Abstract
Central nervous system (CNS) diseases are a major cause of disability and death worldwide. Due to the blood-brain barrier (BBB), drug delivery for CNS diseases is extremely challenging. Nano-delivery systems can overcome the limitations of BBB to deliver drugs to the CNS, improve the ability of drugs to target the brain and provide potential therapeutic methods for CNS diseases. At the same time, the choice of different drug delivery methods (bypassing BBB or crossing BBB) can further optimize the therapeutic effect of the nano-drug delivery system. This article reviews the different methods of nano-delivery systems to overcome the way BBB enters the brain. Different kinds of nanoparticles to overcome BBB were discussed in depth.
Keywords: Nanoscale delivery platforms, Nano-sized particles, Disorders of the central nervous system, Cerebrovascular barrier
Graphical abstract
Diagram of nanotechnologies in surmounting the blood-brain barrier in the disorders of the central nervous system.
Highlights
-
•
Nano-delivery systems can overcome the limitations of BBB to deliver drugs to the central nervous system (CNS) diseases.
-
•
Different kinds of nano-delivery systems have great capability to bypass or cross BBB with different molecular mechanism.
-
•
These nano-delivery systems are capable for optimizing the therapeutic effects.
1. Introduction
Central nervous system (CNS) diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), multiple sclerosis (MS), Epilepsy, stroke, and brain cancers, are major cause of disability and death worldwide [1,2]. Approximately, 17 % of the world's population suffers from CNS diseases, which has brought great challenges to human survival and an economic burden to the societies [3]. CNS diseases affect the neuronal structure and function of the brain, which fall into two primary categories: acute brain injury and chronic neurodegenerative diseases [4]. Acute brain injury includes Epilepsy and stroke. Chronic neurodegenerative diseases include AD, PD, and MS. Although technology is constantly improving for many diseases, most CNS diseases lack effective diagnosis and treatment strategies [5]. For example, aducanumab, donepezil, rivastigmine, galantamine, and memantine for AD, and L-DOPA for PD, do not cure the disease, only delay its progression [1]. This is because the presence of the blood-brain barrier (BBB) limits the entry of many therapeutic agents into the CNS [6].
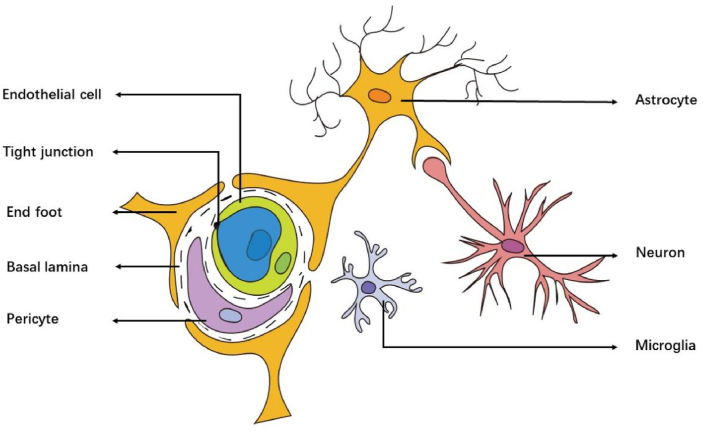
The BBB is a selective permeability barrier, composed of endothelial cells, astrocytes, basal lamina, capillary, interneuron, tight junctions (TJs), microglia, and pericytes [7]. BBB selectively restricts or prevents the passage of many hydrophilic or large molecules, allowing only a few small molecules such as O2 and CO2 to pass, while delivering specific nutrients to the CNS via transporters [6,8]. However, the is a double-edged sword. While protecting the brain tissues, it also limits the delivery of many therapeutics to the CNS. Therefore, overcoming BBB is an immediate neccessity for treating CNS diseases. Nanoparticles (NPs) with diameters ranging from 1 to 100 nm [9]. Have unique physicochemical properties such as small size, large surface to volume ratio and optical behavior [10].Based on these characteristics, nano-delivery systems are expected to help drugs cross the BBB and improve the targeting efficiency of drugs, bringing new hope for the treatment of CNS diseases.
In medicine, nanomaterials can be applied to drug delivery and controlled release, increase permeability, cross biological barriers, and improve biocompatibility [11]. So far, a variety of nanomaterials have been used in delivery systems, such as some NPs (gold, iron, cerium, molybdenum, silver, zinc oxide, silicon dioxide, magnetic materials, trehalose, chitosan, poly (lactic-co-glycolic acid) (PLGA) and PEGylated polylactide (PLA), etc.), liposomes, nanomicelles, and exosomes (EXO), etc [10,[12], [13], [14]]. The routes for administering these nano-delivery systems are mainly divided into two ways: 1) bypassing the BBB and 2) passing through the BBB. Different routes of delivery of NPs to bypass or pass through the BBB can further optimize the therapeutic effect of the nano-drug delivery system. This article reviews the different methods of nano-delivery systems to overcome the way BBB enters the brain. Different kinds of nanoparticles to overcome BBB were discussed in depth.
2. The blood-brain barrier
2.1. Brain barrier
The brain is protected by three main barriers, the BBB, the blood-cerebrospinal fluid barrier (BCSFB), and the arachnoid barrier [6,9]. BCSFB is a shielding structure composed of choroid plexus epithelium and choroid plexus capillary endothelium and its basement membrane. It forms an interface between the cerebrospinal fluid, which can prevent some substances from entering the cerebrospinal fluid from the blood vessels, and the cerebrospinal fluid is replaced about 3–5 times a day, and, therefore, most of the drug is removed each time from the cerebrospinal fluid [15,16]. The arachnoid barrier is located below the dura mater and surrounds the central nervous system. It can separate the extracellular fluid of the central nervous system from the extracellular fluid of other parts of the body. Although the arachnoid membrane can also form a barrier layer, its avascular nature and surface area are relatively small [17]. Based on these characteristics, BCSFB and the arachnoid barrier cannot be good drug delivery targets. BBB is produced by endothelial cells that form capillary walls, and the combined surface area of these microvessels constitutes the body's largest blood-brain exchange interface. In addition, BBB is also the most important barrier protecting the CNS and maintain brain homeostasis [10]. It shows that BBB has more advantages in its structure and function than BCSFB and the arachnoid barrier. Therefore, it is of great significance to focus on the barrier effect of BBB in drug delivery.
2.2. Blood-brain barrier
The conjecture of BBB was first proposed by Paul Ehrlich and proved by Edwin Goldmann [18,19]. The BBB is a special non-fenestrated vascular system, mainly composed of Brain microvascular endothelial cells (BMECs), astrocytes, basal lamina, capillary, interneuron, tight junction, microglia, and pericytes, which controls its permeability (Fig. 1). It maintains CNS homeostasis and prevents peripheral toxins and pathogens from entering the brain [20,21]. Endothelial cells constitute the capillary wall and are the main barrier of the BBB. The endfeet of astrocytes are encased in BMECs, matrix proteins secreted by the cells constitute the basement membrane, and pericytes are embedded in the basement membrane of glial cells and BMECs. Since BBB only allows small molecules (<500 Da and <400 nm) and lipophilic molecules are passively diffused, while other molecules must be actively transported, which limits the delivery of effective drugs and reduces the therapeutic efficiency [22,23]. Therefore, the brain delivery of the nano-delivery system can be enhanced by using the physiological characteristics of BBB. Studies have shown that the function of BBB is mainly attributed to endothelial cells (ECs) [24]. ECs are lipophilic, and lipophilic substances can pass through the BBB through simple diffusion. Therefore, lipophilic nano-delivery platforms can enhance the delivery of brain therapeutic drugs, such as liposomes and polymer NPs. In addition, many transport systems on BMEC have surface receptor and substrate selectivity. For surface receptors, a ligand-based nano-delivery system can be designed to interact with specific homologous receptors on the apical plasma membrane of endothelial cells. For substrate selectivity, a nano-delivery system that can bind to some nutrients (such as glucose, folic acid, etc.) can be designed, so that the system depends on the substrate selectivity of BMEC to nutrients and relies on Carrier-Mediated Transport (CAMT) to cross the BBB. In summary, in order to overcome the limitations of BBB, it is necessary to design a targeted delivery system based on the characteristics of BBB.
Fig. 1.
Schematic illustration of the BBB structure: astrocytes, basal lamina, capillary, endothelial cells, interneuron, tight junction, microglia, and pericytes. Inspired by Ref. [313].
2.3. Blood-tumor barrier (BTB)
BTB is a leakage barrier formed by CNS tumors by changing the tumor microenvironment (TME) and damaging the BBB. A certain type of cell inside the brain grows and proliferates in an abnormal way to form a lump or tumor, which originates in brain tissue and is called a primary brain tumor [25].
A certain type of cell inside the brain grows and proliferates in an abnormal way to form a lump or tumor, which originates in brain tissue and is called a primary brain tumor. Metastatic brain damage refers to the origin of the tumor in non-brain tissue, where the cancer cells extravasate and invade the brain, where they colonize and proliferate, developing into metastatic brain damage [26]. Whether it is primary brain tumor or metastatic brain injury, after cancer cell accumulation, the lesion is in a state of local hypoxia and activate hypoxia-inducible factors, which stimulate the production of VEGF [27]. Secreted VEGF then induces disruption of the existing BBB structure and the growth of structurally altered capillaries in the existing vessels. This newly formed, structurally altered capillary is abnormal, tortuous, poorly shaped, and more permeable than the intact BBB. In addition, existing blood vessels may be compressed by the growing tumor, or they may be absorbed by the tumor, which will also lead to increased leakage of BTB [28]. Although alterations in BTB integrity allow increased paracellular transport of molecules, BBB and BTB efflux processes may also be increased by the presence of tumor cells [26]. For example, many drugs used to treat melanoma and lung cancer brain metastases are actively efflux in preclinical mouse models [29]. This may account for the failure of chemotherapeutic agents in the treatment of CNS tumors.
3. The disorders of the central nervous system (CNS) and nanocarriers
CNS diseases include a variety which lack effective treatment methods [30]. This is because the CNS is highly protected by physiological barriers, especially the BBB, which limit the uptake of most drugs. Although many drugs can cross the BBB, such as temozolomide (TMZ), paclitaxel (PTX), doxorubicin (DOX), etc., their treatment success is still limited due to poor permeability across the BBB, chemotherapy resistance, and cytotoxic effects [[31], [32], [33], [34]]. Many investigators are pursuing a multidisciplinary approach of nanotechnology to overcome the main obstacles in the treatment of the CNS diseases and achieve the best therapeutic effect by designing nano-drug delivery systems with easy-to-control characteristics (such as particle size, shape, and surface characteristics) to control the pharmacokinetic characteristics, BBB crossing ability, cytotoxicity and disease targeting ability of drug carriers. Several approved or ongoing clinical trials of nano-preparations are listed here (Table 1).
Table 1.
Clinical study of nanomedicine in the treatment of central nervous system diseases.
| CNS Disease | nanoformulations | Nanocarrier | Mode of administration | Clinical Status |
|---|---|---|---|---|
| Mild to moderate Alzheimer's disease | APH-1105 | Intranasal nanoparticles | Intranasal | Phase II |
| Parkinson's Disease | CNM-Au8 | Gold nanocrystals | Oral | Phase II |
| Multiple Sclerosis | Copaxone®/Glatopa (Teva) | Polymer NP | Subcutaneous injection | FDA (1996) |
| Multiple Sclerosis | Plegridy® (Biogen) | Polymer NP (PEGylated IFN-β-1a) | Subcutaneous injection | FDA (2014) |
| GBM | EDV-doxorubicin | EDV-doxorubicin + radiation | Intranasal | Phase I |
| GBM | Interleukin-12 | Semliki forest virus vector carrying the human interleukin-12 gene | Intravenous | Phase I, II |
| GBM | Ferumoxytol | Iron oxide NPs | Intravenous | Phase II |
| GBM | Nanothermotherapy | Thermotherapy and magnetic iron-oxide NPs + reduced dose radiotherapy | Intrathecal | Phase II |
| GBM | Doxil®/Caelyx® | Doxorubicin HSPC, cholesterol, and DSPE-PEG2000 | Intravenous | Phase II |
| GBM | SGT-53 (SynerGene Therapeutics) | Cationic liposome with anti-transferrin antibody | Intravenous | Phase II |
| GBM | 5-fluorouracil | 5-fluorouracil-releasing microspheres | Intravenous | Phase II |
| GBM | Caelyx, PEG-Dox | Pegylated liposomal DOX | Phase II | |
| GBM | PEG-Dox | Temozolomide (TMZ) and Pegylated liposomal DOX | Oral | Phase II |
| GBM | Nanotherm® (MagForce) | Inorganic and metallic nanoparticles | Intravenous injection | FDA (2010) |
| GBM | Paclitaxel polyglutamate (Opaxio) | Paclitaxel covalently linked to solid lipid NPs | Intravenous | FDA (2014) |
3.1. Chronic neurodegenerative diseases
3.1.1. Alzheimer's disease
AD is characterized by a progressive decline in two or more cognitive domains, including memory, language, executive and visuospatial functions, personality, and behavior, resulting in the loss of ability to execute tools and/or basic activities of daily life [35]. A hallmark pathology of AD is the progressive accumulation of the protein fragment beta-amyloid (plaques) outside neurons in the brain and the twisted strands of the protein tau (tangles) inside neurons, these changes are eventually accompanied by the loss of synapses and neurons [36,37]. Both abnormal metabolism of Aβ and hyperphosphorylation of tau protein lead to neurodegeneration, and the reduction of acetylcholine (A neurotransmitter) and oxidative stress in the body may also be related factors in the occurrence of AD [38]. Therefore, the occurrence of AD may be a consequence of the interaction of multiple causes. Aβ plaques around the brain, soluble Aβ and tau protein in cerebrospinal fluid are the main biochemical markers of AD, which have been used for clinical diagnosis [39]. Current treatments for AD focus on inhibiting the formation of Aβ plaques/tau tangles and neutralizing their aggregation around neurons. Some clinically approved drugs can only relieve symptoms and delay the progression of AD by providing neurotransmitters [39]. Therefore, new AD markers and nanomedicines targeting AD markers are urgently needed.
3.1.2. Parkinson's disease
PD is a severe age-related neurodegenerative disease involving progressive impairment of voluntary motor control, often accompanied by symptoms such as depression, cognitive decline, and sleep changes, and its prevalence increases with age [[40], [41], [42]]. The underlying cause of the disease is caused by the loss of dopaminergic neurons in the substantia nigra of the midbrain [43,44]. At present, it is believed that Alpha-synuclein (α-Syn) misfolding and subsequent aggregation are the main reasons for dopaminergic neurons degradation in PD [45,46]. Due to various factors, α-Syn misfolds and aggregates into pathological forms. The imbalance between the formation and clearance of the pathological form leads to further deposition of α-Syn. In addition, α-Syn aggregates simultaneously propagate through intercellular and synaptic transmission and invade various brain regions, leading to cytotoxic effects in neurons and ultimately to the onset of PD. Currently, drug therapy (including but not limited to monoamine oxidase type B inhibitors, dopamine receptor agonists, catechol-O-methyltransferase inhibitors, etc.) is the mainstay of clinical treatment for PD. Unfortunately, current treatments do not cure PD. The non-targeted delivery of levodopa, a classic treatment for PD, can attack the peripheral system, leading to a series of adverse reactions, such as cardiovascular adverse reactions and movement disorders [47]. Therefore, encapsulating PD therapeutic drugs in a nano-delivery system, passing through the BBB and targeting the release of drugs into the brain, is a promising method for the treatment of PD.
3.1.3. Multiple sclerosis
MS is a chronic autoimmune disease characterized by multifocal inflammatory demyelinating lesions accompanied by neuronal and oligodendrocyte loss [[48], [49], [50], [51]]. Since myelin can isolate nerve fibers from their surroundings, the loss of myelin can lead to dysfunction of affected neurons in transmitting electrical signals, resulting in sensory and motor disorders [52]. MS shows different clinical phenotypes. In most cases, patients will experience recurrent clinical symptoms and then partially or completely recover, known as relapsing-remitting MS. Within 10–15 years, the frequency of relapse decreases, and the CNS gradually deteriorates, resulting in continuous progression in 50 % of RRMS patients, known as secondary progressive MS [53]. Accumulation of disability from the beginning, no recurrence is called primary progressive MS, accounting for 15 %–20 % of MS patients. For relapsing-remitting MS, it is more common to use corticosteroids (i.e.methylprednisolone) for treatment to reduce inflammation and end recurrence faster [54]. The phase III EXPAND trial led to the approval of Cinemod for the treatment of patients with secondary progression of active disease [55]. The phase III ORATORIO trial led to the approval of Ocrelizumab for the treatment of patients with primary progressive MS [56]. However, most treatments are associated with adverse reactions, partly due to the route of administration. It is mainly due to the poor permeability of BBB to drugs, resulting in low accumulation of drugs into CNS. Therefore, in order to overcome the limitations of traditional MS treatment methods, the use of drug delivery systems to deliver MS therapeutic drugs to the lesion site and improve the ability to penetrate BBB and brain targeting is a hot topic in current research.
3.2. Acute neuroinvasive sicknesses
3.2.1. Epilepsy
Epilepsy is a brain disorder characterized by large-scale abnormal synchronous firing of neurons, mainly due to an imbalance between excitatory and inhibitory neurotransmission [57]. At present, the first and second generation of antiepileptic drugs (AEDs) are used in clinical management of the disease, which include voltage-dependent sodium channel blockers, activators for extended opening time of chloride channels, and drugs for increased γ-aminobutyric acid (GABA) synthesis or decreased GABA degradation [58]. These drugs act by inhibiting excitatory currents or enhancing inhibitory currents. However, research data show that about 30 % of epilepsy patients are resistant to AEDs [59]. Based on the drug resistance of epilepsy patients to AEDs, two main hypotheses are proposed to explain. The first hypothesis suggests that resistance develops because AEDs cannot penetrate the BBB and enter the brain [59,60]. This is due to the overexpression of drug efflux transporters in the BBB of epileptic patients resistant to AEDs, which limit the access of AEDs to the brain. The second hypothesis is that the target receptor sites of AEDs are changed in some way in the brains of epileptic patients, resulting in their decreased sensitivity to drugs [61]. Current evidence supports that these two hypotheses are not mutually exclusive and both play a key role in the study of resistance to AEDs. Therefore, the efficacy of AEDs depends on their ability to cross the BBB and bind to target receptor sites [61]. Recently, nano-delivery systems have attracted attention for their ability to cross the BBB and improve the effectiveness of AEDs, opening up potential prospects for epilepsy treatment.
3.2.2. Stroke
Stroke is one of the most serious neurological diseases that pose a threat to human health, causing substantial long-term disability and even death worldwide. In terms of its physiological and pathological characteristics, stroke is often caused by cerebral vascular stenosis, occlusion or hemorrhage, which leads to the interruption or obstruction of blood supply to brain tissue, resulting in an insufficient supply of nutrition and O2 in the brain region, causing brain cell death and neurological dysfunction, and eventually causing permanent damage to brain tissue [62,63]. Stroke can be divided into three types, namely ischemic stroke, hemorrhagic stroke and transient ischemic attack. A majority of the of patients (87 %) suffer from ischemic stroke [[64], [65], [66]]. Ischemic stroke is caused by obstruction of blood flow in the CNS. Hemorrhagic stroke is caused by blood leakage due to rupture of blood vessels. A transient ischemic attack occurs due to the formation of a small embolism of a clot that temporarily blocks blood flow. The lack of effective measures for diagnosing and treatment of ischemic stroke still remain challenging [66]. Currently, restoring blood flow through thrombolysis and/or thrombectomy are the only approved treatments for ischemic stroke [67]. However, both methods need to be treated within the time window after the onset of the disease to ensure the effectiveness of the treatment, and only a small number of patients can be effectively managed by them [[67], [68], [69]]. In addition to the above two methods, the use of antiplatelet and anticoagulant drugs and neuroprotective agents in ischemic stroke treatment are possible alternatives, however, they all have certain limitations and obvious side effects [66,67,70]. Preclinical studies mostly focus on the neuroprotective agents in ischemic stroke at various stages of development and reperfusion. However, most of them are poorly soluble, their bioavailability is low due to BBB, and the therapeutic efficacy is limited by their short half-life [68,70]. Nanotherapy provides great advantages for the effective treatment of stroke in terms of crossing the BBB, improving drug bioavailability, increasing - drug accumulation, and reducing systemic toxicity. Therefore, the nano-drug delivery system has great application prospects as an effective and safe system for delivering therapeutic agents across BBB.
