Abstract
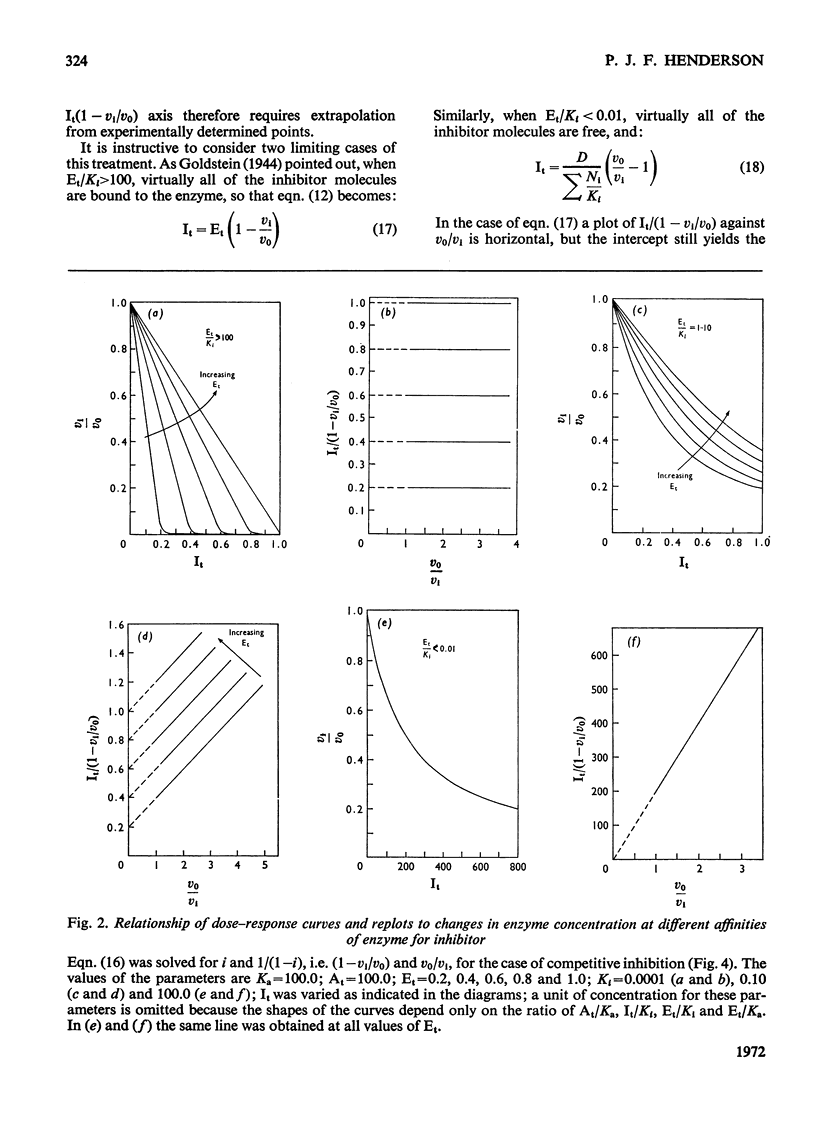
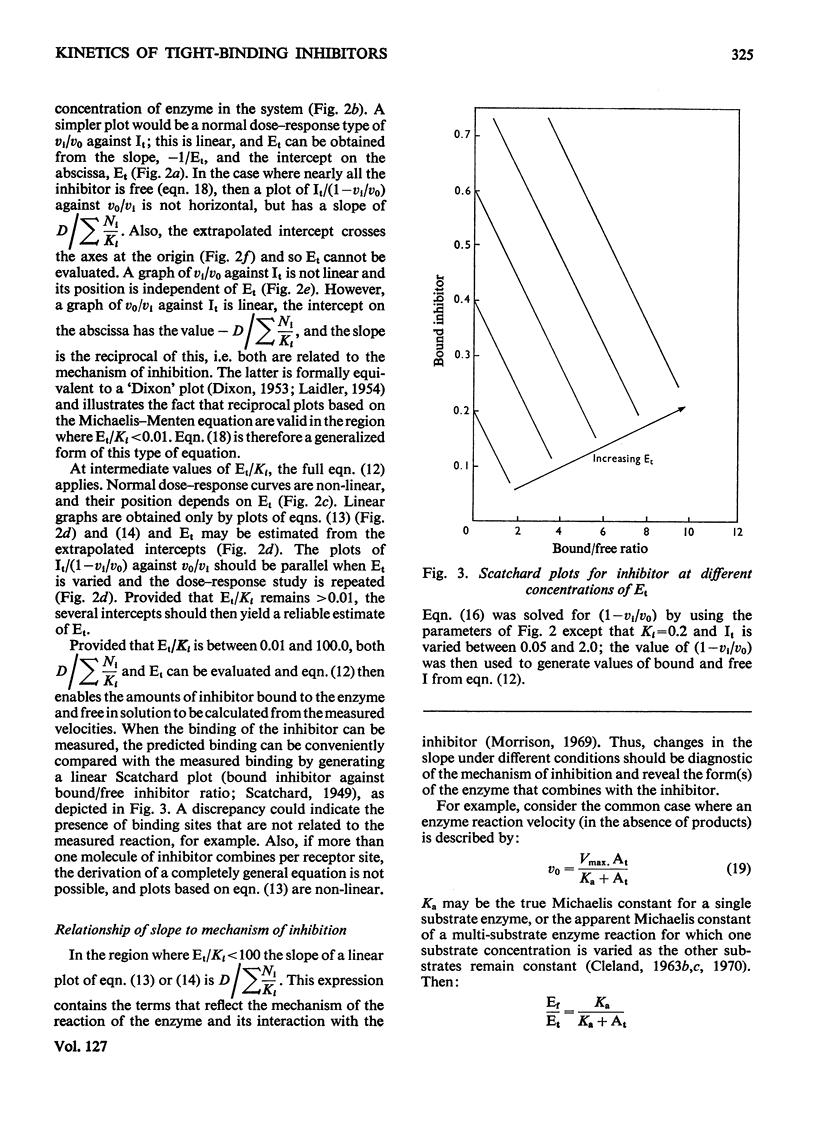
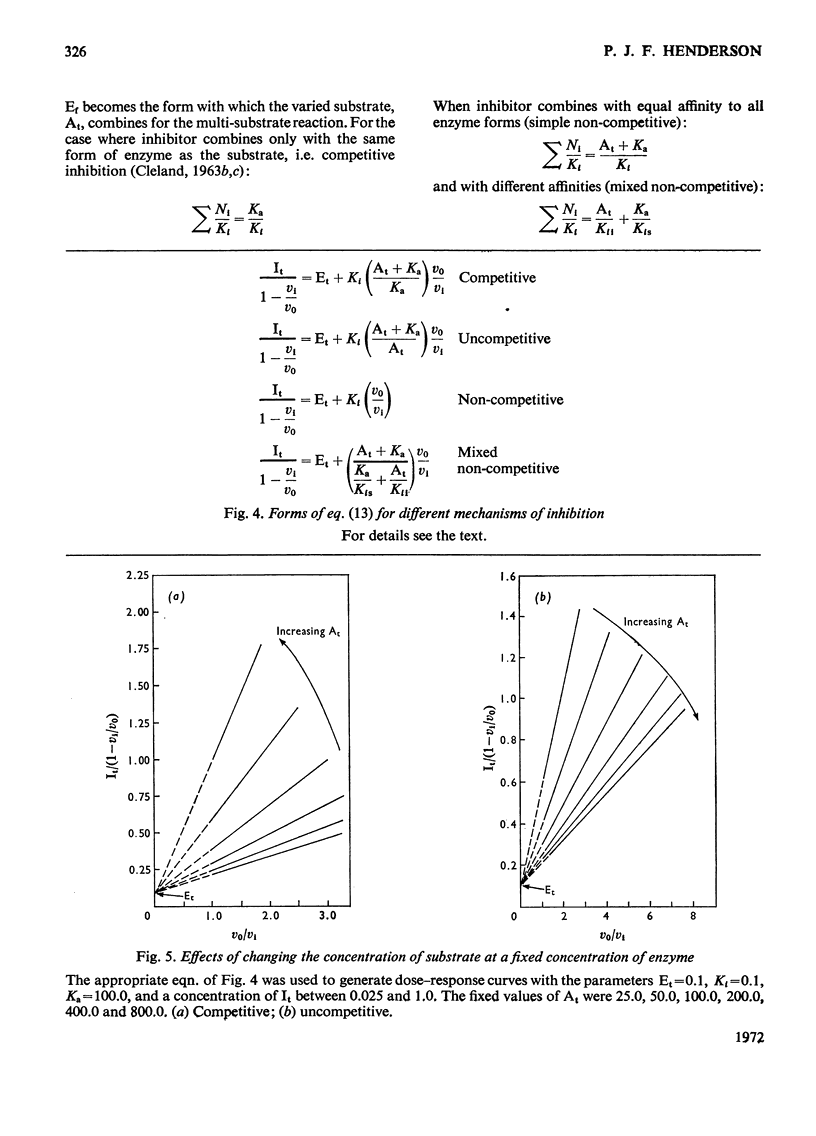
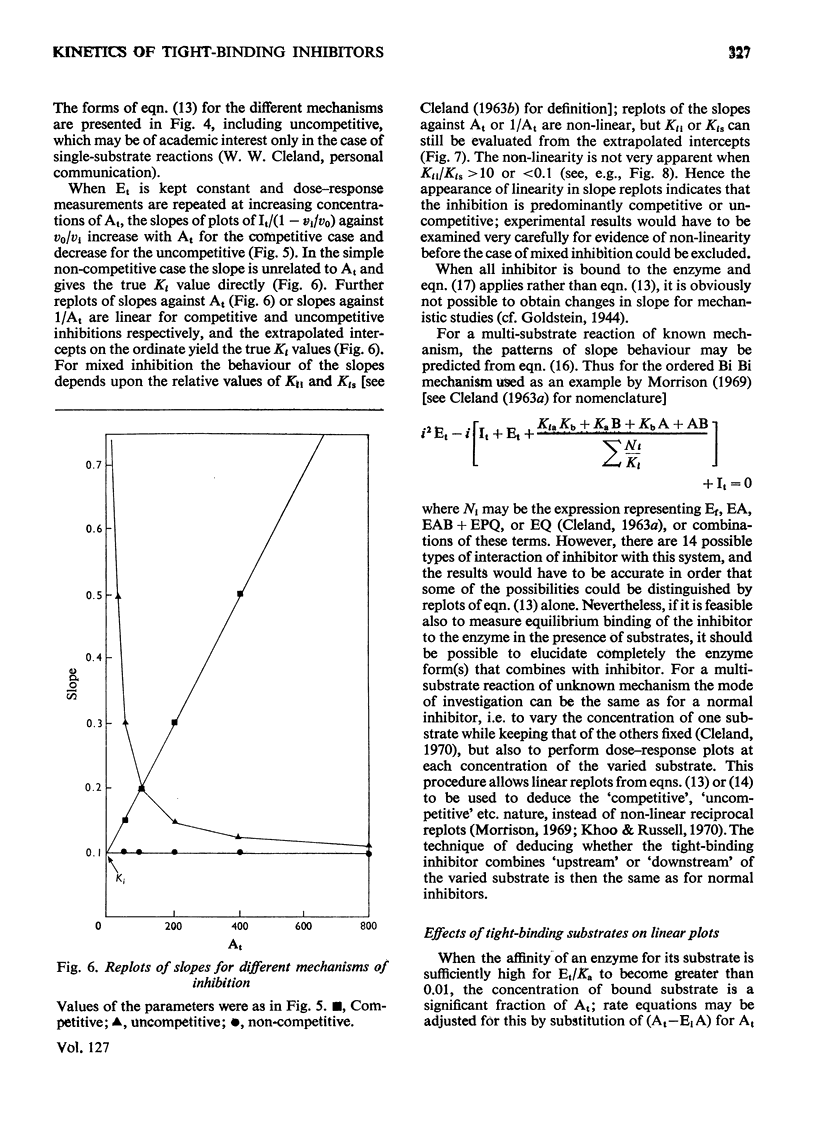
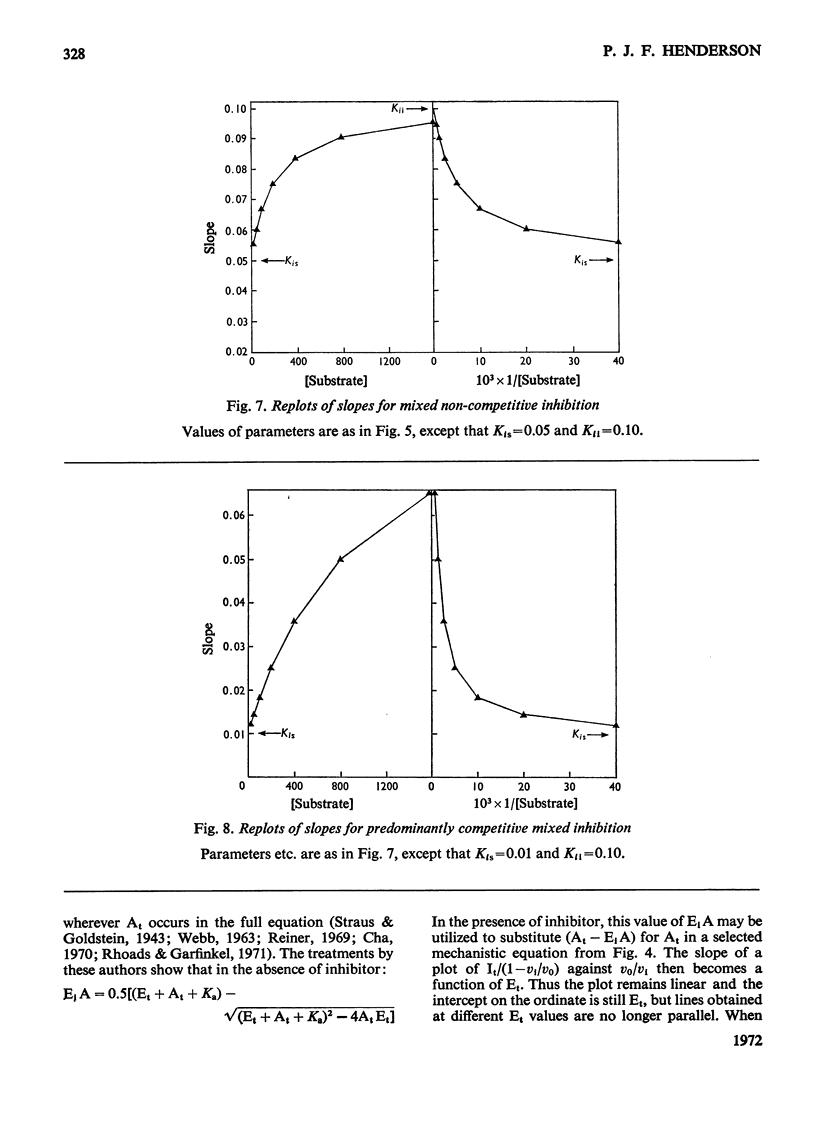
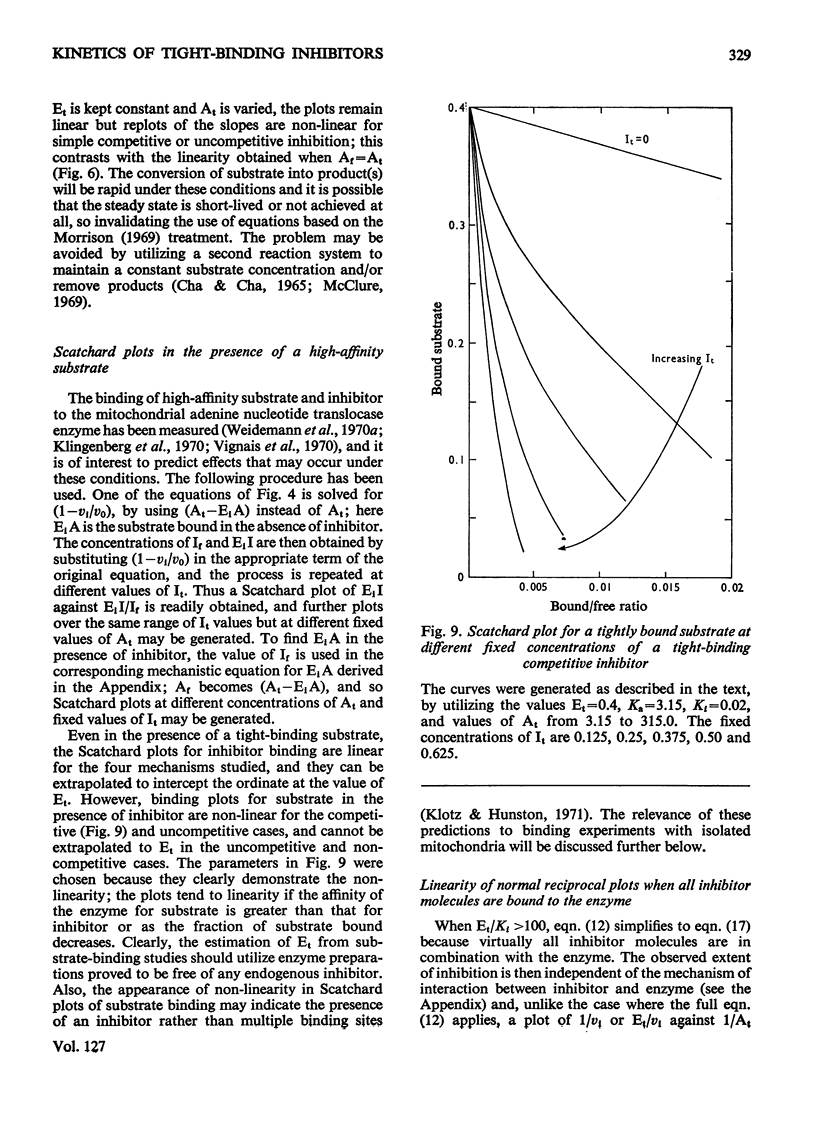
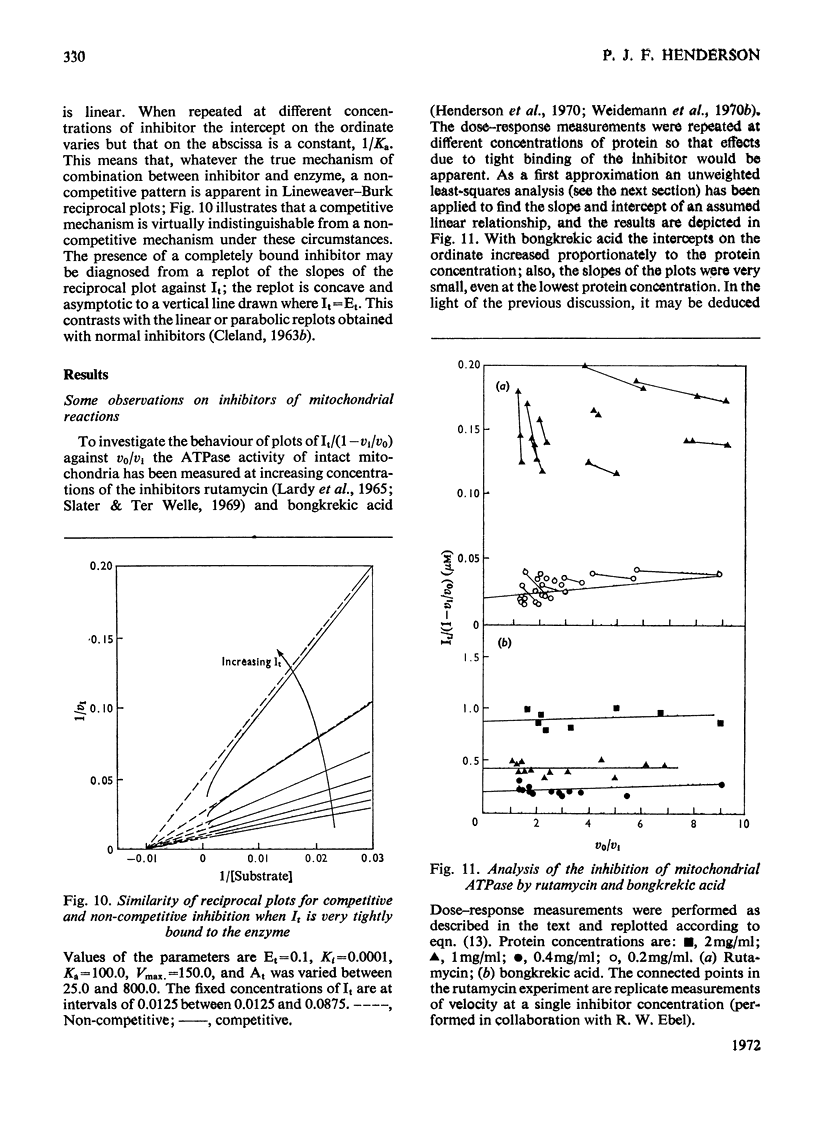
When an enzyme exhibits a high affinity for an inhibitor, the steady-state analysis of the mechanism is complicated by the non-linearity of normal dose–response plots or of reciprocal replots. It is shown here that dose–response measurements generate a linear plot of inhibitor concentration divided by degree of inhibition against velocity without inhibitor divided by velocity with inhibitor; the concentration of enzyme may be derived from the extrapolated intercept