosinase related protein 1), encoding a rate-limiting gene in melanin biosynthesis, and MITF, a master regulator of pigmentation genes (Fig. 1).
Fig. 1.
RTF treatment on skin rejuvenation and skin renewal activity. RTF treatment upregulated collagen and elastin and downregulated MMP1 (matrix metalloproteinase 1) expression. RTF also upregulated AQP3 (hydration gene), PER1 (a circadian rhythm marker), and downregulated TYRP1 (encoding a rate-limiting enzyme in melanin biosynthesis) and MITF (transcription factor, master regulator of pigmentation genes). RTF was added to cells in triplicate for assay of each of the genes. Data is shown as % upregulation/downregulation ± SEM for each gene. ► Significant change in gene expression in RTF-treated samples compared to vehicle control
Clinical Evaluation of Effect of Topical RTF on Skin Attributes
Clinical study results obtained via Corneometer measurements show the topical application of RTF statistically improved hydration after one use and with continual use (weeks 1, 2, 4, and 8). Transepidermal water loss (measured via Tewameter) was statistically reduced after using RTF for 2 weeks. Skin elasticity measured with Cortex DermaLab instrumentation demonstrated statistically significant improved results over baseline at all time points (Table 1).
Table 1.
Effects of topical RTF on skin attributes
| Attribute | Instrument | Post-application | Week 1 | Week 2 | Week 4 | Week 8 |
|---|---|---|---|---|---|---|
| Hydration | Corneometer | 10%* | 4% | 16%* | 14%* | 20%* |
| Transepidermal water loss | Tewameter | − 1% | 0% | − 17%* | − 31%* | − 38%* |
| Elasticity | DermaLab | 20%* | 48%* | 46%* | 61%* | 64%* |
*Statistically significant vs baseline (p ≤ 0.05)
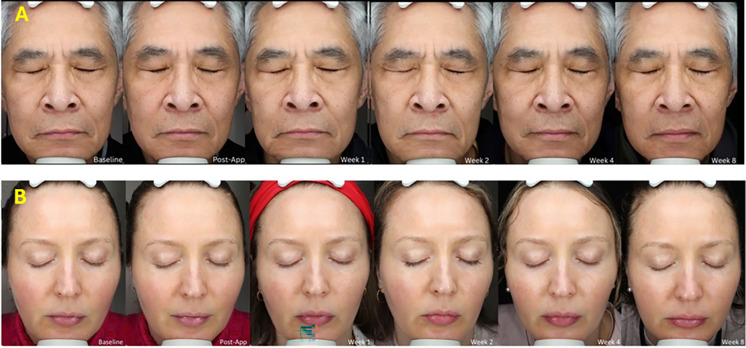
Additionally, subjects saw visible improvements in nine skin parameters (from hydration to even skin tone) that were evaluated as early as week 1, and the subjects continued to see these improvements amplified throughout the study duration (Figs. 2, 3). All results were statistically significant over baseline p ≤ 0.05.
Fig. 2.
Subjects self-perception assessment of skin attributes at week 1, 2, 4, and 8
Fig. 3.
Representative images of two subjects, one male (A) and one female (B), showing improvement in skin hydration, radiance, hydration, clarity, radiance/glow, smoothness, brightness, texture, and skin tone evenness from baseline immediately after retinol-peptide application and progressive improvements in skin attributes from week 1 to week 8
Safety Outcomes
Overall, the RTF in the clinical study was well tolerated. There were no adverse events reported during the clinical study, and the self-reports (feedback) from all the subjects were positive. The test subjects were pleased with the positive outcomes in the improvements in their quantified skin parameters and dermal health.
Discussion
In Vitro Evidence of the Effectiveness of RTF
Numerous studies have shown the effectiveness of retinol in treating skin conditions including photoaging [1, 2, 7, 12]. However, with repeated use topical retinol applications cause skin irritation, which has motivated consumers to seek effective alternative cosmetic active ingredients that are less inflammatory and improve skin health [1, 2, 12, 19]. In the present studies we report a novel RTF composed of retinol (at low concentration) and its analogue (HPR), plus a natural peptide (Pep_RTE62G) from Pisum sativum (pea) extract, and an antioxidant blend that in vitro stimulates skin renewal.