3.3. Brain cancers
Brain cancers are divided into two major categories, namely primary brain cancers that originate in brain tissue and secondary brain cancers that developed as a result of the metastatic spread of cancer cells from other regions. Among primary brain cancers, glioma caused by glial cells are the most prevalent type accounting for about 80 % of all malignant brain cancers, including glioblastoma (GBM), astrocytoma, oligodendrocyte glioma and ependymona [71,72]. GBM, a grade IV astrocytoma, is the most common and aggressive form of brain tumor, exhibiting heterogeneous, highly angiogenic, invasive, and migratory features [73,74]. The current clinical standard of care for brain cancers is maximally safe surgical resection followed by graded radiotherapy and chemotherapy (oral Temozolomide, and/or implanted Carmustine wafer) [75,76]. However, surgical resection always has numerous limitations because it does not completely remove infiltrating tumor cells within normal brain parenchyma and because of the location of brain tumors corresponding to sensitive or inoperable areas of the brain. In addition, residual tumor cells after surgical resection are protected by BBB, hindering drug treatment of the tumor [77]. Therefore, to maximize the treatment of brain tumors, it is necessary to overcome the protective effect of BBB on the brain.
4. Pathways to access the brain
Transporting nanoparticles to overcome the blood-brain barrier (BBB) can be categorized into two ways: bypass the BBB and cross the BBB (see Fig. 2).
Fig. 2.
The way nanoparticles overcome the blood-brain barrier in central nervous system diseases. Inspired by [38].
4.1. Bypass the BBB
4.1.1. Intranasal delivery (ID)
In 1989, Professor W.H.Frey II 's pioneering research on ID made great progress in the treatment of diseases such as brain cancer and infection, stroke and extensive CNS diseases [78]. ID is a minimally invasive and safe administration method, which can bypass the BBB through the olfactory nerve pathway between nasal mucosa and CNS [79]. ID is a noninvasive and safe method of drug administration that is able to bypass the BBB directly through the olfactory mucosa [79]. The nasal cavity is divided into three areas: vestibular, respiratory and olfactory. Drugs are absorbed into the systemic circulation through the respiratory zone and into the CSF through the olfactory zone [80]. When drug molecules enter the nasal cavity, they reach the brain via two main routes: the primary route through the neuronal pathway and the secondary route through the systemic circulation and the blood-brain barrier [81]. Therapeutic agents of ID enter the brain through three pathways [82]: (a) the Olfactory route: When the therapeutic agent reaches the olfactory mucosa through the ID, it makes direct contact with olfactory receptors located on the cilia at the end of olfactory receptor neurons, crosses axons and nerve bundles through the cribriform plate into the olfactory bulb and CSF. (b) the Trigeminal route: occurs between the branches of the trigeminal nerve that innervate the respiratory and olfactory mucosa, transferring drug molecules/agents directly to the brain stem and other connected structures. For example, lidocaine, insulin-like growth factor 1, and interferon-β-1b enter the brain through this pathway. In addition, this pathway can be transported intracellularly via axons and diffusely and massively extracellularly via perineural channels, perivascular spaces, or lymphatic channels connecting cerebrospinal fluid and brain tissue, but extracellular and intracellular transport depends on the physical and chemical properties of the drug molecule/formulation [83]. (c) the Systemic route: The uptake of therapeutic agents from the nasal cavity to the brain may also occur through the blood circulation. Because the airway epithelium is more rich in blood vessels than the olfactory mucosa, a small part of the drug will be absorbed into the systemic circulation. Therefore, a correct understanding of the transport mechanism of ID drugs to the brain is crucial for the treatment of diseases.
ID, a non-invasive method, is a promising strategy for bypassing the BBB to deliver neurotherapeutic agents. This has a more precise drug targeting, faster onset of action, avoidance of drug first-pass metabolism in the gastrointestinal tract and liver, larger drug absorption area, dose reduction, minimal side effects, noninvasive, convenient and patient-friendly route of administration [84]. However, the surface area of the nasal cavity is smaller than that of the mouth, and the contact area of the drug is reduced, reducing the concentration of the drug in the blood. The protective barrier of the nasal mucosa limits the efficiency of ID treatment. In addition, insufficient drug exposure time, mucociliary clearance, nasal mucosal irritation, drug enzymatic degradation, and poor drug retention are also barriers to intranasal administration [85]. Therefore, the clinical application of ID still needs to go a long way.
4.1.2. Intrathecal and intraventricular delivery (IID)
Intrathecal drug administration is a technique for delivering drugs that circumvents the blood- Intrathecal drug delivery is a method of drug delivery that bypasses the BBB and is suitable for analgesia, spasticity and the treatment of CNS tumors [86]. During IID, drugs are administered directly into CSF contained in the subarachnoid space of the CNS. Pulsatile CSF blood flow induces micromixing of CSF and intrathecal therapeutic drugs, resulting in their rapid dispersion. IID bypasses the BBB, but once injected, the drug is usually rapidly dispersed away from the target site, so the treatment results are not satisfactory [87]. Nano-delivery systems have emerged as a promising approach to overcome the limitation that drugs cannot be adequately delivered to target tissues or cells. Compared with freely administered drugs, the application of nanoparticles can prolong the half-life of the drug, prolong the retention time of the drug in CSF, and make it reach the brain parenchyma more effectively after intrathecal administration.
However, it is worth mentioning that IID is an invasive procedure. Most patients need repeated lumbar subarachnoid puncture to release CSF and intrathecal injection to achieve a certain curative effect, which is complicated and brings great pain to patients. At the same time, repeated puncture is easy to cause reinfection. In addition, the unidirectional flow of CSF restricts the entry of intrathecal drugs into the ventricular system, and it can also easily cause persistent infection of the ventricular system. Bacteria can continue to enter the CSF and maintain active infection. Because of this, it is necessary to carefully select IID drugs for treatment to prevent the occurrence of some complications, such as infection, neurotoxicity, and increased intracranial pressure.
4.1.3. Intratumoral delivery (ITD) and convection-enhanced distribution (CED)
In recent years, more and more nano-drug delivery systems have been deeply explored and used for cancer treatment due to their passive tumor targeting, enhanced permeability and retention [88]. However, some complications of their systemic administration are inevitable, such as inferior tumor targeting efficiency, systemic toxic effects, and weakened anti-tumor activity [89]. To overcome the limitations of systemic administration, some investigators have proposed the intratumoral administration as an alternative approach [90]. Intratumoral therapy allows tumor-killing locally. This method can improve the targeting efficiency of drugs, enhance the concentration of drugs in the tumor and the tumor death, and reduce the off-target toxicity of drugs [88,91,92].
CED is a promising technique that creates a pressure gradient at the tip of an infusion catheter inserted into the brain parenchyma to deliver therapeutic agents directly through the interspace of the CNS [93]. CED has several potential advantages over traditional delivery methods: (a) bypassing the BBB, where it can be used for the delivery of therapeutic agents of high or low molecular weight through massive interstitial flow. (b) a targeted delivery of therapeutic agents according to the placement of the catheter, with means of real-time monitoring of the distribution of therapeutic agents and intelligent regulation of their flow and velocity; (c) an enhanced interstitial drug distribution driven by pressure gradients [94,95]. CED was first proposed in 1994 by Bobo et al. as a promising technique to bypass BBB and deliver in a diffusion-independent manner [96,97]. This mechanism of bypassing the BBB dramatically increases the number of therapies that can feasibly be used to treat glioma [97]. Because of this, CED has significantly improved the efficacy of drug, macromolecular, and nanocarriers delivery to the brain [98].
ITD and CED are common invasive drug delivery strategies in cancer therapy. These strategies aim to efficiently deliver drugs to tumor tissues and minimize damage to healthy tissues. However, there are some limiting factors that may affect the efficacy of these strategies [89,99,100]: (a) The effective administration of ITD and CED is highly dependent on the technique of the operator. (b) One of the challenges of CED is reflux. Reflux reduces the chance of achieving therapeutic drug concentrations in the target structures and increases the risk of off-target side effects. (c) Rapid interstitial clearance of the brain may hinder the distribution of some therapeutic agents in the brain. (d) Tumor-specific factors also have the potential to limit CED. Tumors with elevated interstitial pressure can block the positive pressure gradient that drives drug to the tissue site, limiting uniform drug distribution. To resolve these problems, extensive experimental validation and clinical implementation are needed to maximize the efficacy of these drugs and reduce the adverse reactions of patients receiving these invasive dosing strategies.
4.2. Crossing the BBB
The transport mechanism across the blood-brain barrier (BBB) can be categorized into the Receptor-Mediated Transport (RMT), Carrier-Mediated Transport (CAMT), Cell-Mediated Transport (CeMT), and Tight Junction (TJ) Modulation. Among these, RMT and CAMT are the primary endogenous transport systems of BBB. Our discussion will center on RMT and CAMT.
4.2.1. Receptor-mediated transport
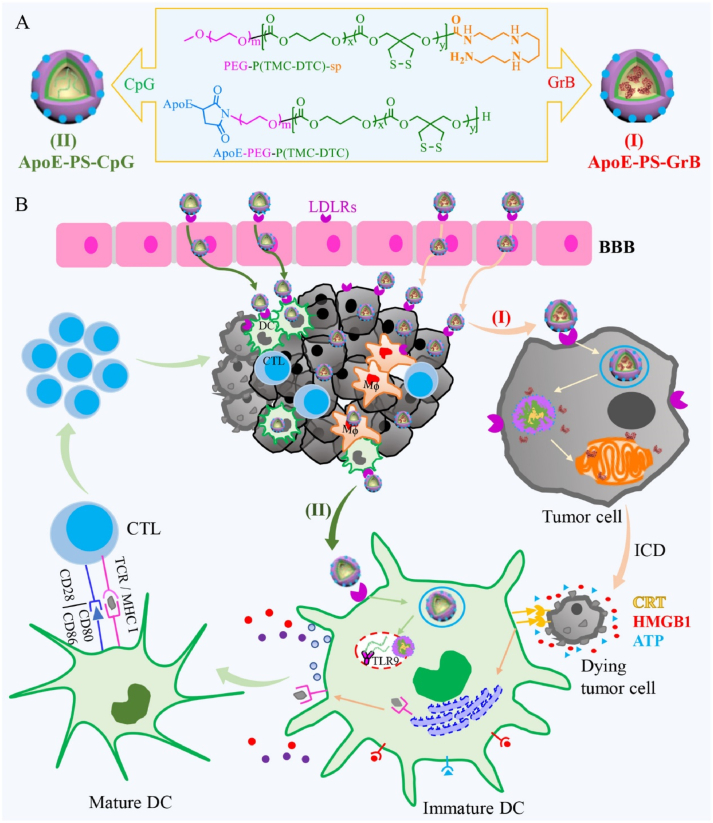
A promising strategy to overcome the BBB to deliver therapeutic agents for CNS diseases is RMT. The mechanism by which it crosses the BBB involves the vesicle transport system of the brain endothelium [101,102]. That is, RMT across the BBB requires ligand interaction with specific cognate receptors at the apical plasma membrane of endothelial cells. Intracellular transport and vesicle sorting then proceed, and finally vesicles containing receptor-ligand complexes or vesicles containing dissociated ligands are delivered to the basolateral side of polarized endothelial cells, where they are released. In this way, molecules can cross the endothelium and enter the brain without disrupting BBB properties. Because it uses vesicle-based transport, the delivery of many biological agents can cross the BBB and enter the brain through the RMT mechanism, including monoclonal antibodies, recombinant proteins, RNA, DNA, and nanomedicine [103]. For example, in a study by Jhaveri et al. [104], RES was loaded into PEGylated liposomes with a surface modified by Tf and used the passive and active targeting capabilities of TF-modified liposomes to effectively treat GBM. Tf ligands on the surface of liposomes can specifically bind TfR, which is upregulated in GBM, thus TF-modified nanoparticles are cancer cell specific. This binding can enter the brain through receptor-mediated endocytosis, unconstrained by the BBB, and enhances the therapeutic capacity against tumors. In addition, ApoE peptide can specifically bind to LDL-R. Jiang et al. [105] prepared an ApoE peptide-functionalized polymersomal granzyme B, the binding of ApoE peptide on the surface of the nanoparticle to LDLR effectively crossed the BBB through transendocytosis, it strongly inhibited growth and caused immunogenic cell death in murine glioma cells (Fig. 3). Pinheiro et al. [106] prepared a RVG29 functionalized lipid nanoparticle loaded with quercetin, and the RVG29 ligand on its surface could bind to nAChR. It can enhance the penetrating ability of BBB, improve the drug concentration of quercetin through BBB, inhibit amyloid-β aggregation, and achieve the purpose of treating AD. In addition, Cui et al. [107] employed a strategy of constructing TanIIa-gL micelles (TGM) by self-assembly of TanIIA and GL and loading them into serum EXO to prepare CpG-EXO/TGM (Fig. 4). They used intravenous injection to deliver CpG-EXO/TGM into the body for therapy of mice. TfR on the surface of the exosome membrane can bind to free Tf in the blood and cross the BBB in a RMT-dependent manner. CpG-EXO/TGM is effective in two ways. First, release of TanIIA and GL inhibited GBM cell growth. Second, CpG-EXO/TGM induced anti-GBM immune responses in TME. CpG-EXO/TGM can effectively prevent postoperative recurrence by using chemotherapy and immunotherapy in the treatment of GBM. It is important to note that because the receptor is not static in vivo, many factors need to be considered when delivering drugs by RMT, which are closely related to the expression of the receptor under pathological conditions, the receptor before and after treatment, and the efficiency of age-dependent RMT transport [108].
Fig. 3.
Illustration of fabrication of ApoE peptide-functionalized polymersomes encapsulating GrB or CpG ODN (ApoE-PS-GrB and ApoE-PS-CpG) (A) and highly enhanced immunotherapy of murine orthotopic LCPN glioma (B). Reprinted with permission from Ref. [105].
Fig. 4.
(A) Preparation of CpG-EXO/TGM. (B) process of CpG-EXO/TGM crossing the BBB. (C) the process of CpG-EXO/TGM using chemotherapy and immunotherapy to synergistically treat GBM. Reprinted with permission from Ref. [107].
4.2.2. Carrier-mediated transport (CAMT)
Due to the high transport affinity between transporters and substances, CAMT is one of the most promising methods to promote the entry of drugs into the brain. Many transport systems on brain endothelial cells are substrate-selective, which provides the brain with essential nutrients and endogenous substrates. The transport of nutrients such as glucose, amino acids and nucleosides is divided into two cases. One is the transport without energy driven by electrochemistry or concentration gradient, and the other is the transport of solute in the direction of its electrochemical potential increase, accompanied by promoted diffusion. In this way, nutrient molecules bind to specific transporters on the cavity side, and transporters change their conformation and transfer nutrients to the brain parenchyma. Therefore, drugs that bind to endogenous substances such as nucleosides, glucose, and amino acids can be absorbed and delivered to the brain through four major transporters on the BBB (i.e., amino acid transporters, monocarboxylate transporters, peptide transporters, and clearance receptors). In addition, in the design of nanocarriers, endogenous substances such as nucleosides, glucose and amino acids can also be used to modify nanocarriers to promote nanocarriers to effectively cross the BBB and play a therapeutic role. Similarly, Ginsenoside Rg3 (Rg3) contains two glucosyl residues in the hydrophilic glycoside chain, which are substrates for glucose transporters (GLUTs). Zhu et al. [109] developed a multifunctional ginsenoside Rg3-based liposomal system (Rg3-LPs) (Fig. 5). These studies revealed that Rg3-LPS could be actively delivered to the brain and overcome the BBB because of its ligand Rg3. When Rg3-LPs loaded with PTX, the proliferation of glioma cells was significantly inhibited and the median survival time of the model mice was significantly prolonged. Therefore, Rg3-PTX-LPs can be used as a multifunctional potential drug for the treatment of glioma.
Fig. 5.
Multifunctional ginsenoside Rg3-based liposomes for glioma targeting therapy. Reprinted with permission from [314].
4.2.3. Cell membrane-mediated transport (CMMT)
Both Cryptococcus neoformans and immune cells such as monocytes or macrophages can enter the brain via CMMT [110]. It indicates that natural CMMT, such as macrophages, neutrophils, tumor cells, red blood cells, mesenchymal stem cells, platelets and endothelial cells are good carriers [[111], [112], [113], [114], [115]]. This feature promotes the effective penetration of biomimetic nanoparticles modified by immune cells through the BBB through CEMT, targeting brain tumors. CMMT nanoparticles can effectively cross the BBB and target brain tumors. For example, Yin et al. [114] developed an engineered macrophage-membrane-coated nanoplatform with enhanced programmed cell death-1 (PD-1) expression (PD-1-MM@PLGA/RAPA) (Fig. 6). Since macrophages can respond to the GBM microenvironment and return home, macrophage membrane-modified nanoparticles can efficiently cross the BBB reach TME, where nanoparticles are delivered to the tumor site. He et al. [116] designed a biomimetic nanomedicine (AM@NP (ABT/A12)) (Fig. 7). AM@NP (ABT/A12) consists of two parts, ABT and A12 co-loaded pH-sensitive acetal-grafted dextran (a-dextran) as core (NP (ABT/A12)) and ApoE peptide-functionalized RBC membrane (ApoE-RBCm, AM) was used as the outer shell. Due to the intrinsic biocompatibility and non-immunogenic properties of BC membranes, it is easy to penetrate the BBB and target GBM to maintain prolonged blood circulation. These studies show that CMMT can transport any type of molecule or substance or drug, which offers new possibilities for crossing the BBB.
Fig. 6.
Schematic Illustration of Preparing Engineered Macrophage-Membrane-Coated Nanoparticles with Enhanced PD-1 Expression. Reprinted with permission from Ref. [114].
Fig. 7.
Brain-Targeted Codelivery of Bcl-2/Bcl-xl and Mcl-1 Inhibitors by Biomimetic Nanoparticles for Orthotopic Glioblastoma Therapy. Reprinted with permission from Ref. [116].
4.2.4. Tight junction (TJ) modulation
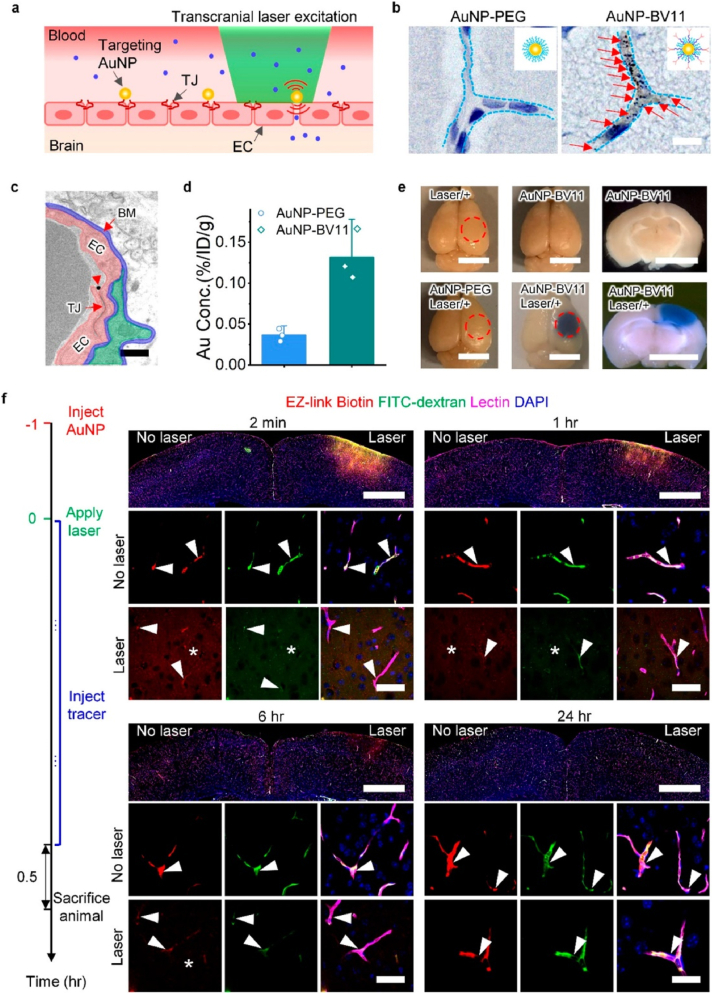
The BBB is one of the most powerful physical barriers in the body and is composed of TJ proteins in brain microvascular endothelial cells [117] TJ plays a crucial role in maintaining the integrity of the BBB. Disruption of the TJ, impairs BBB integrity. Previous studies have emphasized that certain methods can be used to destroy TJ proteins between endothelial cells, break through the BBB and enter the central nervous system, thereby changing the permeability of the BBB [110]. Chen et al. [118] used focused ultrasound to partially destroy TJ proteins such as claudin-5 in brain endothelial cells, thereby changing the permeability of BBB. Li et al. [119] designed and synthesized AuNPs and used picosecond-laser to excite TJ-targeted plasma AuNPs and regulate BBB permeability (Fig. 8). This technique allows the nanoparticles to enter the brain parenchyma without causing any apparent damage to the vascular dynamics and brain parenchyma. This is a promising strategy for safe and effective delivery of therapeutic agents to the CNS. Therefore, using minimally invasive methods to temporarily disrupt TJ to change BBB permeability and deliver therapeutic agents is of great significance for the development of CNS disease treatment.
Fig. 8.