of such plots, and the mechanism of inhibition from replots of the variation of the slope with substrate concentration. The limiting cases where virtually all inhibitor molecules are bound or virtually all are free are described, together with the situation when a significant proportion of the substrate becomes bound. This type of analysis indicates that the inhibitors of oxidative phosphorylation, rutamycin and bongkrekic acid, are tightly bound to rat liver mitochondria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Cha S., Cha C. J. Kinetics of cyclic enzyme systems. Mol Pharmacol. 1965 Sep;1(2):178–189. [PubMed] [Google Scholar]
- Cha S. Kinetic behavior at high enzyme concentrations. Magnitude of errors of Michelis-Menten and other approximations. J Biol Chem. 1970 Sep 25;245(18):4814–4818. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes G. G., Hilborn D. A. Steady state kinetics of soluble and membrane-bound mitochondrial ATPase. Biochim Biophys Acta. 1971 Jun 1;233(3):580–590. doi: 10.1016/0005-2736(71)90156-8. [DOI] [PubMed] [Google Scholar]
- Harris E. J. Mitochondrial ATP contents during phosphorylation. J Bioenerg. 1971 May;2(2):93–99. doi: 10.1007/BF01648924. [DOI] [PubMed] [Google Scholar]
- Henderson P. J., Lardy H. A., Dorschner E. Factors affecting the inhibition of adenine nucleotide translocase by bongkrekic acid. Biochemistry. 1970 Aug 18;9(17):3453–3457. doi: 10.1021/bi00819a026. [DOI] [PubMed] [Google Scholar]