For example, from our in vitro results, several positive skin biomarkers were quantified by gene expression analysis, the results of which support previous reports. For instance, Kennedy showed that treatment of skin co-cultures with Pep_RTE62G twice a day for 5 days stimulated collagen and elastin production [13], while RTF also upregulated retinoic acid receptor genes, RXR-α and RXR-γ, indicating that the regulation of epidermal and dermal gene expression is potentiated by transactivation of retinoid receptors [20]. Our results suggest that RTF stimulated skin rejuvenation and renewal by upregulating collagen and elastin expression and at the same time downregulating MMP1 gene expression, findings similar to those reported previously, where authors also showed that retinol stimulated TIMP-1 activity [1, 2, 7]. Plus, the activation of collagen and elastin by retinol was shown by Quan et al. to occur through the stimulation of the TGFβ pathway [21]. Furthermore, the effectiveness of topical retinol-induced expression of hyaluronan synthase (HAS) gene and hyaluronan (hyaluronic acid) production is also known to enhance dermal parameters [22]. The present data are supported by similar studies where combining retinol with growth factors, cytokines, promoted skin rejuvenation [20]. Moreover, Brown et al., in 2023, showed that retinol analogues potentiated the effects of retinol on aged and photodamaged skin [23]. Along this perspective photoaging involves diverse effects of ultraviolet radiation (UVR) and how UVR regulates the body, brain, and immune system has been reported [10]. Also, neuroendocrine signaling in the skin with a special focus on epidermal neuropeptides appears to play an important role in the skin’s responses to stress [11].
It was also observed that skin circadian rhythm may influence the activity of RTF as shown by upregulation of PER1 clock gene expression. However, more research is warranted to optimize the time of day or night when the application of RTF will provide the maximum skin-rejuvenating activity even though it is known that clock genes are expressed during the day for protection and at night for skin maintenance and repair [24]. Thus, the present in vitro RTF results are consistent with other in vitro findings on the antiaging functional activity of retinol [19–22, 25].
Other studies have shown that topical phytochemicals and retinoids are effective in reducing in post-inflammatory hyperpigmentation (PIH) (via Nrf2 activation) that occurs as a secondary outcome in the resolution of patients with acne [26, 27]. The mechanism of retinol in reducing PIH is not well understood, but most theories suggest that retinol stimulates epidermal cell turnover and thus reduces PIH. While there is also no clear picture on the mechanism of how retinol reduces basal pigmentation and hyperpigmented spots, our results from co-culture studies suggest that retinol reduces melanin levels in skin by downregulating MITF expression. MITF is a helix-loop-helix transcription factor that transactivates pigmentation genes TYR and TYRP1 by binding to its promoter sequences and is hence considered the master regulator of melanocyte differentiation and melanogenesis [28]. We found that RTF downregulated expression of the MITF gene as well as TYRP1, which encoded a rate-limiting enzyme in the melanin biosynthesis pathway [28].
Clinical Evidence of RTF’s Effectiveness for Cosmetic Applications
The present clinical investigation validated the functional activity observed on skin co-culture by improving skin hydration (10% over baseline) and elasticity (20% over baseline) with just one application of the RTF formula, and over twofold improvement in hydration and threefold improvement in skin elasticity with continuous use over 8 weeks of assessment. This aligns with previous results by other authors [1, 2, 7, 12, 19, 29] that are reviewed elsewhere [30, 31]. The incremental improvement in skin hydration correlated with reduction in TEWL, as much as 38-fold over baseline at 8 weeks, also confirms and extends previous reports [1, 7, 30, 31].
The instrumental analysis correlated with subjects’ self-perception of visible improvement in the skin attributes including hydration, skin clarity, radiance, brightness, hyperpigmented spots, and skin tone evenness (Table 1). Our results are consistent with published studies with topical formulations containing retinol and HPR [30, 32]. For example, in a 12-month study clinical study with a combination of topical HPR and retinol as a maintenance therapy on patients with acne, who relapsed on orally administered isotretinoin, it was found that the relapse rate was significantly reduced with only 15% of subjects reporting relapse on the HPR and retinol maintenance therapy [29]. In a recent study, five retinoids, retinol (ROL), retinol acetate (RAc), retinol propionate (RP), retinol palmitate (RPalm), and HPR, were evaluated for their anti-inflammatory, antioxidant, and anti-photoaging activities using in vitro and in vivo methods [9]. The results revealed that four retinoids (RPalm, RP, HPR, and ROL) applied at a dose of 5 μg/ml were effective in reducing photoaging through their antioxidant and anti-inflammatory actions [9].