(a) Schematic for transcranial laser stimulation of TJ-targeted AuNP for BBB modulation. (b) AuNPs are visualized. (c) Detection of TJ co-localization. (d) The accumulation of AuNP-BV11 and AuNP-PEG in the brain was determined. (e) Observation of BBB modulation. (f) Detection of BBB permeability. Reprinted with permission from Ref. [119].
4.3. Temporarily open BBB
4.3.1. Focused ultrasound (FUS)
In recent years, FUS-mediated BBB opening has been widely used in the delivery of drugs to the brain [120].However, the irregular shape and large acoustic impedance of the skull can cause beam aberrations, resulting in poor penetration of ultrasound through the skull. Therefore, clinical tests still require craniotomy to perform ultrasound therapy in the brain [121,122]. In response to these obstacles, it has been proposed that when combined with the use of acoustic simulation based on skull CT scans to determine the phase and amplitude correction of phased arrays [123] and MR temperature imaging (MRTI) to monitor fever, it can completely non-invasively replace brain surgery [124]. This method has been able to pass the human skull focused ultrasound, and the magnetic resonance imaging (MRI) -guided system is currently undergoing clinical phase I trials of thermal ablation of malignant brain tumors [125]. In 2001, Hynynen et al. [126] first proposed that FUS combined with micron-scale microbubbles (MBs) can enhance the effect of FUS. When FUS is used in combination with MB, the thermal effect is not the main mechanism for BBB opening, and the mechanical effect is the main mechanism for more substantial BBB opening [127]. In short, under the action of ultrasound, microbubble oscillation causes an instantaneous increase in cell membrane permeability, that is, acoustic perforation, which opens the tight junction in BBB. However, under low sound pressure, the microbubble oscillates in a stable motion mode, that is, stable cavitation; at higher sound pressure, microbubbles grow and collapse violently, that is, inertial cavitation. Stable cavitation has the least negative impact on the tissue, but the microbubbles in the stable oscillation will also generate sufficient pressure to rupture the blood vessel wall. The severe collapse of inertial cavitation will lead to cell apoptosis, tissue necrosis and vascular wall rupture [128]. Therefore, inertial cavitation needs to be reduced when using focused ultrasound combined with microbubbles to increase BBB permeability. In addition, apoptosis induced by focused ultrasound opening BBB may also be related to the dose of microbubbles. Therefore, in the process of combining FUS with MB, the parameters of ultrasound and the dose of microbubbles needed to be controlled may be able to minimize the risk of cell and tissue damage on the basis of maximizing the opening of BBB.
4.3.2. Magnetic Field Force
Magnetic Field Force (MNPs) refers to magnetic nanoparticles, which are usually made of magnetic materials such as ferrite (such as Fe3O3 or γ-Fe2O3), cobalt, nickel and so on. MNPs have been used for brain drug delivery [129]. The study of magnetic NP is mainly based on three mechanisms: functional modification of ligands, the application of external magnetic fields, and the use of low RF fields [130]. Based on the functional modification of ligands similar to RMT, peptides, antibodies and small molecules are used as NP-linked ligands to interact with specific homologous receptors on the apical plasma membrane of endothelial cells to promote their transport to the brain. The application of external magnetic fields is to use external magnetic forces to move MNPs so that they can be guided from the vascular lumen to the brain parenchyma. The internal MNPs can temporarily destroy the endothelial cell-cell connection, activate the paracellular transport pathway, and promote the local extravasation of circulating substances. The third is to use the heat generated by magnetic NPs under MNPs to open BBB instantaneously and locally. Although the application of magnetic NPs has great potential in the fields of medicine and biotechnology in terms of drug delivery and medical impact, its biocompatibility, safety, control of MNPs and manufacturing cost and scale are still a challenge in practical applications.
4.3.3. Osmotic agent
BBB permeability is achieved using hypertonic agents (such as mannitol, ethanol, and dimethyl sulfoxide), which cause dehydration and contraction of brain capillary endothelial cells BCEC, resulting in tight junction dysfunction and transient destruction of BBB [131]. These penetrants can promote, increase and improve the entry of different drugs into the brain tissue. Intra-arterial mannitol-mediated permeability opening is most commonly used in preclinical and clinical studies [132]. In 1973, Brightman et al. [133] used this method for the first time to demonstrate that intra-arterial mannitol injection induces endothelial contraction and endothelial tight junction opening, causing drugs to enter the brain. In the next few decades, intra-arterial mannitol-mediated therapy has been the main method used in preclinical models and clinical studies to temporarily open the BBB before infusion of therapeutic agents. However, the effect of intra-arterial mannitol-mediated treatment on opening BBB is not a constant layer, but is related to mannitol dose, injection speed, vascular anatomy and cerebral hemodynamics of individual patients or experimental animals [132]. Therefore, improper mannitol injection may not affect the BBB state by safely opening the tight junction of the endothelium, but by directly damaging brain cells. Studies [134] have shown that using dynamic susceptibility can predict the opening of BBB and ensure that the drug is administered to the correct position after BBB opening, which is expected to improve the therapeutic effect and reduce complications in the clinical environment.
5. Nanomaterial-mediated strategies for overcoming BBB
5.1. Liposome
Liposomes are nanoparticle vesicles with good biocompatibility and biodegradability, consisting of phospholipid bilayers with hydrophilic and hydrophobic properties [135]. Due to their amphiphilic character, lipophilic drugs can be encapsulated in the phospholipid bilayer or adsorbed on the surface of liposomes, while hydrophilic drugs can be encapsulated through the aqueous internal encapsulation of vesicles [136]. In recent years, great progress has been made in the research on liposomes as carriers of various substances (such as anti-tumor drugs, antibacterial agents, proteins and peptides, volatile oils, steroids, vaccines, and DNA, etc.) [137]. Since their phospholipid bilayer structure is similar to physiological membranes, making it easier to target and cross the BBB, and facilitating drug delivery into the brain [138]. Liposomes are the earliest discovered nano-drug carriers, which have outstanding characteristics such as targeted delivery, high biocompatibility, biodegradability, easy functionalization, low toxicity, and immunogenicity, which greatly improve the sustained release and therapeutic index of drugs [139,140]. However, some limitations of traditional liposomes hinder their application in drug delivery. These include problems such as rapid systemic elimination, low drug encapsulation efficiency, and transport rate, stability after long-term storage, and sustained drug release [98,141]. To overcome these limitations on the therapeutic effect of brain diseases, liposomes can be enhanced by enhancing their surface modification ability to improve their targeting ability to the brain. For example, with the help of some ligands (like transferrin (Tf), lactoferrin (Lf) etc.), molecular carriers (such as glutathione, glucose etc.), specific peptides, etc., liposomes can quickly and effectively cross the BBB and deliver drugs to designated sites. Here we present three common modified liposomes and summarize the articles published after 2020 on overcoming BBB liposomes in the field of CNS disease treatment (Table 2).
Table 2.
Recent research on liposomes to overcome BBB in the treatment of CNS diseases.
| Surface modify | Targeting ligands | Receptors | Model drug | Use | Others | Refs |
|---|---|---|---|---|---|---|
| glucose-functionalized liposome @TMZ&PAP(gLTP) | glucose | glucose transporter 1 (GLUT1) | TMZ and pro-apoptotic peptide (PAP) | GBM | gLTP can easily penetrate the BBB and effectively deliver drugs to brain tumors, significantly improving overall survival. | [142] |
| n-Butylidenephthalide (BP)/polycationic liposomal polyethylenimine and polyethylene glycol PEG complex (LPPC) | / | / | BP | GBM | LPPC transported BP through the BBB, and uptake through clathrin-dependent, caveolae-mediated and macropinocytosis led to the accumulation of large amounts of BP in tumors and triggered strong anticancer activity. | [143] |
| muscone/RI7217 co-modified docetaxel (DTX) liposomes | RI7217 | TfR | DTX | glioma | The RI7217 monoclonal antibody recognizes TfR through receptor-mediated transendocytosis, which facilitates BBB penetration to the tumor site and enhances brain targeting. muscone modification increased BBB permeability. Therefore, the constructed musk/RI7217 co-modified long-circulating DTX liposomes not only increased the drug concentration in the brain, but also enhanced the anti-glioma effect of DTX. | [144] |
| transferrin-modified Ost liposomes (Tf-Ost-Lip) | Tf | TfR | Osthole(Ost) | AD | Tf-Ost-Lip increased the intracellular uptake of hCMEC/D3 cells and APP-SH-SY5Y cells, and improved the efficiency of BBB delivery. It has a protective effect on APP-SH-SY5Y cells. The retention time of Ost in mice was prolonged, and the concentration of Ost in brain was increased. | [145] |
| Paclitaxel-loaded Rg3- based liposomal system (Rg3-PTX-LiPs) | glucosyl residues of Rg3 | GLUTs | PTX | glioma | Ginsenoside Rg3 can be used as a better substitute for cholesterol in the preparation of liposomes. In addition to improving the stability and mobility of liposomes, ginsenoside Rg3 can also enhance the BBB penetration ability, tumor targeting ability, tumor cell killing activity, TME remodeling ability and PTX chemotherapy effect. | [109] |
| T7-modified liposomes/vincristine (T7-LS/VCR) | T7 | TfR | VCR | glioma | T7-LS/VCR enhanced BBB permeability and showed high anti-glioma effect by promoting cytotoxicity and apoptosis in vitro. | [146] |
| DCDX liposomes (HSPC/cholesterol/PEG2000-DSPE/DCDX -PEG3400-DSPE (52/43/3/2, molar ratio)) | D-type polypeptide (DCDX) | Nicotinic acetylcholine receptors (nAChRs) | / | glioma | DCDX liposomes in the cells of the transport is very complex, involving a variety of ways, such as the endocytic pathways and organelles. This study optimized the structure of the brain-targeted drug delivery system and improved the efficiency of drug delivery. | [147] |
| RVGMAN and PenMAN liposomes encapsulating ApoE2/chitosan complex | mannose (MAN), rabies virus glycoprotein peptide | glut-1; acetylcholine receptor | plasmid encoding ApoE2 (pApoE2) | AD | RVGMAN and PenMAN liposomes encapsulation of ApoE2/chitosan complexes enhanced BBB penetration and brain targeting, resulting in efficient delivery of ApoE2 encoding plasmid DNA. | [148] |
| DOX-terpolymer-lipid-hybrid nanoparticle (DOX-TPLN) | polysorbate 80 (PS 80) | Low-density lipoprotein receptor (LDL-R) | DOX | GBM | The efficacy of DOX was better than that of TMZ. However, the application of DOX in GBM is limited due to its difficulty in penetrating the BBB. The DOX-TPLN formulation enables the penetration of DOX, a brain-impermeable anticancer drug, across the BBB for the treatment of GBM. | [149] |
| Brain derived neurotrophic factor AntagoNAT (BDNF AT) cationic liposomes | / | / | / | PD | BDNF is the most intensively studied target for PD, which can reverse the progression of the disease. BDNF AT is able to up-regulate BDNF expression, so it can be targeted to increase endogenous BDNF expression to treat PD. However, BDNF AT cannot cross the BBB, and BDNF AT cationic liposomes can overcome this problem. | [150] |
| Ost liposomes with modified CXCR4 on the surface (CXCR4-Ost-Lips) | Stromal cell-derived factor-1 (SDF-1) | CXCR4 | Ost | AD | Although Ost can improve AD, its neuroprotective effect is limited by various limitations, such as poor solubility, poor stability, poor brain targeting, and low bioavailability. In addition, SDF-1 is highly expressed in the brain of AD patients and is the only ligand of CXCR4, which has the potential to become a brain-targeting molecule. The results showed that CXCR4-Ost-Lips was able to enhance brain targeting, increase cellular uptake, and prolong the blood circulation time of the drug. | [151] |
| Lf and muscone dual‐modified DTX long‐circulating liposomes (Lf‐LiP‐Mu‐DTX) | Lf | Lactoferrin receptors (LfR) | DTX | glioma | Lf was modified to actively target LfR, and muscone was modified to increase BBB permeability on the liposome surface. Thus, LF-LiP-MU-DTX can increase cellular uptake, enhance BBB penetration, and enhance brain targeting and anti-glioma efficacy in vitro and in vivo. | [152] |
| PenTf-Liposomes Containing Chitosan–plasmid nerve growth factor (pNGF) | Tf | TfR | pNGF | AD | PenTf-Liposomes Containing chitosan-PNGF can protect pNGF from enzymatic degradation, promote NGF transfection, and overcome BBB, which has the potential to treat AD. | [153] |
| GSH-ApoE-Cur-QU-EGCG-RA-PC-liposomes | GSH; ApoE | glutathione receptors (GSHR); LDL-R | Cur; Quercitin (QU); Epigallocatechin gallate (EGCG); Rosmarinic acid (RA) | AD | GSH and ApoE on the liposomes enhanced BBB penetration, and ApoE and PC recognized Aβ in the delivery of the four drugs. | [154] |
| Aniopep-2- icariin/tanshinone IIA liposomes (Ang2-ICA/TSIIA liposomes) | Angiopep-2 (A2) | Low-density lipoprotein receptor-related protein-1 (LRP1) | Icariin (ICA) and tanshinone IIA (TSIIA) | AD | Ang2-ICA/TSIIA liposomes have brain targeting ability and are able to cross the BBB. | [155] |
| SNA-Liposome-ApoE/RVG | ApoE; rabies virus glycoprotein (RVG) | LDL-R; nAChRs | oligonucleotide miRNA inhibitors (OMIs); | GBM | Compared with Sn-liposome-RVG, SN-liposome-apoe is more likely to advance mirNA-based GBM therapies as well as the transformation of other CNS diseases. | [156] |
| Epirubicin (EPI) plus resveratrol (RES) liposomes modified with p-aminophenyl-α-D-manno-pyranoside (MAN) and wheat germ agglutinin (WGA) | MAN; WGA | GLUT; WGA receptors | EPI; RES | glioma | The EPI plus RES liposomes modified with MAN and WGA can significantly improve the transport of EPI and RES across the blood-brain barrier and the survival of brain tumor animals with multifunctional targeting. | [157] |
| ApoE- artesunate-phosphatidylcholine (ARTPC)@TMZ | ApoE | LDLR | artesunate (ART); TMZ | GBM | The ApoE-ARTPC@TMZ nanoplatform enhances BBB penetration for effective delivery of ART and TMZ chemotherapy drugs. ART and TMZ synergistically reduced the tumor burden and prolonged the survival of mice, indicating that they could improve the prognosis of patients with CNS tumors treated with TMZ chemotherapy. | [158] |
| polyethylene glycol-cyclic Arg-Gly-Asp- emodin liposomes (PEG-cRGD-Em-Lip) | cRGD | αvβ3 receptor | Em | Ischemic Stroke | PEG-cRGD-Em-Lip achieves brain-targeted delivery of Em and significantly improves the therapeutic effect of Em in ischemic stroke. | [159] |
| Tf- Vitamin B12 (VB12)- liposomes | Tf | TfR | VB12 | AD | The NPs were stable under storage conditions for 2 months and sustained release of VB12 for up to 9 days. | [160] |
| ApoE- resveratrol/salidroside (Res/Sal)- liposomes | ApoE | LDL-R | Res/Sal | AD | ApoE-Res/Sal-Lips can alleviate AD pathological symptoms, reduce learning and memory impairment, and improve brain function. | [161] |
| RVG29-nAChR-liposomes | RVG29 | nAChR | Quercetin | AD | RVG29-nAChR-liposomes can improve Quercetin permeability through BBB and inhibit amyloid β aggregation. | [162] |
| PTX-CHO-RVG15-Lipo | RVG15 | nAChR | PTX | glioma | PTX-CHO-RVG15-Lipo improved BBB penetration and anti-glioma efficacy of the drug. | [163] |
| novel cascade-targeted liposomes (Lip-TPGS) using glucose and triphenylphosphonium (TPP) as targeting moieties | The PEGylated glucose modified ligand (Chol-TPG) | GSHR | doxorubicin (DOX) prodrugs (SDOX) and chemotherapeutic sensitizer lonidamine (LND) | glioma | Lip-TPGS release is a combination of SDOX and LND to effectively treat glioma. | [164] |
| MAN/CPP- vgf-liposomes | glucose transporter-1 targeting ligand and brain targeted cell-penetrating peptide (CPP) | GLUT-1 | plasmid encoding vgf | AD | MAN/CPP-vgf-liposomes promote the brain targeting ability, transfection efficiency and biocompatibility of plasmacyto-encoding vgf. | [165] |
| Tf-Pep63-Lip | Tf | TfR | Pep63 | AD | Tf-Pep63-Lip can bypass the BBB to enter the brain and rescue cognitive impairment in AD mice. | [166] |
| PTX-Bio2+Glu3-Lip | Glu3-Chol; biotin 2 (Bio2)-Chol | GLUTR; SMVT receptor | PTX | glioma | Multi-target ligands can significantly enhance the tumor targeting ability of liposomes. | [167] |
| PEG-Lip-DOX/CB | / | / | DOX, carboplatin (CB) | GBM | Peg-lip-dox/CB can penetrate the BBB and enhance the cytotoxic effect of the drug on rat glioma cells, and PEG is more effective in increasing the therapeutic effect and reducing the side effects of the drug. | [168] |
| WGA-Lep-AS-IV-NF-1-PS-Lip | WGA; Leptin (Lep); Phosphatidylserine (PS) | N-acetyl-D-glucosamine and sialic acid; Lep receptor (LEPR); α-synuclein (α-Syn); | Astragaloside IV (AS-IV); Nesfatin-1 (NF-1) | PD | WGA-Lep-AS-IV-NF-1-PS-Lip increased BBB permeability of AS-IV and NF-1 and showed promising effects in reducing neurotoxicity. | [169] |
| Angiopep-2-modified calcium arsenite liposome (A2-PEG-Lip@CaAs) | A2 | LRP | arsenic trioxide (ATO) | glioma | A2-PEG-Lip@CaAs has high drug loading, high embedding efficiency, and can effectively target anti-glioma. | [170] |
| pH-sensitive multi-targeted liposomes (Lip-CTPP) | p-hydroxybenzoic acid (p-HA) | dopamine/sigma receptors | DOX and LND | glioma | Lip-CTPP has a good pharmacokinetic behavior, can cross the BBB, and has a strong tumor cell targeting ability. Moreover, the synergism of the two drugs increased the anti-tumor efficacy. | [171] |
| cisplatin- (Cispt) loaded PEGylated liposomes, targeted with OX26 monoclonal antibody | OX26 | TfR | Cispt | GBM | In this study, encapsulation of drugs into liposome nanoparticles can reduce nephrotoxicity, hearing toxicity and neurotoxicity, and enhance the anti-tumor activity of drugs. | [172] |
| Caffeic acid (CA)-Tf-Lip | Tf | TfR | CA | AD | Ca-tf-lip can promote the sustained release of CA. | [173] |
| Tf-functionalized liposomes for the delivery of gallic acid (GA) | Tf | TfR | GA | AD | The liposome prepared in this study can improve the ability to penetrate the BBB, enhance the colloidal stability, promote the sustained slow and stable release of drugs, and have neuroprotective effects. This indicated that the liposome could enhance the anti-tumor activity of the carried GA. | [174] |
| temozolomide magnetic temperature-sensitive liposomes (TMZ/Fe-TSL) | / | / | TMZ | GBM | TMZ/Fe-TSL exposed to an alternating magnetic field (AMF) could significantly induce GBM cell death and promote ROS production. | [175] |
| A2-Sal/Ica-Lip | A2 | LRP | Salidroside (Sal) and Icariin (Ica) | AD | A2-Sal/Ica-Lip can cross the BBB to increase drug accumulation in the brain and increase cellular uptake. In addition, it can reverse neuronal and synaptic damage, inhibit neuroinflammation and oxidative stress, and improve learning and cognitive function. | [176] |
| folate-modified TPGS-transfersomes containing DTX (TF-DTX-FA) | FA | folic acid receptor (FAR) | DTX | GBM | Transfersomes are different from traditional liposomes, They are ultradeformable (ultra-flexible) vesicles that consist of at least one inner water core wrapped in a lipid bilayer composed of phospholipids and surfactants (edge activators). | [177] |
| atorvastatin-loaded PEGylated liposomes (PEGylated LipoStatin) | / | / | atorvastatin | acute ischemic stroke | PEGylated LipoStati can be effectively delivered to the ischemic brain and has a significant neuroprotective effect. | [178] |
| folic acid (FA) derivatives and mitochondria-targeting berberine (BBR) derivatives co-modified liposome coated with Tween 80 loading paclitaxel (PTX-Tween 80-BBR + FA-Lip) | FA | FAR | PTX | glioma | PTX-Tween 80-BBR + FA-Lip can be aggregated in tumor tissues and targeted to mitochondria. | [179] |
| Lep/RES-EGCG-PA-liposomes (PA: cholesterol and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate) | Lep | Lep receptor | RES and epigallocatechin gallate (EGCG). | PD | Lep/RES-EGCG-PA-liposomes increased the drug loading rate and decreased the drug release rate. | [84] |
| Functionalized nanoparticles for brain targeted BDNF gene | MAN | GLUT1 | plasmid encoding BDNF | AD | Liposomes were surface-functionalized with brain targeting ligand, MAN, and cell-penetrating peptides (CPP) (rabies virus-derived peptide or penetratin). CPP, rabies virus-derived peptide (RDP) or penetratin (Pen), was used in combination with MAN | [180] |
| RVG-Lf-AZD5582-SM-164-liposomes | Lf; RVG | LfR; nAChR | AZD5582; SM-164 | GBM | This liposome improved the embedding efficiency of AZD5582 and SM-164 and reduced the release rate. Moreover, it promotes the synergistic activity between AZD5582 and SM-164 to facilitate its transit through the BBB. | [181] |
| α5β1 integrin selective liposomes of RGDK-lipopeptide | RGDK | α5β1 integrin receptors | WP1066 (a small-molecule STAT3 inhibitor) and STAT3siRNA | GBM | Intravenous administration of liposomes containing coencapsulated STAT3siRNA and WP1066 resulted in a significant increase in the overall viability of orthotopic established glioblastoid-bearing mice. | [182] |
| neurotransmitter-modified liposomes (NTs-LIP): tryptamine (Tryp) + Camptothecin (CPT) + Cur | / | / | CPT; CUR | glioma | The introduction of Tryp promotes the delivery efficiency of CPT and CUR across the BBB | [74] |
| procaine-loaded liposome modified with cRGDyK (Pro/cRGDyK-L) | pentapeptide cyclic (arginine-glycine-aspartic acid-tyrosine-lysine) (cRGDyK) | αvβ3 receptor | procaine | glioma | The cRGDyK peptide significantly facilitated the ability of liposomes to transfer procaine across the BBB and improved the cellular uptake of procaine by glioma cells. | [183] |
| gH625-lipoPACAP | / | / | pituitary adenylate cyclase-activating polypeptide (PACAP) | PD | PACAP neuroprotective effects when delivered by gH625-liposome on MPP-damaged SH-SY5Y spheroids gh625 (Liposome-delivered PACAP provides neuroprotection in MPP-injured SH-SY5Y spheres). | [184] |
| FA-modified lidocaine-carrying liposome (Lid-FA-Lip) | FA | FAR | lidocaine-carrying | glioma | Lid-FA-Lip is a promising liposomal preparation of lidocaine, which can be mediated through the PI3K/AKT pathway to improve the therapeutic effect on glioma. | [185] |
5.1.1. Surface functionalization of liposomes with transferrin
Tf is an iron-binding glycoprotein that can specifically bind to TfR. TfR is one of the main proteins expressed on the lumen side of the BBB [186]. Therefore, it can be combined with Tf-modified liposomes to achieve specific targeting of Tf-modified liposomes. Yang et al. [166] used the ability of TfR to target BBB to construct Tf-modified liposomes, which were loaded with a small peptide Pep63 that has neuroprotective effects on synaptic plasticity and memory. In vivo experiments have shown that Tf-Lip can bypass the BBB and allow the therapeutics to enter the brain. Ashrafzadeh et al. [172] TfR-targeted liposomes loaded with Cispt were used to transport Cispt to the BBB and deliver Cispt to brain tumors. The results of in vitro and in vivo experiments showed that Tf-modified liposomes could effectively cross the BBB and deliver Cispt to brain tumors, improving the therapeutic effect. Among the numerous potential targets of the BBB, TfR remains the most common target for ensuring adequate drug delivery to the brain. However, TfRs are expressed not only on the blood-brain barrier, but also on normal cells such as red blood cells, hepatocytes, intestinal cells, choroid plexus epithelial cells, and neurons [186]. Liposomes targeting TfR also reach the liver and kidney. In addition, the expression of TfR on the cell surface is physiologically regulated, which may limit the localization of liposomes targeting TfR. Therefore, the design and optimization of TfR as a target delivery system need to be re-considered to ensure the safety and effectiveness of treatment.