- JOHANSEN G., LUMRY R. Statistical analysis of enzymic steady-state rate data. C R Trav Lab Carlsberg. 1961;32:185–214. [PubMed] [Google Scholar]
- Khoo J. C., Russell P. J. On kinetic treatments of enzyme-antienzyme reactions. Biochim Biophys Acta. 1970 Nov 11;220(2):239–243. doi: 10.1016/0005-2744(70)90009-4. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Hunston D. L. Properties of graphical representations of multiple classes of binding sites. Biochemistry. 1971 Aug 3;10(16):3065–3069. doi: 10.1021/bi00792a013. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., WELLMAN H. The catalytic effect of 2,4-dinitrophenol on adenosinetriphosphate hydrolysis by cell particles and soluble enzymes. J Biol Chem. 1953 Mar;201(1):357–370. [PubMed] [Google Scholar]
- LARDY H. A., WITONSKY P., JOHNSON D. ANTIBIOTICS AS TOOLS FOR METABOLIC STUDIES. IV. COMPARATIVE EFFECTIVENESS OF OLIGOMYCINS A, B, C, AND RUTAMYCIN AS INHIBITORS OF PHOSPHORYL TRANSFER REACTIONS IN MITOCHONDRIA. Biochemistry. 1965 Mar;4:552–554. doi: 10.1021/bi00879a027. [DOI] [PubMed] [Google Scholar]
- MYERS D. K. Studies on cholinesterase. VIII. Determination of reaction velocity constants with a reversible inhibitor of pseudocholinesterase. Biochem J. 1952 Sep;52(1):46–53. doi: 10.1042/bj0520046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure W. R. A kinetic analysis of coupled enzyme assays. Biochemistry. 1969 Jul;8(7):2782–2786. doi: 10.1021/bi00835a014. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Activation and inhibition of mitochondrial adenosine triphosphatase by various anions and other agents. J Bioenerg. 1971 Feb;2(1):1–11. doi: 10.1007/BF01521319. [DOI] [PubMed] [Google Scholar]