In addition to the positive antiaging effects of retinol (its analogue, HPR) and pea peptide extract, and undoubtably the antioxidant blend, which contains Oryza sativa (rice) bran extract [15, 33], rosemary leaf extract [16], sunflower extract [17], and tocopherol (vitamin E) [16], these ingredients are known to have antioxidant, moisturizing, anti-inflammatory and antiaging activities along with increasing elasticity [15–18]. None of the subjects in our clinical study reported any skin irritation from the daily topical application of the RTF, which is consistent with the safety profile of low dose retinoids plus the other active ingredients [1, 2, 7, 9, 30, 31]. Finally, the present clinical results demonstrated that the RTF was not only effective in women but it also improved skin health in men. This is meaningful because skin healthcare in men is increasing in popularity worldwide, but especially in Asian countries [34, 35].
Study Strengthens and Limitations
The present study provided in vitro evidence that was supported by clinical findings with a wide range of skin parameters, which were quantified and analyzed. The effectiveness of the RTF topical applications included both objective instrumentation and subjective assessments of skin appearance and improvements in dermal outcomes not only in women but also in men of varying age from 35 to 70 years old. Notably, some of the women in the clinical study were menopausal, which strengthens the present findings when estrogen-deficient skin conditions were presumably present.
The main limitations of this study include (a) while 2D in vitro cultures are not ideal for all the mechanisms of inherent cellular properties and essential cell–cell interactions, they are valuable for the identification and evaluation of skin biomarker expression [36]; (b) the B16 melanoma cell line has its own limitations due to its murine origin and may not provide a true reflection of using human primary melanocytes, and a similar concern could be addressed by using normal primary keratinocytes; (c) although several important skin biomarkers were identified and quantified, a broader range of endpoints should be included in follow-up studies with better in vitro conditions; and (d) for the clinical investigation, the small number of participants, while adequate, needs to be enlarged (especially the number of male patients studied), the lack of a placebo-control group need to be addressed, and expert skin graders should be used to control for the subjective assessments of the skin parameters (by self-reporting and instrumentation analysis).
Conclusion
Numerous studies have shown the effectiveness of retinol in treating skin conditions including photoaging [1, 2, 7, 30, 31]. However, skin irritation associated with repeated use of topical retinol applications has motivated consumers to seek effective alternative cosmetics with active ingredients which are less inflammatory and improve skin health [1, 2, 7, 30]. The present study reported a novel RTF composed of retinol (at low concentration) and its analogue (HPR), plus a natural peptide from Pisum sativum (pea) extract, and an antioxidant blend that stimulates renewal and rejuvenating dermal health, without irritation. In brief, RTF treatment of skin in vitro co-cultures upregulated aquaporin-3, PER1 (a circadian rhythm marker), antiaging dermal markers collagen and elastin, and downregulated expression of MMP1 and the pigmentation genes TYRP1 and MITF, indicating the capacity of RTF to reduce melanin production in skin. Moreover, our clinical assessment validated the functional activity observed on the skin co-cultures by improving skin hydration and elasticity with just one RTF application followed by incremental but significant increases in nine skin parameters (hydration, clarity, radiance/glow, smoothness, brightness, texture, appearance of pores, dark spots/hyperpigmentation, and skin tone evenness from baseline) with continuous use over 8 weeks of assessment compared to baseline values. The quantified instrumental analysis correlated with the subjects’ self-perception of visible improvement in the nine skin attributes. In summary, RTF is fast-acting and effective in improving visible signs of skin aging without any signs of skin irritation.
Acknowledgements
We thank Krys Bojanowski at Sunny Biodiscovery Inc., Santa Paula, CA for performing the co-culture experiments and collecting data; and thank the participants of the clinical study.
Author Contributions
Brian Cook, Melanie Riggs, K.C Holley, Helen Knaggs, Ganesh Diwakar and Edwin Lephart were involved in the design, supervision, performance and writing of this manuscript (plus revisions) and have given their approval for this version of the paper to be published.
Funding
This study was funded by Nu Skin Enterprises (NSE) Inc. and, in part, by the Life Sciences College, grant # 19-2215 at Brigham Young University (BYU). No funding or sponsorship was received for this study or publication of this article.
Data Availability
The data/study results cited in this article are contained within the text and supplemental material is available online.