5.1.2. Surface functionalization of liposomes with specific peptide
Liposomes modified with RGD peptide (Arg-Gly-Asp) showed excellent brain-targeted drug delivery ability [187]. Li et al. [188] developed the RGD tripeptide-modified vinorelbine plus tetrandrine liposomes, which can overcome BBB, reverse multidrug resistance, and target glioma cells. The in vitro and in vivo studies showed that the liposome could significantly overcome the BBB, accumulate the drug in glioma cells, and inhibit the ability of glioma formation in mice. However, RGD peptides are frequently combined with other peptides or ligands to create multifunctional liposomes. This allows the RGD peptides not only to exert their own abilities but also to enhance the uptake and penetration of the liposomes through the BBB and brain targeting. For example, Liu et al. [189] prepared multifunctional liposomes, where in a specific ligand cyclic RGD (cRGD) peptide coupled to a cell-penetrating peptide R8. These multifunctional liposomes exhibited enhanced uptake, and effectively passed through the BBB, entered the brain, and accumulated in the glioma lesions of mice. These liposomes appear to serve as a potential anti-glioma drug delivery system. Similarly, Chen et al. [159] also prepared a multifunctional liposome, using PEG and cRGD-modified liposomes to encapsulate emodin. Their experiments showed that compared to the emodin-alone, the multifunctional liposomes significantly reduced brain edema and BBB decomposition, and achieved the goal of treating ischemic stroke. Their research provides a targeted drug delivery vehicle for the treatment strategies of ischemic stroke and other brain injuries. In summary, RGD is a promising therapeutic method for CNS diseases, whether it is modified liposomes alone or coupled with other peptides or ligands.
5.1.3. Multifunctional liposomes
Multifunctional liposomes can overcome BBB and increase drug concentration in the brain. Wu et al. [190] constructed a BBB-targeted delivery system of Tf-PEG PEGylated cationic liposomes, which was be used to deliver protamine-labeled NGF. Tf and PEG can target BBB and increase drug circulation time, respectively. The cationic liposomes containing protamine have a nuclear localization function, which further enhances nuclear localization and gene expression. The multifunctional liposomes prepared by Kuo et al. [154] are triple liposomes of PC liposomes and cross-linked GSH and ApoE liposomes. These liposomes had improved the stability, increased the drug-encapsulation, prolonged the release time of the drug, enhanced the ability to pass through the BBB, recognized the brain microvascular endothelial cells and Aβ, and reduced the hyperphosphorylation of tau protein to treat AD.
5.2. Nanoparticles
NPs are submicroscopic particles whose diameter is usually between 1 and 100 nm [9]. Due to the unique physicochemical properties, including their small size, large surface-to-volume ratio, and optical behavior, NPs areincreasingly used in drug delivery and bioimaging applications [10]. As already said, BBB is a major challenge in treating the CNS diseases. To overcome this limitation, NPs can be loaded to carry the drugs across the BBB. Since not all NPs can pass through the BBB, and many factors need to be considered, such as the material, size, and surface properties of the NPs [191]. One of the most important factors is the choice of nanomaterials. The materials of NPs include inorganic materials (gold, iron, cerium, molybdenum, silver, zinc oxide, silica, magnetic materials, etc.) and organic materials (trehalose, chitosan, PLGA and PLA, etc.) [10,12]. The appropriately chosen NPs can improve the efficiency drug transport into the CNS and reduce elimination by the peripheral circulation and systemic side effects [10,192]. It is worth noting that NPs may induce cytotoxicity, immunotoxicity and neurotoxicity, so they should be minimized while treating the CNS diseases [193]. Table 3 shows some inorganic and organic materials for common nanoparticles used for overcoming BBB.
Table 3.
Recent research on nanoparticles (inorganic materials and organic materials) to overcome BBB in the treatment of CNS diseases.
| Surface modify | Targeting ligands | Receptors | Model drug | Use | Others | Refs |
|---|---|---|---|---|---|---|
| Prussian blue/polyamidoamine (PAMAM) dendrimer/Angiopep-2 (PPA) nanoparticles | A2 | LRP1 | Prussian blue | AD | PPA NPs have superior BBB penetration and are able to exert a synergistic effect of ROS scavenging and restoring mitochondrial function in microglia. PPA NPs effectively reduced the neurotoxic Aβ aggregates and rescued the cognitive function of APP/PS1 mice. | [194] |
| Lacosamide- gold nanoparticles (LCM-AuNP) | glucose | GLUT1 | LCM | epilepsy | The binding of LCM to AuNP not only inhibited seizure activity but also reduced the dosage of antiepileptic drugs. | [195] |
| CTHG-Lf NPs | Lf | LfR | TMZ | glioma | CTHG-Lf NPs is an intelligent BBB permeable nano platform with hollow mesoporous copper sulfide nanoparticles (HM-CuS NPs) as TMZ carrier and hyaluronic acid (HA) as gatekeeper. Further modified with glucose oxidase (GOx) and Lf to achieve efficient synergistic treatment of in situ GBM. | [196] |
| siRNA-loaded synthetic protein nanoparticles (SPNPs) | iRGD | SPARC34/GP60 | STAT3 siRNA | GBM | Sirna-spnps equipped with the cell-penetrating peptide iRGD, capable of delivering siRNA STAT3, were able to improve the survival of GBM mouse models. | [197] |
| rotigotine-loaded chitosan nanoparticles (RNPs) | / | / | rotigotine | PD | Delivery of RNPs by ID enhanced brain targeting efficiency and drug bioavailability. | [198] |
| dodecamer peptide (G23)-functionalized polydopamine (pD)-coated curcumin-loaded zein nanoparticles (Cur-ZpD-G23 NPs) | / | / | Cur | GBM | Cur-ZpD-G23 NPs increased cellular ROS production and induced apoptosis in glioma cells. | [199] |
| integrin α2β1-targeting H-ferritin (2D-HFn)-based drug delivery system | H-ferritin | TfR1 | ntegrin α2β1 | glioma | 2D-HFn not only significantly improved DOX drug loading capacity, but also improved BBB penetration and brain targeting ability. 2D-HFn loaded with DOX significantly inhibited subcutaneous and orthotopic tumor progression and prolonged survival in the orthotopic glioma mouse model. | [200] |
| PLGA functionalized magnetic Fe3O4 nanoparticle (MNP) with L-carnosine peptide (LMNP) composite loaded with dexamethasone (dm@LMNP) | / | / | dexamethasone | ischemic stroke | L-carnosine functionalized iron oxide nanoparticles loaded with dexamethasone can not only improve BBB permeability but also improve the therapeutic effect of ischemic stroke. | [201] |
| Fe3O4 nanozymes | / | / | / | Cerebral ischemic stroke | dietary PEG-modified Fe3O4 nanozymes can facilitate blood–brain-barrier reconstruction and protect neurons following ischemic stroke. | [85] |
| a macrophage loaded with a photothermal nanoprobe (MFe3O4-Cy5.5) | / | / | / | glioma | Fe3O4-Cy5.5 can perform fluorescence, photoacoustic and magnetic resonance imaging, which can be used for precise tumor resection. It can also effectively induce local photothermal therapy and inhibit postoperative glioma recurrence. | [202] |
| DOX- ethylenediamine triacetic acid- iron oxide nanoparticles (DOX-EDT-IONPs) | / | / | DOX | GB, | The combination of magnetically enhanced convective diffusion and cadherin-binding peptides to transiently open the BBB TJ is expected to improve the efficacy of GBM chemotherapy using DOX-EDT-IONPs. | [203] |
| Tween 80- rhynchophylline loaded methoxy poly -poly nanoparticles (T80-NPS-RIN) | apolipoprotein E | LRPs | RIN | AD | T80-NPS-RIN has an enhanced therapeutic effect on nerve injury, with higher brain RIN accumulation concentration and higher bioavailability. | [204] |
| Chiral gold nanoparticles (Chiral AuNPs) | chiral GSH ligand | GSH transporters | / | AD | D3.3 AuNPs have greater binding affinity for Aβ42 and higher brain biodistribution, thereby having A stronger inhibitory effect on Aβ42 fibrillation in AD model mice. | [205] |
| angiopep-2 modified lipid-coated mesoporous silica nanoparticle loading paclitaxel (ANG-LP-MSN-PTX) | A2 | LRP1 | PTX | glioma | ANG-LP-MSN-PTX can promote the passage of PTX through the BBB and induce more apoptosis of glioma cells, which is a promising targeted delivery system in the treatment of glioma. | [206] |
| angiopep-2-lipid-based magnetic nanovectors + nutlin-3a (A2-LMNVs + Nut-3a) | A2 | LRP1 | nutlin-3a | GBM | A2-LMNVs have a strong affinity for GBM cells with respect to other healthy cell lines. | [207] |
| ultra-small, large pore silica nanoparticles (USLP)-Lf + DOX | Lf | LfR | DOX | GBM | USLP-Lf enhanced the efficacy of DOX-mediated apoptosis in GBM cells. | [208] |
| monoclonal antibodie (mAb)-conjugated human apoferritin (HFn) nanoparticles (mAb-HFn) | Tf | TfR1 | Trastuzumab (TZ); Cetuximab (CTX) | brain malignancies (such as GBM) | Hfn-conjugated TZ and CTX are promising treatments for brain cancer. | [209] |
| a ROS-responsive ruthenium nanoplatform (R@NGF-Se-Se-Ru) | / | / | NGF | AD | R@NGF-Se-Se-Ru has the ability to inhibit Aβ aggregation and has the potential to be A multifunctional drug for the treatment of AD. | [210] |
| internalized RGG/TGN- polyethylene glycol- poly(amidoamine) dendrimer- Arsenic trioxide (iRGD/TGN-PEG-PAMAM-ATO) | TGN; iRGD | neuropilin-1 receptor; αvβ/αvβ5 | ATO | glioma | Functional conjugation of PAMAM to PEG can reduce the potentially harmful effects of PAMAM, reduce its clearance in the blood, and prolong its retention time in the circulation. Enhancing BBB crossover efficiency and enhancing anti-tumor efficacy, thus showing great potential for glioma treatment. | [211] |
| ultra-small, large pore silica nanoparticles (USLP)-NH2-PEG-TMZ | Lf | LfR | TMZ | GBM | USLP-NH2-PEG-TMZ is effective in blood extravasation and penetration of the BBB and has the potential to improve the efficacy of TMZ in the treatment of GBM. | [212] |
| Yb3+ and Er3+ double-doped CeO2–x upconversion nanoparticles (Yb/Er/CeO2–x UCNPs) | / | / | Yb/Er/CeO2–x | PD | Yb/Er/CeO2–x UCNPs cross the blood–brain barrier and exhibit biocompatibility and antioxidant catalytic properties, which decrease the ROS and effectively help in treating PD. | [213] |
| TGN-CGA@SeNCs | lipopolysaccharide (LPS) | LPS receptor | chlorogenic acid (CGA) | AD | TGN-CGA@SeNCs is a novel flowerlike selenium nanocluster (TGN-CGA@SeNCs) prepared using a brain-targeting peptide (TGN peptide) and CGA, which is able to improve the bioavailability of CGA and reduce the dose of CGA to prevent AD progression. | [214] |
| a Fe3O4 magnetic nanoparticles modified through the growth of Fe-based Metal-organic frameworks of the Materials Institute Lavoiser (MNPs@MIL) + TMZ | / | / | TMZ | GBM | The use of TMZ-loaded MIL@MNPs improved the efficiency of TMZ delivery in the brain. | [215] |
| LCM-AuNP | glucose | GLUT1 | LCM | epilepsy | Lcm-aunp can overcome the BBB and efficiently deliver LCM into the brain parenchyma at an effective dose to reduce seizure severity and frequency in a rat model of KA-induced TLE. | [216] |
| peptide-functionalized gold nanoparticles (Pep-AuNPs) | heptapeptide NIDPNAV | lysophosphatidylcholine | AuNPs | MS | Pep-AuNPs were modified using heptapeptide NIDPNAV that binds specifically to the demyelinating region. Subsequently, intravenous delivery of peptide-modified gold nanoparticles has enhanced the ability to target lesions in the brain, and is more effective in anti-inflammatory and neuroprotection. | [217] |
| levetiracetam-loaded albumin nanoparticles- polysorbate 80 (LEV-NPs-PS 80) | / | / | LEV | epilepsy | LEV-NPs-PS 80 minimized side effects, especially peripheral side effects. In addition, there is an increase in the duration of anticonvulsant activity. | [218] |
| Angiopep-2-conjugated FeTi@Au core-shell nanoparticles (FeTi@Au-A2 NPs) | A2 | LRP1 | FeTi@Au-A2 NPs | glioma | FeTi@Au-A2 NPs hold great promise as a targeted delivery strategy for glioma treatment using hyperthermia. | [219] |
| MNP@BQR@A2-EXO-siGPX4 | A2 | LRP1 | siGPX4 | GBM | MNP@BQR@A2-EXO-siGPX4 is a novel composite therapeutic platform combining the magnetic targeting features and drug delivery properties of magnetic nanoparticles with the BBB penetration abilities and siRNA encapsulation properties of engineered EXO for GBM therapy. | [220] |
| NIR830-RGD- ultrafine iron oxide nanoparticles (uIONP)/SN38 | RGD | αvβ3 | ultrafine iron oxide nanoparticles | GBM | NIR830-RGD-uIONP/SN38 exerted tumor-specific cytotoxicity on U87MG GBM cells and prolonged the survival of GBM mice. | [221] |
| NBP-loaded cerium oxide nanoparticles (NBP–CeO2 NPs) | / | / | Dl-3-n-butylphthalide (NBP) | ischemic stroke | NBP-CeO2NPs have antioxidant and neurovascular repair abilities. | [222] |
| magnetic graphene oxide (NGO/SPIONs) nanoparticles coated with PLGA polymer as a dynamic nanocarrier for 5-iodo-2-deoxyuridine (IUdR) | / | / | IUdR | glioma | In addition to the high accumulation of IUdR/MNPs at targeted tumor sites, IUdR/MNPs demonstrated the ability of IUdR/MNPS to significantly enhance radiosensitizing effect, improve therapeutic efficacy, and increase toxicity in glioma-bearing rats. | [223] |
| Zinc oxide-nanoparticle (ZnO-NP) | / | / | ZnO-NP | neuroblastoma | Brain delivery of ZnO-NP is a potential therapeutic approach for neurodegenerative diseases. | [224] |
| PTX/R-flurbiprofen-PLGA-NPs | / | / | PTX; R-flurbiprofen | GBM | PLGA NPs can effectively carry their payload to glioma tissue, and the combination of anticancer and anti-inflammatory drugs can exert additional anti-tumor activity. | [225] |
| dual-targeted magnetic mesoporous silica nanoparticle (HA-MMSN-1F12) through surface-coupled Aβ42-targeting antibody 1F12 and CD44-targeting ligand hyaluronic acid (HA) | HA | antibody 1F12 and CD44 | anti-Aβ monoclonal antibody (mAb) | AD | HA-MMSN-1F12 facilitated mAb transit across the BBB and enhanced the ability of mAb to depolymerize Aβ aggregates into monomers. | [226] |
| Dopamine-loaded poly (butyl cyanoacrylate) nanoparticle (DA-PBCA NPs) | / | / | DA | PD | Da-pbca NPs can increase the release of DA in the brain and improve the brain structure and behavioral function in the PD rat model. | [227] |
| Chitosan-coated selenium nanoparticles (Cs-SeNPs)+ 5-fluorouracil (5-FU) | / | / | 5-FU | Brain Cancer (such as GBM) | Cs-SeNPs can effectively inhibit the proliferation, migration and invasion of GBM cells. The application of Cs-SeNPs can improve the sensitivity of GBM cells to 5-FU. | [228] |
| covalently conjugated hydroxyl PAMAM dendrimer–siGFP (D-siGFP) | GSH | GSHR | siGFP | GBM | D-siGFP is able to protect the siRNA payload from plasma proteins and endonucleases. | [229] |
| Temozolomide-conjugated gold nanoparticle functionalized with an antibody against the ephrin type-A receptor 3 (anti-EphA3-TMZ@GNPs) | anti-EphA3 | EphA3 | TMZ | GBM | anti-EphA3-TMZ@GNPs can be used as an intranasal delivery system to effectively treat GBM. | [230] |
| DOX-A2- carboxymethyl chitosan nanogel (CMCSN) | A2 | LRP1 | DOX | GBM | the strategy of combining the A2 and marine polysaccharide CMCS for brain targeting will have a promising application in GBM treatment. | [231] |
| Salinomycin- polyethylenimine-polyethylene glycol- iron oxide nanoparticles (Sali-PEI-PEG-IONPs) | / | / | Salinomycin | GBM | Sali-PEI-PEG-IONPs in combination with permeability enhancers such as hypertonic mannitol and external magnetic fields can provide effective and site-specific magnetic targeting for GBM chemotherapy. | [232] |
| iron oxide magnetic nanoparticles (NPs) as carrier system for helianthin (He/NPs) | / | / | helianthin | GBM | Helianthin-coated NPs were cytotoxic to GBM cells in vitro. | [233] |
| synthetic protein nanoparticles (SPNPs) coated with the transcytotic peptide iRGD (AMD3100-SPNPs) | / | / | AMD3100 | glioma | AMD3100-SPNP blocks CXCL12/CXCR4 signaling in vitro, Inhibition of GBM proliferation, reduction of CXCR4 monocyte myeloid-derived suppressor cells infiltration into the TME, restoration of BBB integrity, as well as induction of immunogenic cell death sensitize the tumor to radiotherapy and lead to anti-GBM immunity. In addition, the combination of AMD3100-SPNPs with radiation resulted in long-term survival. | [234] |
| gold nano-architectures- human serum albumin-DOX (NA-HSA-Dox) | / | / | DOX | Diffuse Intrinsic Pontine Glioma | NA-HSA-Dox has a better anti-tumor effect. | [235] |
5.2.1. Inorganic nanoparticles
Iron pxide nanoparticles (IONPs) (magnetite (Fe3O4) or maghemite (γ-Fe2O3)) are one of the common iron nanoparticles. The great advantage of IONPs in applications is that IONPs can be driven through BBB using magnetic methods [236]. For example, in the study of Huang et al. [237], it was shown that in the presence of an external magnetic field, Tween 80-modified Superparamagnetic IONPs accumulated significantly in the cortex near the magnet and gradually decreased in the brain tissue away from the magnet. And transmission electron microscope images of ultra-thin slices of rat brains show Tween 80-modified Superparamagnetic IONPs crossing the BBB. It shows that Superparamagnetic IONPs can effectively pass through BBB in the presence of external magnetic field. Magnetic resonance-guided focused ultrasound is a physical strategy to temporarily destroy the BBB. In the study of Chen et al. [238], to evaluate the effect of focused ultrasound pressure on BBB opening, focused ultrasound was applied immediately after microbubbles were injected. The BBB opening was then observed by Evans blue dye and MR imaging. The results showed that MRgFUS can destroy intact BBB and BBTB instantaneously, successfully enhancing the delivery of nanoparticles. In addition to using magnetic methods to promote IONPs through BBB, surface modification can also be carried out by PEG, PEI, etc., to improve IONPs biocompatibility and water dispersion, and extend its circulation time in the blood. Norouzi et al. [239] developed DOX (dauxorubicin)-loaded ethylenediamine triacetic acid-iron oxide nanoparticles (DOX-EDT-IONPs), which can promote the delivery of DOX to GBM cells. They found that under the basic condition of magnetic targeting, IONPs will cross the BBB and target the brain lesion. In addition, the BBB osmotic enhaser mannitol given under the basic conditions of magnetic targeting can destroy BBB instantaneously and increase the chance of magnetic targeting of drugs into the brain. Among them, PEG and PEI modified IONPs to improve their biocompatibility and prolong their circulation time in the blood. In conclusion, in the presence of an external magnetic field, the surface modification of Iron nanoparticles and the combination of iron nanoparticles with other peptides or ligands, etc., may open up a new avenue for the treatment of CNS diseases.