- Morrison J. F. Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim Biophys Acta. 1969;185(2):269–286. doi: 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- Morrison J. F. Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim Biophys Acta. 1969;185(2):269–286. doi: 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- Shug A., Lerner E., Elson C., Shrago E. The inhibition of adenine nucleotide translocase activity by oleoyl CoA and its reversal in rat liver mitochondria. Biochem Biophys Res Commun. 1971 May 7;43(3):557–563. doi: 10.1016/0006-291x(71)90650-4. [DOI] [PubMed] [Google Scholar]
- Vignais P. V., Vignais P. M., Colomb M. G. 35S-Atractyloside binding affinity to the inner mitochondrial membrane. FEBS Lett. 1970 Jul 3;8(6):328–332. doi: 10.1016/0014-5793(90)80006-5. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann M. J., Erdelt H., Klingenberg M. Adenine nucleotide translocation of mitochondria. Identification of carrier sites. Eur J Biochem. 1970 Oct;16(2):313–335. doi: 10.1111/j.1432-1033.1970.tb01086.x. [DOI] [PubMed] [Google Scholar]
- Weidemann M. J., Erdelt H., Klingenberg M. Effect of bongkrekic acid on the adenine nucleotide carrier in mitochondria: tightening of adenine nucleotide binding and differentiation between inner and outer sites. Biochem Biophys Res Commun. 1970 May 11;39(3):363–370. doi: 10.1016/0006-291x(70)90585-1. [DOI] [PubMed] [Google Scholar]