Declarations
Conflict of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of date; in writing of the manuscript; or in the decision to publish the results. Brian Cook, Melanie Riggs, K.C. Holley, Helen Knaggs and Ganesh Diwakar are employed by NSE Inc. Edwin Lephart is an Editorial Board member of Dermatology and Therapy. Edwin Lephart was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
This clinical study was approved by the Allendale Institutional Review Board on March 21, 2024, with the assigned study number (NSE-18-24). The study was conducted in accordance with the ethical principles of the 1975 Declaration of Helsinki and its subsequent amendments [37]. Prior to the performance of any study-specific procedures, subjects were provided with an explanation of the nature of the study, including the purpose, procedures, expected duration, and potential risk. Prospective subjects were informed of their right to withdraw from the study at any time without being obliged to give a reason. If consent was obtained, the subject signed and dated the informed consent form. Specific written consent to use the patient’s facial photos was obtained.
References
- 1.Ramos-E-Silva M, Hahn DM, Rutowitsch MS, Zechneister M. Hydroxy acids and retinoids in cosmetics. Clin Dermatol. 2001;19:460–6. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Intervent Aging. 2006;1:327–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temova Rakusa Z, Skufca P, Kristl A, Roskar R. Retinoid stability and degradation kinetics in commercial cosmetic products. J Cosmet Dermatol. 2021;20:2350–8. [DOI] [PubMed] [Google Scholar]
- 4.Antille C, Tran C, Sorg O, Saurat J-H. Penetration and metabolism of topical retinoids in ex-vivo organ-cultured full-thickness human skin explants. Skin Pharmacol Physiol. 2004;17:124–8. [DOI] [PubMed] [Google Scholar]
- 5.Szymanski L, Skopek R, Palusinska M, et al. Retinoic acid and is derivatives in skin. Cells. 2020;9:2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Byrne SM, Blaner WS. Retinol and retinyl esters: biochemistry and physiology. J Lipid Res. 2013;54:1731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zasada M, Budzisz E. Retinoids: active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol Alergol. 2019;36:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thielitz A, Gollnick H. Topical retinoids in acne vulgaris: update on efficacy and safety. Am J Clin Dermatol. 2008;9:369–81. [DOI] [PubMed] [Google Scholar]
- 9.Shu P, Jiang L, Li M, et al. Comparison of five retinoids for anti-photoaging therapy: evaluation of anti-inflammatory and anti-oxidant activities in vitro and therapeutic efficacy in vivo. Photochem Photobiol. 2024;100:633–45. [DOI] [PubMed] [Google Scholar]
- 10.Slominski RM, Chen JY, Raman C, Slominski AT. Photo-neuro-immuno-endocrinology: how the ultraviolet radiation regulates the body, brain, and immune system. Proc Natl Acad Sci (USA). 2024;121:e2308374121. 10.1073/pnas.2308374121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slominski AT, Slominski RM, Raman C, Chen JY, Athar M, Elmets C. Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides. Am J Physiol Cell Physiol. 2022;323:C1757–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira R, Napoli J, Enver T, Bernardino L, Ferreira L. Advances and challenges in retinoid delivery systems in regenerative and therapeutic medicine. Nat Commun. 2020;11:4265. 10.1038/s41467-020-18042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy K, Cal R, Casey R, et al. The anti-aging effects of a natural peptide discovered by artificial intelligence. Int J Cosmet Sci. 2020;42:388–98. [DOI] [PubMed] [Google Scholar]
- 14.Veraldi S, Barbareschi M, Guanziroli E, et al. Treatment of mild to moderate acne with a fixed combination of hydroxypinacolone retinoate, retinol gycospheres and papain glyspheres. G Ital Dermatol Venereol. 2015;150:143–7. [PubMed] [Google Scholar]
- 15.Chaikul P, Kanlayavattanakul M, Khongkow M, Jantimaporn A, Lourith N. Anti-skin ageing activities of rice (Oryza sativa) bran soft and hard waxes in cultured skin cells. Int J Cosmet Sci. 2024;46:162–74. [DOI] [PubMed] [Google Scholar]