Gold (Au)NPs are customizable for size, have unique optical properties, facile surface modification and good biocompatibility [240]. In the study of Sela et al. [241], blood samples of rats with and without AuNPs were collected to detect the gold content in the blood samples, and it was found that the concentration of gold decreased significantly after perfusion, indicating that AuNPs penetrated into BBB after perfusion. In addition, by injecting AuNPs into the abdominal cavity of the rats, cerebrospinal fluid collected 6 h later was tested for gold content in the cerebrospinal fluid, further confirming that AuNPs penetrated the BBB. This indicates that BBB penetration of AuNPs is spontaneous and does not require an external field like IONPs. However, perfusion facilitates BBB penetration of AuNPs. However, there are different views [242]. Prades et al. [243] reported that AuNPs had a low or zero ability to cross the BBB. For this reason, they coupled the peptide to AuNPs with a size of 13 nm, increasing the AuNPs homoBBB permeability. Cheng et al. [244] concluded that AuNPs in the range below 100 nm were more likely to cross the BBB after systemic administration. Therefore, they selected AuNPs with a small nuclear size of only 5 nm and coupled them with peptides, enhancing the BBB permeability of AuNPs in two ways. In conclusion, the BBB permeability of AuNPs can be effectively enhanced by particle size, surface modification and other aspects, which is conducive to drug delivery.
The potential of silica nanoparticles in drug delivery systems has been paid more and more attention by researchers. In the CNS diseases, silica nanoparticles interact with BBB through multiple mechanisms to transport therapeutic drugs. The mechanisms include (a) acting as both an enhancer through BBB and an inducer of transient relaxation of TJs; (b) Transfer of silica nanoparticles from cerebrovascular endothelial cells; (c) silica nanoparticles generate endocytosis from cerebral endothelial cell lumen side, followed by exocytosis into the lumen side; In addition, (d) receptor-mediated cross BBB achieved with Silica nanoparticles modified with Tf and Lf [245,246]. For example, Liu et al. [247] found that the transport of silica nanoparticles by cerebral capillary endothelial cells was conducive to crossing the BBB. In addition, PEG-modified silica nanoparticles increase the endothelial permeability of NPs with nanoparticles, thereby promoting the passage of BBB. Song et al. [248] found that Lf-connected silica nanoparticles exhibited higher transmission efficiency on BBB compared to bare silica nanoparticles. The receptor-mediated transfer of silica nanoparticles across brain endothelial cells delivers drugs to the brain. This suggests that silica nanoparticles can open up an emerging platform for the transport of therapeutic drugs through their interaction with BBB.
5.2.2. Organic nanoparticles
Chitosan is a linear polysaccharide extracted from chitin,a natural polymer mainly found in the exoskeleton of crustaceans), after its deacetylation. Chitosan has many useful properties, such as biocompatibility, low toxicity, solubility, degradability, antibacterial and versatility, which make it attractive for application in biomedicine [249]. Anshinone IIA is a lipid-soluble phenanthraquinone compound with anti-inflammatory and anti-oxidant effects extracted from Salvia miltiorrhiza.Tanshinone IIA was loaded onto chitosan-coated nanostructured lipid carriers and administered intranasally, bypassing the BBB to achieve effective drug delivery to the brain for the treatment of PD [250]. Similarly, protein phosphatase 2 (PP2A) activator FTY720 was encapsulated in chitosan-based nanoparticles, which enhanced the bioavailability and protected the neurons [251].
PLGA is a biodegradable copolymer, usually composed of lactic acid and glycolic acid (polyethylene glycol). PLGA is one of the most successful biodegradable polymers. Because these two monomers of PLGA are natural and are easily metabolized by the Krebs cycle, PLGA has very little systemic toxicity when used for drug delivery [252]. R-flurbiprofen is a non-steroidal anti-inflammatory drug with potential anticancer activity. Paclitaxel is a drug used to treat a variety of malignant tumors, but it is fails to penetrate BBB. Therefore, Secil et al. [225] constructed a nanoparticle that combines R-flurbiprofen and Paclitaxel with positively charged chitosan-modified PLGA nanocarriers. In vitro these nanoparticles were effectively taken up by tumor cells and were toxic to tumor cells. they exhibited significantly higher therapeutic activity against glioma in vivo, when compared to either Paclitaxel or R-flurbiprofen alone. It shows that PLGA nanoparticles can overcome NPs physical and chemical shortcomings and effectively carry drugs to glioma tissues to exert their potential anticancer activity.
Another carrier, PEG-PLA, can be modified by Tf and coupled with resveratrol to form a multifunctional nanoparticle (Tf-PEG-PLA-RSV) [253]. In vitro experiments showed that the uptake of Tf-PEG-PLA-RSV by glioma cells and their death was increased compared to the free RSV. Compared to free resveratrol, the Tf-PEG-PLA-RSV NPs significantly reduced tumor volume and accumulated in brain tumors, prolonging the survival time of glioma-bearing rats.
Although organic nanoparticles have certain therapeutic effects, compared with inorganic nanoparticles, some organic nanoparticles have relatively low loading capacity in drug delivery, which limits their efficiency in some drug delivery applications. Therefore, one need to consider these when applying inorganic vs organic nanoparticles to obtain the best results.
5.3. Nanomicelles
Nanomicelles are composed of hydrophilic (polar) and hydrophobic (nonpolar) groups, and amphiphilic molecules are their common subunits [254]. The assembly of these molecules is determined by group orientation to a suitable environment, such as a solvent [255,256]. The hydrophilic part of the conventional micelle faces the outer surface and the hydrophobic part faces the interior. These nanomicelles are used for encapsulating poorly soluble drugs, which enhances their solubility and bioavailability. In contrast, the hydrophobic part of the inverted nanomicelles is oriented toward the surface and the hydrophilic part toward the core. It can be used to encapsulate and deliver hydrophilic drugs and proteins. Nanomicelles possesse unique properties such as structural stability, ability to dissolve hydrophobic drugs, physicochemical properties (amphiphilic copolymers), pH sensitivity, mucosal adhesion properties, specific binding, and sensitivity. Because of this, drug delivery systems based on nano-micelles have some advantages over traditional drug delivery systems, such as, biodegradability, specific targeting, small side effects, low toxicity, increased drug solubility and suitable for intravenous drug delivery [255]. The nanomicelles related studies in overcoming BBB are listed in Table 4.
Table 4.
Recent investigations on nanomicelles for circumventing the blood-brain barrier in the management of central nervous system disorders.
| Surface modify | Targeting ligands | Receptors | Model drug | Use | Others | Refs |
|---|---|---|---|---|---|---|
| DOX/RVG-Chondroitin sulfate-disulfide bonds-curcumin-micelles (DOX/RVG-CSC-micelles) | RVG | nAChRs | DOX; CSC | glioma | The RVG-mediated copolymer micelle containing the chemotherapeutic drug doxorubicin (DOX/RVG-CSC) penetrates the BBB and reaches the tumor cell region, followed by drug release upon stimulation with high concentrations of glutathione in GBM. | [257] |
| antibody 6H4 fragments specific to Aβ oligomers encapsulated in polymeric nanomicelles | glucose | GLUT1 | anti-Aβ oligomer fragment antibody | AD | The polymeric nanomicelles simultaneously recognized A variety of toxic Aβ and reduced their amount, inhibited the Aβ-induced AD pathological process and alleviated cognitive function. | [258] |
| 7-C/siRNA | T7 | TfR | slit2 SiRNA; cholesterol | glioma | T7-C/siRNA can reach the tumor site through ID and regulate the "cold" TME to the "hot" TME, and non-invasive ID can shorten the distance and reduce the loss of gene drugs. | [259] |
| Levodopa and Cur-loaded NH®2–PEO–PCL GSH-coated NPs (LdCurNP) | GSH | GSHR | Cur; Levodopa | PD | LdCurNP, a biodegradable nano-micelle that is compatible with blood and has low cytotoxicity to cells, is a promising approach for the treatment of PD. | [260] |
| Cur- nanomicelles | Epidermal growth factor receptor (EGFR) ligands | EGFR | Cur | GBM | Cur-nanomicelle showed anti-GBM activity either alone or in combination with erlotinib. | [261] |
| paclitaxel PTX@ANG/FA-NPs hybrid novel nanodrug delivery system | A2; folate | LDL-R1; folate receptors | PTX | GBM | The NPs were able to overcome the limitations of difficult penetration of PTX through the BBB, poor water solubility, and rapid elimination. | [262] |
| DOX-loaded polymeric micelles (DOX RVG-15-PMs) | RVG-15 | nAChRs | DOX | GBM | DOX RVG-15-PMs slowed the rate of weight loss in tumor-bearing mice, hindered the metastasis of cancer cells, and promoted the apoptosis of cancer cells. | [263] |
| henylboronic-decorated chitosan-based nanoplatform to deliver myricetin | / | / | myricetin | ischemic disease | The natural cationic polysaccharide chitosan modified micelles interact electrostatic with the anionic epithelium of the BBB and sinusoidal endothelial barrier to enhance endothelial barrier transport. Thus, the feasibility of chemical modification makes chitosan ideal for responsive structural design and targeted payload delivery. | [264] |
| dual-sensitive Fab encapsulated glucosylated polymeric micelle (Gluc-PM-Fab) | glucose | GLUT1 | 3D6 antibody fragments | AD | Gluc-PM-Fab can deliver 3D6 antibody fragment to the brain via GLUT1 and inhibit Aβ1-42 aggregation in AD mice. | [265] |
| HA/DP7-C/siRNA delivery system | HA | HA | SiRNA; | glioma | HA is used as a layer for NPs, which is able to increase drug adhesion, prolong drug residence time in the nasal cavity, and increase drug absorption. | [266] |
| MCC950-loaded transferrin-conjugated poly(2-methyl-2-oxazoline)-block-poly(L-lactic-co-glycolic acid) (M-PMPL@Tf) nanomicelles | Tf | TfR1 | MCC950 | I/R | MCC950 is a novel NLRP3 inflammasome inhibitor synthesized by Prakash et al. | [267] |
5.4. Exosomes
Pan and Johnstone first identified EXO in 1983 and defined these functional vesicles as EXO in 1989 [268]. EXO are bilayer nanovesicles, ranging in size from 30 to 150 nm. They can be released from a variety of cells (fibroblasts, immune system cells (T cells, B cells, dendritic cells), adipocytes, and tumor cells, among others). They were also isolated from all normal and diseased body fluids (blood, urine, breast milk, amniotic fluid and bronchoalveolar lavage fluid, etc. [269,270]. Secretes EXO body formation included three different stages: 1) from the plasma membrane formation endocytic vesicles, and 2) inward budding of endocytic vesicles. These lead to the formation of multi-vesicular body (MVB) composed of intraluminal vesicles; Finally the fusion of MVB with the plasma membrane releases the EXOs into the immediate environment [271]. EXO are considered as promising drug delivery vectors and therapeutic targets due to their good biocompatibility, stability, ability to penetrate the blood-brain barrier, minimal toxicity, and low immunogenicity [272]. Interestingly, EXO released by cell types from different sources differ in their ability to penetrate BBB. EXO from stem cells, neural origins, and macrophages can achieve higher BBB penetration [273]. Compared with EXO isolated from other types of somatic cells, BBB endothelial cells are highly reactive to EXO isolated from mesenchymal stem cells, endothelial cells, macrophages, normal nerve cells, and healthy or cancer cells, facilitating BBB penetration [273]. For example, Chen et al. [274]found that EXO secreted by human umbilical cord mesenchymal stem cells reach the substantia nigra via BBB in vivo, alleviating apomorphine-induced asymmetric rotation, reducing loss and apoptosis of dopaminergic neurons in the substantia nigra, and upregating dopamine levels in the striatum. These results indicate that the exocrine secreted by human umbilical cord mesenchymal stem cells can effectively cross BBB and treat PD. Morad et al. [275] showed that EXO secreted by tumor cells break through the complete blood-brain barrier through transcytosis. This suggests that the ability of EXO to penetrate BBB depends on the source cell that secretes it. In addition, EXO can carry substances such as small molecules, proteins and nucleic acids across the BBB [276]. For example, Wu et al. [277] formed CSI by encapsulating catalase (CAT) into silica nanoparticles (CAT@SiO2) and then loading the sonosensitizer indocyanine green (ICG). Then, inspired by the ability of macrophages to cross the BBB, CSI was further coated with AS1411 aptamer-modified macrophage EXO to form CSI@Ex-A. CSI@Ex-A has efficient BBB penetration, good cancer cell targeting capacity as well as good biocompatibility and long circulation time. Zhang et al. [278] found that EXO coated DOX-loaded nanoparticles have great potential to penetrate the BBB and induce tumor cell apoptosis and ICD, as well as enhance the survival of GBM -bearing mice. This indicates that EXO have a strong ability to carry drugs and cross the BBB. Nonetheless, the practical application of EXO still encounters significant obstacles. For exa. However, the application of EXO in clinical practice still faces great challenges. For example, the current method of EXO isolation produces a small number of EXO, resulting in high costs for application into clinical research and mass production after drug approval [271]. Thus, although EXO have unparalleled advantages over other nanoparticles, they have still a long way to go before they can enter in clinical practice. We summarize studies published on EXO for overcoming BBB in the field of CNS disease treatment (Table 5).
Table 5.
Recent studies on extracellular vesicles to breach the blood-brain barrier for central nervous system disorders.
| Surface modify | Targeting ligands | Receptors | Model drug | Use | Others | Refs |
|---|---|---|---|---|---|---|
| CpG-exosomes -TanIIA-GL nanomicelles (CpG-EXO/TGM) | Tf | TfR | Tanshinone IIA; Glycyrrhizic acid; CpG oligonucleotides | GBM | In this study, a TGM was constructed, and the TGM was then loaded into serum EXO to form CpG-EXO/TGM. CpG-EXO/TGM can effectively prevent postoperative recurrence by using chemotherapy and immunotherapy in the treatment of GBM. | [107] |
| macrophage-derived exosomes loaded with curcumin (EXO-Cur) | / | / | Cur | I/R | EXO-Cur has inflammation-related targeting properties. EXO-Cur has a neuroprotective effect by eliminating ROS generation, reducing mitochondria-mediated apoptosis, and alleviating the damage of BBB caused by cerebral ischemia. | [279] |
| cEM@DEP-siRNA | / | / | Panobinostat; PPM1D-siRNA | glioma | cEM@DEP-siRNA is a nano drug delivery system based on functionalized macrophage EXO with panobinostat and PPM1D‐siRNA. | [280] |
| magnetic nanoparticles@ brequinar@A2-EXO- siGPX4 (MNP@BQR@A2-EXO-siGPX4) | A2 | LRP1 | GPX4 SiRNA; brequinar | GBM | MNP@BQR@A2-EXO-siGPX4 enhances ferroptosis synergistically to treat GBM by disintegrating the DHODH and GPX4 ferroptosis defense axes. | [220] |
| EXO-metformin/sicPLA2 | Tf | TfR | Metformin; sicPLA2 | glioma | EXO-metformin/sicPLA2 impairment Mitochondrial energy metabolism in GBM. | [281] |
| (CSI@EXO-AS1411; CSI: catalase @SiO2- catalase | / | / | catalase | GBM | CSI@Ex-A has efficient BBB penetration, good cancer cell targeting, good biocompatibility, and long circulation time. | [277] |
| Neutrophils (NEs)-EXO/DOX | / | / | DOX | glioma | In vivo zebrafish and glioma mouse models showed that NEs-EXO/DOX rapidly penetrated the BBB and migrated to the brain. In addition, NEs-EXO/DOX can respond chemoattractively to inflammatory stimuli. Intravenous administration of NEs-EXO/DOX effectively inhibited tumor growth and prolonged survival. | [282] |
| exosome coated DOX-loaded nanoparticles | / | / | DOX | GBM | Exosome coated DOX-loaded nanoparticles have great potential to penetrate the blood-brain barrier and induce tumor cell apoptosis and ICD, as well as survival in tumor-bearing GBM mice. | [278] |
| a dual-receptor-specific exosome loaded with TMZ and BG (termed as EXO-An2-Apt-TMZ and EXO-An2-Apt-BG) | A2; CD133 RNA aptamers (Apt) | LRP1; CD133 | TMZ; O6-benzylguanine (BG) | GBM | After intravenous injection of EXO-An2-Apt-TMZ and EXO-An2-Apt-BG, due to the synergistic effect of An2 and Apt, the nanocomposites could cross the BBB and actively reach GBM cells and GSC, exerting the corresponding efficacy of TMZ and BG. | [283] |
| DTX@A2- exosomes-mimetics (EM) | A2 | LRP1 | DTX | GBM | DTX@A2-EM showed significant inhibitory effect on GBM growth in situ and reduced the side effects of chemotherapy drugs. | [284] |
| HSSP-BMSCEXO-TMZ; HSSP-BMSCEXO-SiRNA | HMOX1 targeting ligand | HMOX1 | TMZ; STAT3 SiRNA | GBM | The multifunctional NNPS can cross the BBB and target GBM cells, and the synergistic effect between TMZ released by the NPS and SiSTAT3 can restore TMZ sensitivity of TMZresistant glioma for TMZ chemotherapy. | [285] |
| ExoP -Bryostatin-1 (Bryo) | the ligand of platelet-derived growth factor receptor α (PDGFRα) | PDGFRα | Bryo | MS | ExoP means that EXO is derived from NSC expressing PDGFRα ligands. | [286] |
| brain microvascular endothelial cells (HBMVECs) derived exosomes (HBMVECs-Ex) | / | / | P-glycoprotein | AD | HBMVECs-Ex, which has inherited P-glycoprotein from its parent cells, functions to promote P-glycoprotein-mediated Aβ clearance under AD conditions. | [287] |
| DOX and PTX-loaded EXO | / | / | DOX; PTX | glioma | Microfluidics assisted DOX and PTX loading into EXO. | [288] |
| reassembly-exosomes (R-EXO)-TMZ/DHT | / | / | TMZ; Dihydrotanshinone (DHT) | glioma | R-EXO-TMZ/DHT can target glioma cells by virtue of its natural homologous targeting ability. DHT can effectively overcome the drug resistance induced by TMZ and has a good therapeutic effect on glioma. | [289] |
| MicroRNA loaded CXCR4 mpEV | CXCL12 | CXCR4 | miRNA-100; Cy5-AmiRNA-21 | GBM | They used the ID method to deliver microRNA-loaded CXCR4 mpEV into the brain, release the carried miRNA-100 and Cy5-AmiRNA-21 to improve the sensitivity of GBM cells to TMZ, inhibit tumor growth, and improve the overall survival of model mice. | [290] |
| atorvastatin-loaded hEnMSCs exosomes | / | / | atorvastatin | GBM | hEnMSCs EXO are derived from human endometrial mesenchymal stem cells. | [291] |
| EXO-Silibinin (Slb) | / | / | Slb | AD | Slb has dual effects of reducing Aβ aggregation by binding to Aβ monomers and inhibiting astrocyte activation, which favors its neuroprotective effect as A treatment for AD. | [292] |
| anti‐microRNA‐21 oligonucleotides (AMO21c)-T7 peptides -cell membrane nanovesicles (AMO21c-T7-CMNVs) | cholesterol-linked T7 peptides | TfR | AMO21c | GBM | Delivery of AMO21c to the brain was more effective in T7 modified CMNV than in CMNV. | [293] |
| curcumin loaded human endometrial stem cells derived exosomes (hEnSCs EXOs-Cur) | / | / | Cur | PD | hEnSCs EXOs-Cur crossed the BBB by increasing BCL2 expression level as well as reducing BAX and caspase-3, enhanced enhanced motor incongruency movement, inhibited αS protein aggregation and rescued neuronal cell death. | [294] |
| NSC-EXOs loaded with miR-124-3p | / | / | small molecule miR-124-3p | glioma | NSC-EXO loaded with miR-124-3p significantly inhibited the proliferation, invasion and migration of glioma cells. | [295] |
| EXO-miR-1208 | / | / | miR-1208 | glioma | After FUS irradiation, Exo-Mir-1208 improved the ability to penetrate the BBB, promoted the uptake of miR-1208 in EXO by glioma cells, and effectively inhibited glioma. | [296] |
| T7-siYY1-exo | T7 | TfR | SiYY1 | GBM | 7-siYY1-exo can enhance the sensitivity to chemoradiotherapy and reverse drug resistance. In addition, T7-siYY1-exo and TMZ/Irradiation exerted a synergistic anti-GBM effect and significantly improved the survival time of GBM mice. | [297] |
| atorvastatin (Ato) loaded EXOs (AtoEXOs) | / | / | atorvastatin | GBM | EXO is derived from human endometrial stem cells. | [298] |
5.5. Biomimetic nanoparticles
Nanoparticles are usually classified as biocompatible nanoparticles or biomimetic nanocarriers. Nanoparticles are obtained by modifying natural materials or chemically imitating the key characteristics of biological structures. They are chemically, physically or morphologically similar to biological structures and can be used for more efficient and safe drug delivery. At present, more and more biomimetic nanoparticles have been used in the treatment of CNS diseases because of their ability to help drugs penetrate BBB better [299]. For example, Wang et al. [299] inspired by the natural structure and properties of high-density lipoprotein (HDL), designed a perforated penetrated HDL fused with penetrating peptide tLyP-1 (Fig. 9). The tLyP-1 peptide is fused to the surface of HDL to promote BBB permeability, tumor homing ability, and site accumulation of photosensitizers and siRNAs. The results show that lipoprotein-mimicking nanoparticles can not only effectively cross the BBB and carry the payload to the in situ glioma, but also regulate the tumor microenvironment, thereby inhibiting tumor growth with biosafety. Cao et al. [300] designed a biomimetic macrophage membrane camouflage nanoparticles. Such biomimetic nanoparticles can not only reduce the clearance of mononuclear phagocyte system, showing immune camouflage, but also increase the ability of the complex to cross BBB during in situ GBM specific targeted therapy, so as to promote the ferroptosis of GBM cells. In summary, biomimetic nanoparticles can give the NP for drug delivery the privilege of immune camouflage in vivo and can easily pass through the BBB. At present, the biomimetic material technology for drug delivery is still in the early stage of novelty, and the preparation stage of the material is not yet mature, which makes it difficult to produce on a large scale, and there is no example for clinical application [299]. Therefore, potential safety and biocompatibility issues in the clinical environment cannot be ruled out, and rigorous biosafety assessments are required during use. We summarize studies published on biomimetic nanoparticles for overcoming BBB in the field of CNS disease treatment (Table 6).
Fig. 9.
Schematic illustration of ptHDL/siHIF-ICG and site-specific release for augmenting the FI imaging-guided photo-gene therapy of glioma. ptHDL/siHIF-ICG accumulated in tumor site by targeting peptide-guided tumor-homing routine, and be internalized into glioma cells via receptor-mediated endocytosis. ptHDL/siHIF-ICG disassembled in glioma cells upon NIR laser irradiation, and the released ICG serves not only imaging agent but also photosensitizer for phototherapy. Reprinted with permission from Ref. [304].
Table 6.
Recent studies on biomimetic nanoparticles to breach the blood-brain barrier for central nervous system disorders.
| Biomimetic material | inspiration | Model drug | Use | Refs |
|---|---|---|---|---|
| MNPs@TMZ + cispt | cancer cells readily pass the BBB and localize with homologous cells | TMZ; cispt | GBM | [301] |
| PP@PCL | the rapid BBB penetrability of 4T1 tumor cells upon their brain metastasis and natural roles of platelet in targeting injured vasculatures | Paeonol; polymetformin | ischemic stroke | [302] |
| RFA NPs | a folate-modified erythrocyte-cancer cell-macrophage hybrid membrane as the shell | lomitapide | GBM | [303] |
| ptHDL/siHIF-ICG | the natural structure and properties of HDL | HIF-1α siRNA | glioma | [304] |
| SR717@RGE-HFn NPs | As a natural iron storage protein, ferritin shows excellent biocompatibility and biodegradability | non-nucleotide STING agonist (SR717) | glioma | [305] |
| TGN-RBC-NPs-Cur | The complete RBC membrane is coated on the surface of NPs, so that RBC-NPS has both the physical and chemical properties of synthetic materials and the complex biological functions of endogenous materials | Cur | glioma | [306] |
| macrophage cell membrane cloaked biomimetic nanomedicines | / | JQ1 | GBM | [307] |
| IL-12 mRNA@cRGD-CM-CaCO3NPs | Biomimetic calcium carbonate nanoparticles delivered IL-12 mRNA | IL-12 | GBM | [308] |
| R-NKm@NP | a Trojan-horse-like nanoparticle system | TMZ; IL-15 | GBM | [309] |
| M-NPs@G | cancer cell-mitochondria hybrid membrane | gboxin | GBM | [310] |
| an neural stem cell membrane -coated Apt 19S-conjugated BBB penetrable NP | / | antisense oligonucleotide (ASO) | PD | [311] |
| Biomimetic Macrophage Membrane-Camouflaged Nanoparticles | Macrophage membrane camouflage nanoparticles can reduce the clearance of mononuclear phagocyte system, showing immune camouflage. | Small activating RNA ALOX15 | GBM | [300] |
| RVG/TPP- maslns | macrophage membranes camouflaged the SLNs from being eliminated by RES-rich organs by inheriting the immunological characteristics of macrophages | antioxidants | AD | [312] |
6. Conclusion
Many CNS malfunctions can cause a wide range of diseases, such as AD, PD, MS, epilepsy, stroke, brain cancer, etc. These diseases have seriously harmed human health and brought heavy burden to the society. Although the present technology has made significant progress compared to the past, the current strategies for CNS diseases did not advance into cure, but can only temporarily relieve. The main reason for this difficulty the BBB. Therefore, overcoming BBB is the key to the treatment of CNS diseases.
However, there are still several hindrances for overcoming BBB. First, BBB is a selective permeability barrier. Due to the selective permeability of BBB to different substances, many drugs cannot effectively cross the CNS to achieve the purpose of treating CNS disease. Second, the size and chemical properties of most drug molecules may limit their ability to pass through the BBB. Third, the permeability of BBB may change during different states or progression of the disease, so it is difficult to accurately predict the efficay of the delivered drugs. Fourth, individuals may have different degrees of BBB permeability. Fifth, the methods used to overcome BBB may have some side effects or lead to some new adverse reactions. For example, increasing BBB permeability may cause harmful substances to enter the brain. Therefore, to treat diseases more safely and effectively, we should overcome these problems.
There are four common strategies to overcome BBB. First, the use of nano-drug delivery systems improves the biodistribution and stability of drugs, thereby improving their efficiency when crossing the BBB. Second, some methods of passing through or bypassing the BBB were used to improve the distribution of drugs in the CNS. Third, the structure of the drug was modified to facilitate its crossing of the BBB. For example, designing small molecule drugs or using deliverable prodrugs is one strategy. Fourth, a combination of h the above strategies, may be ued to improve the therapeutic benefits.
Although many nanoparticles can effectively overcome the BBB, their shape, size, hydrophobicity, coating, chemical and surface charge properties can determine the outcomes of drug effects in clinical practice. Here we will discuss the advantages and disadvantages of various nanomaterials in the application process and application scenarios. The first is liposomes. Liposomes are usually made of biocompatible materials, such as phospholipids, which can effectively reduce immune response and toxicity. Liposomes can effectively cross the BBB and promote the effective delivery of drugs to the central nervous system due to their membrane structure characteristics and surface modification. In addition, the drug loading capacity, adjustable release characteristics and good stability of liposomes can more effectively promote the use of therapeutic drugs in the body. Liposomes have a wide range of application scenarios, such as drug delivery (delivery of antidepressants, antipsychotics and antiepileptic drugs, etc.), gene therapy (delivery of nucleic acid drugs such as mRNA, siRNA, etc.), vaccine delivery (mRNA vaccine), neuroprotection, imaging, etc. Although, lipid nanoparticles have broad application potential in the treatment of CNS, which can effectively improve the bioavailability and targeting of drugs. However, its production and safety issues still need to be further studied to ensure its effectiveness and safety in clinical applications.
For inorganic nanoparticles, they usually have high chemical stability and durability, can maintain stability in the body environment, and protect drugs or therapeutic molecules from degradation. And because of its excellent optical properties, it can be used as a contrast agent for imaging technology to improve the clarity and sensitivity of imaging. Its adjustable surface properties can achieve targeted delivery, improve biocompatibility, and enhance cell uptake by adjusting the surface modification of inorganic nanoparticles. In addition, some inorganic nanoparticles have multiple functions, such as simultaneous treatment, imaging and diagnostic functions, and can perform multiple tasks. For example, First, Inorganic nanoparticles (such as quantum dots, ferrite nanoparticles) are widely used in medical imaging (such as MRI, CT, optical imaging) to improve the resolution and sensitivity of imaging. Second, Inorganic nanoparticles such as magnetic nanoparticles can be used for magnetothermal therapy to treat cancer or other diseases by external magnetic field heating. 3.Inorganic nanoparticles (such as silver nanoparticles) have antibacterial properties and can be used for the treatment and prevention of infectious diseases. However, due to the biocompatibility of inorganic nanoparticles with the body, inorganic nanoparticles may cause immune response or toxicity, especially in long-term accumulation or high-dose use, which may cause harm to the body. Compared with liposomes, its biodegradability is usually poor, which may lead to long-term existence in the body and increase the risk of toxicity. At the same time, the preparation of high-quality inorganic nanoparticles may involve complex processes and high costs, limiting their large-scale application. Although, inorganic nanoparticles have significant application potential in the treatment of central nervous system diseases, especially in imaging, drug delivery and versatility. They also face challenges such as biocompatibility, long-term safety, and production costs.
For organic nanoparticles, they are usually made of biodegradable organic materials, with good biocompatibility and low toxicity, and can be used safely in the body. By surface modification or design of organic nanoparticles, the ability to cross the blood-brain barrier can be improved, and the controlled release and targeted delivery of drugs in the brain can be achieved after crossing the BBB. It is worth mentioning that organic nanoparticles can combine therapeutic and imaging functions, for example, combining drugs and imaging agents in the same nanoparticle to achieve multiple functions. Compared with inorganic nanoparticles, the production process of organic nanoparticles is usually more mature and simple, and the cost is relatively low. It is more common in targeted drug delivery, medical imaging (such as MRI or optical imaging), gene and nucleic acid delivery, vaccine delivery, etc. Although the biodegradability of organic nanoparticles is usually good, they may face great chemical stability challenges in vivo, such as excessive degradation or rapid clearance in vivo, affecting their efficacy. Secondly, although the production process is relatively simple, it may still face challenges in large-scale production and quality control. Moreover, although the production process is relatively simple, it may still face challenges in large-scale production and quality control. In summary, although organic nanoparticles have the advantages of good biocompatibility, efficient blood-brain barrier penetration, adjustable drug delivery and versatility in the treatment of central nervous system diseases. However, they also face challenges such as stability, biodegradability and large-scale production. With the advancement of technology and the deepening of research, the application prospects of organic nanoparticles will be further expanded, bringing more innovative solutions for the treatment of central nervous system diseases.
For biomimetic nanoparticles, because biomimetic nanoparticles usually mimic the natural components or structures in organisms, they have excellent biocompatibility, can be better compatible with the biological environment, and reduce immune response and toxicity. By mimicking the characteristics of cell membranes, viruses, or other organisms, biomimetic nanoparticles can have a better ability to cross the BBB, more accurately target specific cells or tissues, and improve the efficiency and specificity of drug delivery. In addition, it can improve the drug delivery performance, reduce the impact of drugs on non-targeted tissues, and improve the efficacy and safety of treatment. It is commonly used in targeted drug delivery, medical imaging, gene and nucleic acid delivery, vaccine and immunotherapy. However, the preparation of biomimetic nanoparticles may involve complex processes and techniques, which require highly precise control and relatively high production costs. Because it imitates the natural components or structures in organisms, its biodegradability may be inconsistent with that in natural systems, and may cause immune responses in practical applications. Long-term use may have potential safety problems. In summary, biomimetic nanoparticles provide many advantages in the treatment of central nervous system diseases, but they also face production and technical challenges, biodegradability and immune response.
In general, idealized nanoparticles should have the following characteristics:First, nanoparticles must be safe and stable, and can be degradable in vivo. Second, nanoparticles must pass through or bypass the blood-brain barrier non-invasively. Third, the drugs carried by nanoparticles must be concentrated in the target area. Fourth, The production time for nanoparticles is short and cost effetive, and large quantities can be produced for therapeutic applications. the methods for nanoparticles to bypass BBB include ID, IID, ITD, and CED, and the methods for nanoparticles to cross BBB include RMT, CAMT, CMMT, and TJM. Amogn these IID, ITD, and CED are invasive. Invasive methods are not only cumbersome and may cause pain and inconvenience to patients, but also may lead to infection, bleeding, neurotoxicity, increased intracranial pressure, and the risk of immune rejection. Therefore, to the use of non-invasive methods should be prioritized. However, when the same nanoparticle is applied in different ways to overcome BBB, it will have different outcomes for depending on the disease. Therefore, when applying nanotechnology to treat CNS disease, one should not only consider the choice of nanomaterials, but also consider the method of nanoparticles overcoming BBB. In summary, although nanotechnology has great potential in overcoming BBB, it is still a difficult road to transform the experimental research of nanotechnology into clinical application.
CRediT authorship contribution statement
Qian Luo: Writing – original draft, Software, Data curation, Conceptualization. Jiaying Yang: Supervision. Mei Yang: Investigation. Yingtong Wang: Data curation. Yiran Liu: Data curation. Jixuan Liu: Project administration. Dhan V. Kalvakolanu: Supervision. Xianling Cong: Methodology. Jinnan Zhang: Data curation. Ling Zhang: Supervision, Investigation, Conceptualization. Baofeng Guo: Writing – review & editing, Investigation, Conceptualization. Yanhong Duo: Writing – review & editing, Supervision, Conceptualization.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No. 82273479 to L.Z.) and Research Fund of Jilin Provincial Science and Technology Department (20240305079YY to L.Z) and China-Japan Union Hospital and College of Basic Medical Sciences, Jilin University Union Project (Grant No. KYXZ2022JC05 to L.Z.). DVK was supported by funds through the Maryland Department of Health's Cigarette Restitution Fund Program (Grant No. CH-649-CRF).
Declaration of Competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributor Information
Ling Zhang, Email: zhangling3@jlu.edu.cn.
Baofeng Guo, Email: gbf@jlu.edu.cn.
Yanhong Duo, Email: yanhongduo@seas.harvard.edu.
Abbreviations
- CNS
Central nervous system
- BBB
blood-brain barrier
- AD
Alzheimer's disease
- PD
Parkinson's disease
- MS
multiple sclerosis
- NPs
Nanoparticles
- BCSFB
blood-cerebrospinal fluid barrier
- BMECs
Brain microvascular endothelial cells
- EC
Endothelial cells
- TJ
tight junction
- TJs
tight junctions
- TJP
tight junction proteins
- RMT
Receptor-Mediated Transport
- ApoE
Apolipoprotein E
- VEGF
vascular endothelial growth factor
- BTB
Blood-tumor barrier
- TMZ
temozolomide
- PTX
paclitaxel
- DOX
doxorubicin
- NGF
Nerve growth factor
- Ru NPs
ruthenium nanomaterial
- PCM
phase change material
- DA
dopamine
- α-Syn
Alpha-synuclein
- AOS
antisense oligonucleotide
- NSCM
neural stem cell membrane
- ROS
reactive oxygen species
- NCPs
nanoscale coordination polymers
- Cur
Curcumin
- AEDs
antiepileptic drugs
- GABA
γ-aminobutyric acid
- LCM
lacosamide
- AuNPs
gold nanoparticles
- DHAA
dehydroascorbic acid
- LTG
lamotrigine
- Tf
transferrin
- TfR1
transferrin receptor 1
- NETs
neutrophil extracellular traps
- GBM
glioblastoma
- mpEV
microfluidically processed EVs
- TME
tumor microenvironment
- GLUT1
glucose transporter 1
- DTX
docetaxel
- nAChRs
Nicotinic acetylcholine receptors
- CXCR4
chemokine receptor 4
- Ost
Osthole
- Lf
Lactoferrin
- LfR
Lactoferrin receptors
- GSH
Glutathione
- GSHR
glutathione receptors
- LDL-R
Low-density lipoprotein receptor
- CB
carboplatin
- Lep
Leptin
- MAN
mannose
- RVG
rabies virus glycoprotein
- FA
folic acid
- FAR
folic acid receptor
- MVB
multi-vesicular body biogenesis
- ID
Intranasal Delivery
- CSF
cerebrospinal fluid
- NLCs
nanostructured lipid carriers
- I/R
ischemia/reperfusion
- IID
Intrathecal and Intraventricular Delivery
- ITD
Intratumoral Delivery
- CED
Convection Enhanced Delivery
- GSC
glioblastoma stem-like cells
- CAMT
Carrier - Mediated Transport
- CMMT
Cell - Mediated Transport
- RES
Resveratrol
- LTG
lamotrigine
- EXO
Exosomes
- FUS
Focused Ultrasound
Data availability
Data will be made available on request.