- 16.Nieto G, Ros G, Castillo J. Antioxidant and antimicrobial properties of Rosemary (Rosmarinus officinalis, L.): a review. Medicines. 2018;5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin T-K, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19:70. 10.3390/ijms19010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keen MA, Hassan I. Vitamin E in dermatology. Indian Dermatol Online J. 2016;7:311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorg O, Antille C, Kaya G, Saurat J-H. Retinoids in cosmeticeuticals. Dermatol Ther. 2006;19:289–96. [DOI] [PubMed] [Google Scholar]
- 20.Aldag CD, Teixeria N, Leventhal PS. Skin rejuvenation using cosmetic products containing growth factors, cytokines, and matrikines: a review of the literature. Clin Cosmet Investig Dermatol. 2016;9:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan T, Shao Y, He T, Voorhees JJ, Fisher GJ. Reduced expression of connective tissue growth factors (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J Investig Dermatol. 2010;130:415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li WH, Wong W-H, Serrano J, et al. Topical stabilized retinol treatment induces the expression of HAS genes and HA production in human skin in vitro and in vivo. Arch Dermatol Res. 2017;309:275–83. [DOI] [PubMed] [Google Scholar]
- 23.Brown A, Furmanczyk M, Ramos D, et al. Natural retinol analogs potentiate the effects of retinol on aged and photodamaged skin: results from in vitro to clinical studies. Dermatol Ther. 2023;13:2299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knaggs H, Lephart ED. Enhancing anti-aging through healthy lifestyle factors. Cosmetics. 2023;10:142. 10.3390/cosmetics10050142. [Google Scholar]
- 25.Wang Y, Zhang Q, Wei Y, et al. Retinol semisolid preparations in cosmetics: transcutaneous permeation mechanism and behaviour. Sci Rep. 2024;14:22793. 10.1038/s41598-024-73240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimes PE. A microsponge formulation of hydroquinone 4 % and retinol 0.15% in the treatment of melasma and postinflammatory hyperpigmentation. Cuits. 2004;74:362–8. [PubMed] [Google Scholar]
- 27.Chaipasongsuk A, Panich U. Role of phytochemicals in skin photoprotection via regulation of Nrf2. Front Pharmacol. 2022;13:823881. 10.3389/fphar.2022.823881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawakami A, Fisher DE. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab Investig. 2017;97:649–56. [DOI] [PubMed] [Google Scholar]
- 29.Bettoli V, Zauli S, Borghi A, et al. Efficacy and safety of a 12-month treatment with a combination of hydroxpinacolone retinoate and retinol glycospheres as maintenance therapy in acne patients after oral isotretinoin. G Ital Dermatol Venereol. 2017;152:13–7. [DOI] [PubMed] [Google Scholar]
- 30.Lau M, Mineroff G, Wang JY. Cosmeceuticals for antiaging: a systemic review of safety and efficacy. Arch Dermatol Res. 2024;316:173–89. [DOI] [PubMed] [Google Scholar]
- 31.Mehta N, Gupta S. Skin aging. In: Gupta S, Mehta N, Dudani P, editors. Critical thinking in contemporary dermatology. Singapore: Springer; 2024. 10.1007/s00403-024-02908-2. [Google Scholar]
- 32.Saraiva SM, Miguel SP, Araujo ARTS, Rodrigues M, Ribeiro MP, Coutinho P. Cosmetic industry: natural secondary metabolites for beauty and aging. In: Carocho M, Heleno SA, Barros L, editors. Natural secondary metabolites. Cham: Springer; 2023. 10.1007/978-3-031-18587-8_27. [Google Scholar]
- 33.Zamil D, Khan RM, Braun TL, Nawas ZY. Dermatological uses of rice products: trend or true? J Cosmet Dermatol. 2022;21:6056–60. [DOI] [PubMed] [Google Scholar]
- 34.Aristizabal M, Gold MH. Skin care for males. In: Thaller SR, Cohen MN, editors. A comprehensive guide to male aesthetic and reconstructive plastic surgery. Cham: Springer; 2024. 10.1007/978-3-031-48503-932. [Google Scholar]
- 35.Zhou Z, Samizadeh S. Skin aging and skincare. In: Samizadeh S, editor. Non-surgical rejuvenation of Asian faces. Cham: Springer; 2024. 10.1007/978-3-030-840099-09. [Google Scholar]
- 36.Gabbott CM, Sun T. Comparison of human dermal fibroblasts and HaCat cells cultured in medium with or without serum via a generic tissue engineering research platform. Int J Mol Sci. 2018;19:388. 10.3390/ijms19020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Medical Association. A World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Med Assoc. 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data/study results cited in this article are contained within the text and supplemental material is available online.