References
- 1.Wan W., et al. METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N(6)-methyladenosine modification of PD-L1 mRNA in breast cancer. Mol Cancer. 2022;21(1):60. doi: 10.1186/s12943-021-01447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu H., et al. Cell membrane-based biomimetic vehicles for effective central nervous system target delivery: insights and challenges. J. Contr. Release. 2023;360:169–184. doi: 10.1016/j.jconrel.2023.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Fan Y., Chen Z., Zhang M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J. Transl. Med. 2022;20(1):291. doi: 10.1186/s12967-022-03493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen T.T., et al. Nanotechnology-based drug delivery for central nervous system disorders. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2021;143:112117. doi: 10.1016/j.biopha.2021.112117. [DOI] [PubMed] [Google Scholar]
- 5.Li W., et al. Cell membrane-based nanomaterials for theranostics of central nervous system diseases. J. Nanobiotechnol. 2023;21(1):276. doi: 10.1186/s12951-023-02004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie J., et al. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. 2019;224:119491. doi: 10.1016/j.biomaterials.2019.119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nature Medicine. 2013;19(12):1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X., et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther. 2021;6(1):74. doi: 10.1038/s41392-020-00450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalid K., et al. Advanced in developmental organic and inorganic nanomaterial: a review. Bioengineered. 2020;11(1):328–355. doi: 10.1080/21655979.2020.1736240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanar F., Carugo D., Zhang X. Hybrid nanoplatforms comprising organic nanocompartments encapsulating inorganic nanoparticles for enhanced drug delivery and bioimaging applications. Molecules. 2023;15:28. doi: 10.3390/molecules28155694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Z., et al. Nanomaterials for cancer therapy: current progress and perspectives. J. Hematol. Oncol. 2021;14(1):85. doi: 10.1186/s13045-021-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niu X., Chen J., Gao J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: focus on recent advances. Asian J. Pharm. Sci. 2019;14(5):480–496. doi: 10.1016/j.ajps.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song W., Musetti S.N., Huang L. Nanomaterials for cancer immunotherapy. Biomaterials. 2017;148:16–30. doi: 10.1016/j.biomaterials.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson D., Brissette C.A., Watt J.A. The choroid plexus and its role in the pathogenesis of neurological infections. Fluids Barriers CNS. 2022;19(1):75. doi: 10.1186/s12987-022-00372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson D., Brissette C.A., Watt J.A. The choroid plexus and its role in the pathogenesis of neurological infections. Fluids Barriers CNS. 2022;19(1):75. doi: 10.1186/s12987-022-00372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy S., et al. Recent advances in nano delivery systems for blood-brain barrier (BBB) penetration and targeting of brain tumors. Drug Discov. Today. 2021;26(8):1944–1952. doi: 10.1016/j.drudis.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Abbott N.J., et al. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Wu D., et al. The blood-brain barrier: structure, regulation, and drug delivery. Signal Transduction and Targeted Therapy. 2023;8(1):217. doi: 10.1038/s41392-023-01481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Y., et al. Exosomes: multiple-targeted multifunctional biological nanoparticles in the diagnosis, drug delivery, and imaging of cancer cells. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2020;129:110442. doi: 10.1016/j.biopha.2020.110442. [DOI] [PubMed] [Google Scholar]
- 20.Arvanitis C.D., Ferraro G.B., Jain R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer. 2020;20(1):26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kushwaha R., et al. Reactive astrocytes associated with prion disease impair the blood brain barrier. Neurobiol. Dis. 2023;185:106264. doi: 10.1016/j.nbd.2023.106264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam M.S., et al. 2023. Unveiling the Influence of Tumor Microenvironment and Spatial Heterogeneity on Temozolomide Resistance in Glioblastoma Using an Advanced Human in Vitro Model of the Blood-Brain Barrier and Glioblastoma. Small (Weinheim an Der Bergstrasse, Germany) [DOI] [PubMed] [Google Scholar]
- 23.Huang H.-S., et al. A novel isotope-labeled small molecule probe CC12 for anti-glioma via suppressing LYN-mediated Progression and activating apoptosis pathways. Int. J. Biol. Sci. 2023;19(10):3209–3225. doi: 10.7150/ijbs.82266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng G., et al. Anti-parkinsonian therapy: Strategies for Crossing the blood-brain Barrier and nano-biological Effects of nanomaterials. Nanomicro lett. 2022;14(1):105. doi: 10.1007/s40820-022-00847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Tellingen O., et al. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Arsiwala T.A., et al. Ultrasound-mediated disruption of the blood tumor barrier for improved therapeutic delivery. Neoplasia (New York, N.Y. 2021;23(7):676–691. doi: 10.1016/j.neo.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belykh E., et al. Blood-brain barrier, blood-brain tumor barrier, and fluorescence-guided neurosurgical oncology: delivering optical Labels to brain tumors. Frontiers. Oncology. 2020;10:739. doi: 10.3389/fonc.2020.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcucci F., Corti A., Ferreri A.J.M. Breaching the blood-brain tumor Barrier for tumor therapy. Cancers. 2021;13(10) doi: 10.3390/cancers13102391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sprowls S.A., et al. Improving CNS Delivery to brain Metastases by blood-tumor barrier disruption. Trends cancer. 2019;5(8):495–505. doi: 10.1016/j.trecan.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanif S., et al. Nanomedicine-based immunotherapy for central nervous system disorders. Acta Pharmacol. Sin. 2020;41(7):936–953. doi: 10.1038/s41401-020-0429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu Z., et al. Novel nano-drug delivery System for brain tumor treatment. Cells. 2022;23:11. doi: 10.3390/cells11233761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramalho M.J., et al. Transferrin-Conjugated PLGA Nanoparticles for Co-Delivery of Temozolomide and Bortezomib to glioblastoma cells. ACS applied nano materials. 2023;6(15):14191–14203. doi: 10.1021/acsanm.3c02122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang X., et al. Nanoparticles of 2-deoxy-D-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials. 2014;35(1):518–529. doi: 10.1016/j.biomaterials.2013.09.094. [DOI] [PubMed] [Google Scholar]
- 34.He Y., et al. Enhanced anti-glioma efficacy of doxorubicin with BRD4 PROTAC degrader using targeted nanoparticles. Materials Today. Bio. 2022;16:100423. doi: 10.1016/j.mtbio.2022.100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weller J., Budson A. Current understanding of Alzheimer's disease diagnosis and treatment. F1000Research. 2018:7. doi: 10.12688/f1000research.14506.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.2016 Alzheimer's disease facts and figures. Alzheimer's Dementia : the Journal of the Alzheimer's Association. 2016;12(4):459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Scheltens P., et al. Alzheimer's disease. Lancet (london, england) 2021;397(10284):1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z., Sun T., Jiang C. Recent advances on drug delivery nanocarriers for cerebral disorders. Biomedical Materials (Bristol, England) 2021;16(2):24104. doi: 10.1088/1748-605X/abdc97. [DOI] [PubMed] [Google Scholar]
- 39.Kang Y.J., Cutler E.G., Cho H. Therapeutic nanoplatforms and delivery strategies for neurological disorders. Nano Converg. 2018;5(1):35. doi: 10.1186/s40580-018-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masato A., et al. Impaired dopamine metabolism in Parkinson's disease pathogenesis. Mol. Neurodegener. 2019;14(1):35. doi: 10.1186/s13024-019-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lotankar S., Prabhavalkar K.S., Bhatt L.K. Biomarkers for Parkinson's disease: recent advancement. Neuroscience bulletin. 2017;33(5):585–597. doi: 10.1007/s12264-017-0183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vijiaratnam N., et al. Progress towards therapies for disease modification in Parkinson's disease. Lancet Neurol. 2021;20(7):559–572. doi: 10.1016/S1474-4422(21)00061-2. [DOI] [PubMed] [Google Scholar]
- 43.Frisardi V., Santamato A., Cheeran B. Parkinson's disease: new Insights into Pathophysiology and rehabilitative approaches. Parkinson's disease. 2016;2016:3121727. doi: 10.1155/2016/3121727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li T., Le W. Biomarkers for Parkinson's disease: how good are they? Neuroscience bulletin. 2020;36(2):183–194. doi: 10.1007/s12264-019-00433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen R., Gu X., Wang X. α-Synuclein in Parkinson's disease and advances in detection. Clinica Chimica Acta. International Journal of Clinical Chemistry. 2022;529:76–86. doi: 10.1016/j.cca.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Tolosa E., et al. Challenges in the diagnosis of Parkinson's disease. Lancet Neurol. 2021;20(5):385–397. doi: 10.1016/S1474-4422(21)00030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thanvi B., Lo N., Robinson T. Levodopa-induced dyskinesia in Parkinson's disease: clinical features, pathogenesis, prevention and treatment. Postgrad Med J. 2007;83(980):384–388. doi: 10.1136/pgmj.2006.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson A.J., et al. Multiple sclerosis. Lancet (London, England) 2018;391(10130):1622–1636. doi: 10.1016/S0140-6736(18)30481-1. [DOI] [PubMed] [Google Scholar]
- 49.Marcus R. What is multiple sclerosis? JAMA. 2022;328(20):2078. doi: 10.1001/jama.2022.14236. [DOI] [PubMed] [Google Scholar]
- 50.Bergholt M.S., et al. Correlated heterospectral lipidomics for biomolecular profiling of remyelination in multiple sclerosis. ACS Cent. Sci. 2018;4(1):39–51. doi: 10.1021/acscentsci.7b00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stephenson E.L., et al. Targeting the chondroitin sulfate proteoglycans: evaluating fluorinated glucosamines and xylosides in screens pertinent to multiple sclerosis. ACS Cent. Sci. 2019;5(7):1223–1234. doi: 10.1021/acscentsci.9b00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng Y., et al. Recent advances in nanomedicines for multiple sclerosis therapy. ACS Appl. Bio Mater. 2020;3(10):6571–6597. doi: 10.1021/acsabm.0c00953. [DOI] [PubMed] [Google Scholar]
- 53.Dendrou C.A., Fugger L., Friese M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015;15(9):545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 54.Mwema A., Muccioli G.G. A. des Rieux, Innovative drug delivery strategies to the CNS for the treatment of multiple sclerosis. J Control Release. 2023;364:435–457. doi: 10.1016/j.jconrel.2023.10.052. [DOI] [PubMed] [Google Scholar]
- 55.Kappos L., et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263–1273. doi: 10.1016/S0140-6736(18)30475-6. [DOI] [PubMed] [Google Scholar]
- 56.Lamb Y.N. Ocrelizumab: a Review in multiple sclerosis. Drugs. 2022;82(3):323–334. doi: 10.1007/s40265-022-01672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel D.C., et al. Neuron-glia interactions in the pathophysiology of epilepsy. Nat. Rev. Neurosci. 2019;20(5):282–297. doi: 10.1038/s41583-019-0126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Z., et al. ROS/Electro dual-reactive Nanogel for targeting epileptic Foci to remodel aberrant Circuits and inflammatory microenvironment. ACS nano. 2023;17(8):7847–7864. doi: 10.1021/acsnano.3c01140. [DOI] [PubMed] [Google Scholar]
- 59.Zhang C., et al. The transport of antiepileptic drugs by P-glycoprotein. Adv. Drug Deliv. Rev. 2012;64(10):930–942. doi: 10.1016/j.addr.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Potschka H. Role of CNS efflux drug transporters in antiepileptic drug delivery: overcoming CNS efflux drug transport. Advanced Drug Delivery Reviews. 2012;64(10):943–952. doi: 10.1016/j.addr.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Beck H. Plasticity of antiepileptic drug targets. Epilepsia. 2007;48(1):14–18. doi: 10.1111/j.1528-1167.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 62.Tang L., et al. Harnessing nanobiotechnology for cerebral ischemic stroke management. Biomater. Sci. 2023;11(3):791–812. doi: 10.1039/d2bm01790c. [DOI] [PubMed] [Google Scholar]
- 63.Alkaff S.A., et al. Nanocarriers for stroke therapy: advances and obstacles in translating animal studies. Int. J. Nanomed. 2020;15:445–464. doi: 10.2147/IJN.S231853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao Y., Chen X., Liu H. A facile approach for synthesis of nano-CeO2 particles loaded co-polymer matrix and their colossal role for blood-brain barrier permeability in Cerebral Ischemia. Journal of Photochemistry and Photobiology. B, Biology. 2018;187:184–189. doi: 10.1016/j.jphotobiol.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Kaviarasi S., et al. Emerging paradigms in nanotechnology for imaging and treatment of cerebral ischemia. Journal of Controlled Release. Official Journal of the Controlled Release Society. 2019;300:22–45. doi: 10.1016/j.jconrel.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 66.Lv W., et al. Advances of nano drug delivery system for the theranostics of ischemic stroke. J. Nanobiotechnol. 2022;20(1):248. doi: 10.1186/s12951-022-01450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al-Ahmady Z.S., et al. Selective liposomal transport through blood brain barrier disruption in ischemic stroke reveals two distinct therapeutic opportunities. ACS Nano. 2019;13(11):12470–12486. doi: 10.1021/acsnano.9b01808. [DOI] [PubMed] [Google Scholar]
- 68.Bao Q., et al. Simultaneous blood-brain barrier Crossing and Protection for stroke treatment Based on edaravone-loaded ceria nanoparticles. ACS nano. 2018;12(7):6794–6805. doi: 10.1021/acsnano.8b01994. [DOI] [PubMed] [Google Scholar]
- 69.Fiorelli M., et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort Stroke. 1999;30(11):2280–2284. doi: 10.1161/01.str.30.11.2280. [DOI] [PubMed] [Google Scholar]
- 70.Liao J., et al. Recent advances in targeted nanotherapies for ischemic stroke. Mol. Pharm. 2022;19(9):3026–3041. doi: 10.1021/acs.molpharmaceut.2c00383. [DOI] [PubMed] [Google Scholar]
- 71.Quader S., Kataoka K., Cabral H. Nanomedicine for brain cancer. Adv. Drug Deliv. Rev. 2022;182:114115. doi: 10.1016/j.addr.2022.114115. [DOI] [PubMed] [Google Scholar]
- 72.Zhao X., et al. Remodeling the blood-brain barrier microenvironment by natural products for brain tumor therapy. Acta Pharm. Sin. B. 2017;7(5):541–553. doi: 10.1016/j.apsb.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bouzinab K., et al. Delivery of temozolomide and N3-propargyl analog to brain tumors using an apoferritin nanocage. ACS applied materials & interfaces. 2020;12(11):12609–12617. doi: 10.1021/acsami.0c01514. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y., et al. Brain co-delivery of first-line chemotherapy drug and epigenetic bromodomain inhibitor for multidimensional enhanced synergistic glioblastoma therapy. Exploration (Beijing) 2022;2(4):20210274. doi: 10.1002/EXP.20210274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lyon J.G., et al. Engineering challenges for brain tumor immunotherapy. Adv. Drug Deliv. Rev. 2017;114:19–32. doi: 10.1016/j.addr.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choo M., et al. Involvement of cell shape and lipid metabolism in glioblastoma resistance to temozolomide. Acta Pharmacol. Sin. 2023;44(3):670–679. doi: 10.1038/s41401-022-00984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han L., Jiang C. Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm. Sin. B. 2021;11(8):2306–2325. doi: 10.1016/j.apsb.2020.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Awad R., Avital A., Sosnik A. Polymeric nanocarriers for nose-to-brain drug delivery in neurodegenerative diseases and neurodevelopmental disorders. Acta Pharm. Sin. B. 2023;13(5):1866–1886. doi: 10.1016/j.apsb.2022.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang X., et al. Intranasal delivery of BACE1 siRNA and rapamycin by dual targets modified nanoparticles for Alzheimer's disease therapy. Small. 2022;18(30) doi: 10.1002/smll.202203182. [DOI] [PubMed] [Google Scholar]
- 80.Crowe T.P., et al. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi: 10.1016/j.lfs.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 81.Agrawal M., et al. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Contr. Release. 2018;281:139–177. doi: 10.1016/j.jconrel.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 82.Akel H., Ismail R., Csóka I. Progress and perspectives of brain-targeting lipid-based nanosystems via the nasal route in Alzheimer's disease. Eur. J. Pharm. Biopharm. 2020;148:38–53. doi: 10.1016/j.ejpb.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 83.Formica M.L., et al. On a highway to the brain: a review on nose-to-brain drug delivery using nanoparticles. Appl. Mater. Today. 2022:29. [Google Scholar]
- 84.Kuo Y.-C., Wang I.H., Rajesh R. Use of leptin-conjugated phosphatidic acid liposomes with resveratrol and epigallocatechin gallate to protect dopaminergic neurons against apoptosis for Parkinson's disease therapy. Acta Biomater. 2021;119:360–374. doi: 10.1016/j.actbio.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 85.Yan B.C., et al. Dietary Fe3O4 nanozymes Prevent the Injury of Neurons and blood-brain barrier Integrity from cerebral ischemic stroke. ACS biomaterials science & engineering. 2021;7(1):299–310. doi: 10.1021/acsbiomaterials.0c01312. [DOI] [PubMed] [Google Scholar]
- 86.Partridge B., et al. Advancements in drug delivery methods for the treatment of brain disease. Front. Vet. Sci. 2022;9:1039745. doi: 10.3389/fvets.2022.1039745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Venugopal I., Habib N., Linninger A. Intrathecal magnetic drug targeting for localized delivery of therapeutics in the CNS. Nanomedicine (Lond) 2017;12(8):865–877. doi: 10.2217/nnm-2016-0418. [DOI] [PubMed] [Google Scholar]
- 88.Yun W.S., et al. Recent studies and progress in the intratumoral administration of nano-sized drug delivery systems. Nanomaterials. 2023;15:13. doi: 10.3390/nano13152225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melero I., et al. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat. Rev. Clin. Oncol. 2021;18(9):558–576. doi: 10.1038/s41571-021-00507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chu X.Y., et al. Improving antitumor outcomes for palliative intratumoral injection therapy through lecithin- chitosan nanoparticles loading paclitaxel- cholesterol complex. Int J Nanomedicine. 2019;14:689–705. doi: 10.2147/IJN.S188667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petriev V.M., et al. Nuclear nanomedicine using Si nanoparticles as safe and effective carriers of (188)Re radionuclide for cancer therapy. Sci. Rep. 2019;9(1):2017. doi: 10.1038/s41598-018-38474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tiwari A.P., et al. pH/NIR-Responsive polypyrrole-functionalized fibrous localized drug-delivery Platform for synergistic cancer therapy. ACS appl mater interfaces. 2018;10(24):20256–20270. doi: 10.1021/acsami.7b17664. [DOI] [PubMed] [Google Scholar]
- 93.Mehta A.M., Sonabend A.M., Bruce J.N. Convection-enhanced delivery. Neurotherapeutics. 2017;14(2):358–371. doi: 10.1007/s13311-017-0520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D'Amico R.S., et al. Convection-enhanced drug delivery for glioblastoma: a review. J. Neuro Oncol. 2021;151(3):415–427. doi: 10.1007/s11060-020-03408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vogelbaum M.A., Aghi M.K. Convection-enhanced delivery for the treatment of glioblastoma. Neuro Oncol. 2015;17(2):ii3–ii8. doi: 10.1093/neuonc/nou354. Suppl 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bobo R.H., et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Upadhyayula P.S., et al. Convection enhanced delivery of topotecan for gliomas: a single-center experience. Pharmaceutics. 2020;13(1) doi: 10.3390/pharmaceutics13010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X., et al. Nano carriers for drug transport across the blood-brain barrier. J. Drug Target. 2017;25(1):17–28. doi: 10.1080/1061186X.2016.1184272. [DOI] [PubMed] [Google Scholar]
- 99.Sperring C.P., et al. Convection-enhanced delivery of immunomodulatory therapy for high-grade glioma. Neurooncol Adv. 2023;5(1) doi: 10.1093/noajnl/vdad044. vdad044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barua N.U., Gill S.S., Love S. Convection-enhanced drug delivery to the brain: therapeutic potential and neuropathological considerations. Brain Pathol. 2014;24(2):117–127. doi: 10.1111/bpa.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jones A.R., Shusta E.V. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. (N. Y.) 2007;24(9):1759–1771. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arguello A., et al. Molecular architecture determines brain delivery of a transferrin receptor-targeted lysosomal enzyme. J. Exp. Med. 2022;219(3) doi: 10.1084/jem.20211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lajoie J.M., Shusta E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu. Rev. Pharmacol. Toxicol. 2015;55:613–631. doi: 10.1146/annurev-pharmtox-010814-124852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jhaveri A., et al. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J Control Release. 2018;277:89–101. doi: 10.1016/j.jconrel.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang Y., et al. Apolipoprotein E peptide-directed chimeric polymersomes Mediate an ultrahigh-efficiency targeted protein Therapy for glioblastoma. ACS nano. 2018;12(11):11070–11079. doi: 10.1021/acsnano.8b05265. [DOI] [PubMed] [Google Scholar]
- 106.Pinheiro R.G.R., et al. RVG29-Functionalized lipid Nanoparticles for quercetin brain Delivery and Alzheimer's disease. Pharm res. 2020;37(7):139. doi: 10.1007/s11095-020-02865-1. [DOI] [PubMed] [Google Scholar]
- 107.Cui J., et al. Immune exosomes loading self-assembled nanomicelles traverse the blood-brain barrier for chemo-immunotherapy against glioblastoma. ACS Nano. 2023 doi: 10.1021/acsnano.2c10219. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 108.Li Y.X., et al. Advances in the research of nano delivery systems in ischemic stroke. Front. Bioeng. Biotechnol. 2022;10:984424. doi: 10.3389/fbioe.2022.984424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu Y., et al. Multifunctional ginsenoside Rg3-based liposomes for glioma targeting therapy. Journal of Controlled Release. Official Journal of the Controlled Release Society. 2021;330:641–657. doi: 10.1016/j.jconrel.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 110.Song X., Qian H., Yu Y. Nanoparticles mediated the diagnosis and therapy of glioblastoma: bypass or cross the blood-brain barrier. Small. 2023 doi: 10.1002/smll.202302613. [DOI] [PubMed] [Google Scholar]
- 111.Chiu H.T., et al. Albumin-gold nanorod Nanoplatform for cell-mediated tumoritropic Delivery with homogenous ChemoDrug Distribution and enhanced retention ability. Theranostics. 2017;7(12):3034–3052. doi: 10.7150/thno.19279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lenna S., et al. Mesenchymal stromal cells mediated delivery of photoactive nanoparticles inhibits osteosarcoma growth in vitro and in a murine in vivo ectopic model. J. Exp. Clin. Cancer Res. 2020;39(1):40. doi: 10.1186/s13046-020-01548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xing Y., et al. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale. 2017;9(25):8781–8790. doi: 10.1039/c7nr01857f. [DOI] [PubMed] [Google Scholar]
- 114.Yin T., et al. Engineered macrophage-membrane-coated Nanoparticles with enhanced PD-1 expression induce Immunomodulation for a Synergistic and targeted antiglioblastoma activity. Nano lett. 2022;22(16):6606–6614. doi: 10.1021/acs.nanolett.2c01863. [DOI] [PubMed] [Google Scholar]
- 115.Guo Q.L., et al. Nanosensitizers for sonodynamic therapy for glioblastoma multiforme: current progress and future perspectives. Mil Med Res. 2022;9(1):26. doi: 10.1186/s40779-022-00386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He W., et al. Brain-targeted Codelivery of bcl-2/bcl-xl and Mcl-1 Inhibitors by biomimetic Nanoparticles for orthotopic glioblastoma therapy. ACS nano. 2022;16(4):6293–6308. doi: 10.1021/acsnano.2c00320. [DOI] [PubMed] [Google Scholar]
- 117.Hashimoto Y., Campbell M. Tight junction modulation at the blood-brain barrier: current and future perspectives. Biochim. Biophys. Acta Biomembr. 2020;1862(9):183298. doi: 10.1016/j.bbamem.2020.183298. [DOI] [PubMed] [Google Scholar]
- 118.Chen L., et al. Claudin-5 binder enhances focused ultrasound-mediated opening in an in vitro blood-brain barrier model. Theranostics. 2022;12(5):1952–1970. doi: 10.7150/thno.65539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li X., et al. Reversibly modulating the blood-brain barrier by laser stimulation of molecular-targeted nanoparticles. Nano Lett. 2021;21(22):9805–9815. doi: 10.1021/acs.nanolett.1c02996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang J., et al. Ultrasound-mediated blood–brain barrier opening: an effective drug delivery system for theranostics of brain diseases. Adv. Drug Deliv. Rev. 2022;190:114539. doi: 10.1016/j.addr.2022.114539. [DOI] [PubMed] [Google Scholar]
- 121.Meyers R., et al. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J. Neurosurg. 1959;16(1):32–54. doi: 10.3171/jns.1959.16.1.0032. [DOI] [PubMed] [Google Scholar]
- 122.Hynynen K., Jolesz F.A. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med. Biol. 1998;24(2):275–283. doi: 10.1016/s0301-5629(97)00269-x. [DOI] [PubMed] [Google Scholar]
- 123.Clement G.T., Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol. 2002;47(8):1219–1236. doi: 10.1088/0031-9155/47/8/301. [DOI] [PubMed] [Google Scholar]
- 124.Aryal M., et al. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev. 2014;72:94–109. doi: 10.1016/j.addr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hynynen K., Clement G. Clinical applications of focused ultrasound-the brain. Int J Hyperthermia. 2007;23(2):193–202. doi: 10.1080/02656730701200094. [DOI] [PubMed] [Google Scholar]
- 126.Hynynen K., et al. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220(3):640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 127.Zhao P., et al. Recent advances of focused ultrasound induced blood-brain barrier opening for clinical applications of neurodegenerative diseases. Adv. Drug Deliv. Rev. 2024;209:115323. doi: 10.1016/j.addr.2024.115323. [DOI] [PubMed] [Google Scholar]
- 128.Jones R.M., Hynynen K. Advances in acoustic monitoring and control of focused ultrasound-mediated increases in blood-brain barrier permeability. Br. J. Radiol. 2019;92(1096):20180601. doi: 10.1259/bjr.20180601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim D., Kwon H.J., Hyeon T. Magnetite/ceria nanoparticle Assemblies for extracorporeal Cleansing of amyloid-β in Alzheimer's disease. Adv Mater. 2019;31(19) doi: 10.1002/adma.201807965. [DOI] [PubMed] [Google Scholar]
- 130.Zha S., et al. Functionalized nanomaterials capable of crossing the blood-brain barrier. ACS Nano. 2024;18(3):1820–1845. doi: 10.1021/acsnano.3c10674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bhunia S., et al. Drug delivery to the brain: recent advances and unmet challenges. Pharmaceutics. 2023;15(12) doi: 10.3390/pharmaceutics15122658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chu C., et al. Hyperosmolar blood-brain barrier opening using intra-arterial injection of hyperosmotic mannitol in mice under real-time MRI guidance. Nat. Protoc. 2022;17(1):76–94. doi: 10.1038/s41596-021-00634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brightman M.W., et al. Osmotic opening of tight junctions in cerebral endothelium. J. Comp. Neurol. 1973;152(4):317–325. doi: 10.1002/cne.901520402. [DOI] [PubMed] [Google Scholar]
- 134.Zawadzki M., et al. Real-time MRI guidance for intra-arterial drug delivery in a patient with a brain tumor: technical note. BMJ Case Rep. 2019;12(1) doi: 10.1136/bcr-2018-014469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weiss V.U., et al. Nano electrospray gas-phase electrophoretic mobility molecular analysis (nES GEMMA) of liposomes: applicability of the technique for nano vesicle batch control. The Analyst. 2016;141(21):6042–6050. doi: 10.1039/c6an00687f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lombardo D., Kiselev M.A. Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics. 2022;(3):14. doi: 10.3390/pharmaceutics14030543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mandpe P., Prabhakar B., Shende P. Role of liposomes-based stem Cell for multimodal cancer therapy. Stem Cell Reviews and Reports. 2020;16(1):103–117. doi: 10.1007/s12015-019-09933-z. [DOI] [PubMed] [Google Scholar]
- 138.Jagaran K., Singh M. Lipid nanoparticles: promising treatment approach for Parkinson's disease. Int. J. Mol. Sci. 2022;23(16) doi: 10.3390/ijms23169361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li M., et al. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019;164:640–653. doi: 10.1016/j.ejmech.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 140.Wang S., et al. Liposomes for tumor targeted therapy: a review. Int. J. Mol. Sci. 2023;24(3) doi: 10.3390/ijms24032643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eloy J.O., et al. Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery. Colloids Surf. B Biointerfaces. 2014;123:345–363. doi: 10.1016/j.colsurfb.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y., Qu H., Xue X. Blood-brain barrier penetrating liposomes with synergistic chemotherapy for glioblastoma treatment. Biomater. Sci. 2022;10(2):423–434. doi: 10.1039/d1bm01506k. [DOI] [PubMed] [Google Scholar]
- 143.Lin Y.-L., et al. Encapsulated n-butylidenephthalide efficiently Crosses the blood-brain Barrier and suppresses Growth of glioblastoma. Int. J. Nanomed. 2020;15:749–760. doi: 10.2147/IJN.S235815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kang S., et al. Muscone/RI7217 co-modified upward messenger DTX liposomes enhanced permeability of blood-brain barrier and targeting glioma. Theranostics. 2020;10(10):4308–4322. doi: 10.7150/thno.41322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kong L., et al. Transferrin-modified osthole PEGylated liposomes Travel the blood-brain Barrier and mitigate Alzheimer's disease-related Pathology in APP/PS-1 mice. Int. J. Nanomed. 2020;15:2841–2858. doi: 10.2147/IJN.S239608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mojarad-Jabali S., et al. Comparison of three synthetic transferrin mimetic small peptides to promote the blood-brain barrier penetration of vincristine liposomes for improved glioma targeted therapy. Int. J. Pharm. 2022;613:121395. doi: 10.1016/j.ijpharm.2021.121395. [DOI] [PubMed] [Google Scholar]
- 147.Hu J., et al. Single-cell transcriptome analysis reveals intratumoral Heterogeneity in ccRCC, which Results in different clinical outcomes. Mol. Ther. 2020;28(7):1658–1672. doi: 10.1016/j.ymthe.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arora S., Layek B., Singh J. Design and validation of liposomal ApoE2 gene delivery system to evade blood-brain barrier for effective treatment of Alzheimer's disease. Mol. Pharm. 2021;18(2):714–725. doi: 10.1021/acs.molpharmaceut.0c00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ahmed T., et al. Optimizing the design of blood-brain barrier-penetrating polymer-lipid-hybrid nanoparticles for delivering anticancer drugs to glioblastoma. Pharmaceut. Res. 2021;38(11):1897–1914. doi: 10.1007/s11095-021-03122-9. [DOI] [PubMed] [Google Scholar]
- 150.Pawar G., et al. Endonasal CNS delivery System for blood-brain barrier impermeant therapeutic oligonucleotides using heterotopic mucosal engrafting. Frontiers. Pharmacology. 2021;12:660841. doi: 10.3389/fphar.2021.660841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ni Y.-N., et al. Multifunctional osthole liposomes and brain targeting functionality with potential applications in a mouse model of Alzheimer's disease. J. Liposome Res. 2021;31(3):267–278. doi: 10.1080/08982104.2020.1806872. [DOI] [PubMed] [Google Scholar]
- 152.Qi N., et al. "Guide" of muscone modification enhanced brain-targeting efficacy and anti-glioma effect of lactoferrin modified DTX liposomes. Bioengineering & Translational Medicine. 2023;8(2) doi: 10.1002/btm2.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rodrigues B.D.S., Kanekiyo T., Singh J. Nerve growth factor gene delivery across the blood-brain barrier to reduce beta amyloid accumulation in AD mice. Mol. Pharm. 2020;17(6):2054–2063. doi: 10.1021/acs.molpharmaceut.0c00218. [DOI] [PubMed] [Google Scholar]
- 154.Kuo Y.-C., Ng I.W., Rajesh R. Glutathione- and apolipoprotein E-grafted liposomes to regulate mitogen-activated protein kinases and rescue neurons in Alzheimer's disease. Materials Science & Engineering. C, Materials For Biological Applications. 2021;127:112233. doi: 10.1016/j.msec.2021.112233. [DOI] [PubMed] [Google Scholar]
- 155.Wang J., et al. Multifunctional icariin and tanshinone IIA co-delivery liposomes with potential application for Alzheimer's disease. Drug Deliv. 2022;29(1):1648–1662. doi: 10.1080/10717544.2022.2072543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grafals-Ruiz N., et al. Brain targeted gold liposomes improve RNAi delivery for glioblastoma. Int. J. Nanomed. 2020;15:2809–2828. doi: 10.2147/IJN.S241055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kong D., et al. Multifunctional targeting liposomes of epirubicin plus resveratrol improved therapeutic effect on brain gliomas. Int. J. Nanomed. 2022;17:1087–1110. doi: 10.2147/IJN.S346948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ismail M., et al. Targeted liposomes for combined delivery of artesunate and temozolomide to resistant glioblastoma. Biomaterials. 2022;287:121608. doi: 10.1016/j.biomaterials.2022.121608. [DOI] [PubMed] [Google Scholar]
- 159.Chen Y.-Y., et al. Brain-targeting emodin mitigates ischemic Stroke via inhibiting AQP4-mediated Swelling and neuroinflammation. Translational Stroke Research. 2023;15(4):818–830. doi: 10.1007/s12975-023-01170-4. [DOI] [PubMed] [Google Scholar]
- 160.Andrade S., et al. Transferrin-functionalized liposomes loaded with vitamin VB12 for Alzheimer's disease therapy. Int. J. Pharm. 2022;626:122167. doi: 10.1016/j.ijpharm.2022.122167. [DOI] [PubMed] [Google Scholar]
- 161.Chen M.-H., et al. ApoE-modified liposomes encapsulating resveratrol and salidroside alleviate manifestations of Alzheimer's disease in APP/PS-1 mice. Drug Dev. Ind. Pharm. 2023;49(9):559–571. doi: 10.1080/03639045.2023.2252062. [DOI] [PubMed] [Google Scholar]
- 162.Pinheiro R.G.R., et al. RVG29-Functionalized lipid Nanoparticles for quercetin brain Delivery and Alzheimer's disease. Pharmaceut. Res. 2020;37(7):139. doi: 10.1007/s11095-020-02865-1. [DOI] [PubMed] [Google Scholar]
- 163.Xin X., et al. Efficient anti-glioma therapy Through the brain-targeted RVG15-modified liposomes loading paclitaxel-cholesterol complex. Int. J. Nanomed. 2021;16:5755–5776. doi: 10.2147/IJN.S318266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao Y., et al. pH-redox responsive cascade-targeted liposomes to intelligently deliver doxorubicin prodrugs and lonidamine for glioma. Eur. J. Med. Chem. 2022;235:114281. doi: 10.1016/j.ejmech.2022.114281. [DOI] [PubMed] [Google Scholar]
- 165.Arora S., Singh J. In vitro and in vivo optimization of liposomal nanoparticles based brain targeted vgf gene therapy. Int. J. Pharm. 2021;608:121095. doi: 10.1016/j.ijpharm.2021.121095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang X., et al. Transferrin-Pep63-liposomes accelerate the clearance of Aβ and rescue impaired synaptic plasticity in early Alzheimer's disease models. Cell Death Discovery. 2021;7(1):256. doi: 10.1038/s41420-021-00639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu Q., et al. Biotin and glucose co-modified multi-targeting liposomes for efficient delivery of chemotherapeutics for the treatment of glioma. Bioorg. Med. Chem. 2021;29:115852. doi: 10.1016/j.bmc.2020.115852. [DOI] [PubMed] [Google Scholar]
- 168.Ghaferi M., et al. Impact of PEGylated liposomal Doxorubicin and carboplatin Combination on glioblastoma. Pharmaceutics. 2022;14(10) doi: 10.3390/pharmaceutics14102183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kuo Y.-C., Chen I.Y., Rajesh R. Astragaloside IV- and nesfatin-1-encapsulated phosphatidylserine liposomes conjugated with wheat germ agglutinin and leptin to activate anti-apoptotic pathway and block phosphorylated tau protein expression for Parkinson's disease treatment. Materials Science & Engineering. C. Materials For Biological Applications. 2021;129:112361. doi: 10.1016/j.msec.2021.112361. [DOI] [PubMed] [Google Scholar]
- 170.Xu H., et al. Angiopep-2-modified calcium arsenite-loaded liposomes for targeted and pH-responsive delivery for anti-glioma therapy. Biochem. Biophys. Res. Commun. 2021;551:14–20. doi: 10.1016/j.bbrc.2021.02.138. [DOI] [PubMed] [Google Scholar]
- 171.Lu J., et al. Multiple targeted doxorubicin-lonidamine liposomes modified with p-hydroxybenzoic acid and triphenylphosphonium to synergistically treat glioma. Eur. J. Med. Chem. 2022;230:114093. doi: 10.1016/j.ejmech.2021.114093. [DOI] [PubMed] [Google Scholar]
- 172.Ashrafzadeh M.S., et al. In vivo glioblastoma therapy using targeted liposomal cisplatin. Int. J. Nanomed. 2020;15:7035–7049. doi: 10.2147/IJN.S255902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Andrade S., Pereira M.C., Loureiro J.A. Caffeic acid loaded into engineered lipid nanoparticles for Alzheimer's disease therapy. Colloids and Surfaces. B, Biointerfaces. 2023;225:113270. doi: 10.1016/j.colsurfb.2023.113270. [DOI] [PubMed] [Google Scholar]
- 174.Andrade S., Loureiro J.A., Pereira M.C. Transferrin-functionalized Liposomes for the Delivery of gallic acid: a therapeutic Approach for Alzheimer's disease. Pharmaceutics. 2022;14(10) doi: 10.3390/pharmaceutics14102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yao J., et al. TMZ magnetic temperature-sensitive liposomes-mediated magnetothermal chemotherapy induces pyroptosis in glioblastoma. Nanomedicine. Nanotechnology, Biology, and Medicine. 2022;43:102554. doi: 10.1016/j.nano.2022.102554. [DOI] [PubMed] [Google Scholar]
- 176.Zhang X., et al. Angiopep-2 modified dual drug-loaded liposomes with brain targeting functionality mitigate Alzheimer's disease-related symptoms in APP/PS-1 mice. J. Drug Target. 2023;31(6):634–645. doi: 10.1080/1061186X.2023.2216405. [DOI] [PubMed] [Google Scholar]
- 177.Luiz M.T., et al. Docetaxel-loaded folate-modified TPGS-transfersomes for glioblastoma multiforme treatment. Materials Science & Engineering. C, Materials For Biological Applications. 2021;124:112033. doi: 10.1016/j.msec.2021.112033. [DOI] [PubMed] [Google Scholar]
- 178.Thomas R.G., et al. Treatment of ischemic stroke by atorvastatin-loaded PEGylated liposome. Transl. Stroke Res. 2023;15(2):388–398. doi: 10.1007/s12975-023-01125-9. [DOI] [PubMed] [Google Scholar]
- 179.Yang C., et al. Berberine and folic acid co-modified pH-sensitive cascade-targeted PTX-liposomes coated with Tween 80 for treating glioma. Bioorg. Med. Chem. 2022;69:116893. doi: 10.1016/j.bmc.2022.116893. [DOI] [PubMed] [Google Scholar]
- 180.Arora S., Kanekiyo T., Singh J. Functionalized nanoparticles for brain targeted BDNF gene therapy to rescue Alzheimer's disease pathology in transgenic mouse model. Int. J. Biol. Macromol. 2022;208:901–911. doi: 10.1016/j.ijbiomac.2022.03.203. [DOI] [PubMed] [Google Scholar]
- 181.Kuo Y.-C., Lee Y.-J., Rajesh R. Enhanced activity of AZD5582 and SM-164 in rabies virus glycoprotein-lactoferrin-liposomes to downregulate inhibitors of apoptosis proteins in glioblastoma. Biomater. Adv. 2022;133:112615. doi: 10.1016/j.msec.2021.112615. [DOI] [PubMed] [Google Scholar]
- 182.Vangala V., et al. Combating glioblastoma by codelivering the small-molecule inhibitor of STAT3 and STAT3siRNA with α5β1 integrin receptor-selective liposomes. Mol. Pharm. 2020;17(6):1859–1874. doi: 10.1021/acs.molpharmaceut.9b01271. [DOI] [PubMed] [Google Scholar]
- 183.Li D., et al. cRGDyK-modified procaine liposome inhibits the proliferation and motility of glioma cells via the ERK/p38MAPK pathway. Exp. Ther. Med. 2021;22(2):859. doi: 10.3892/etm.2021.10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Barra T., et al. Neuroprotective effects of gH625-lipoPACAP in an in vitro fluid dynamic model of Parkinson's disease. Biomedicines. 2022;10(10) doi: 10.3390/biomedicines10102644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li D., et al. Lidocaine liposome modified with folic acid suppresses the proliferation and motility of glioma cells via targeting the PI3K/AKT pathway. Exp. Ther. Med. 2021;22(3):1025. doi: 10.3892/etm.2021.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Choudhury H., et al. Transferrin receptors-targeting nanocarriers for efficient targeted delivery and transcytosis of drugs into the brain tumors: a review of recent advancements and emerging trends. Drug Delivery and Translational Research. 2018;8(5):1545–1563. doi: 10.1007/s13346-018-0552-2. [DOI] [PubMed] [Google Scholar]
- 187.Qin J., et al. cRGD mediated liposomes enhanced antidepressant-like effects of edaravone in rats. European Journal of Pharmaceutical Sciences. Official Journal of the European Federation For Pharmaceutical Sciences. 2014;58:63–71. doi: 10.1016/j.ejps.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 188.Li X.-T., et al. The efficacy of RGD modified liposomes loaded with vinorelbine plus tetrandrine in treating resistant brain glioma. J. Liposome Res. 2019;29(1):21–34. doi: 10.1080/08982104.2017.1408649. [DOI] [PubMed] [Google Scholar]
- 189.Liu Y., et al. Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting. Biomaterials. 2014;35(17):4835–4847. doi: 10.1016/j.biomaterials.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 190.Wu S., et al. The blood-brain barrier cell-targeted gene delivery System to enhance nerve growth factor protein Secretion in the brain. ACS Biomater. Sci. Eng. 2020;6(11):6207–6216. doi: 10.1021/acsbiomaterials.0c01113. [DOI] [PubMed] [Google Scholar]
- 191.Lombardo S.M., et al. Key for crossing the BBB with nanoparticles: the rational design. Beilstein J. Nanotechnol. 2020;11:866–883. doi: 10.3762/bjnano.11.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chu J.-J., et al. Nanoparticles-based anti-aging treatment of Alzheimer's disease. Drug Deliv. 2022;29(1):2100–2116. doi: 10.1080/10717544.2022.2094501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hu Y.-L., Gao J.-Q. Potential neurotoxicity of nanoparticles. Int. J. Pharm. 2010;394(1–2):115–121. doi: 10.1016/j.ijpharm.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 194.Zhong G., et al. Blood-brain barrier Permeable nanoparticles for Alzheimer's disease treatment by selective mitophagy of microglia. Biomaterials. 2022;288:121690. doi: 10.1016/j.biomaterials.2022.121690. [DOI] [PubMed] [Google Scholar]
- 195.Temizyürek A., et al. Blood-brain barrier targeted delivery of lacosamide-conjugated gold nanoparticles: improving outcomes in absence seizures. Epilepsy Res. 2022;184:106939. doi: 10.1016/j.eplepsyres.2022.106939. [DOI] [PubMed] [Google Scholar]
- 196.Cao Y., et al. Blood-brain barrier permeable and multi-stimuli responsive nanoplatform for orthotopic glioma inhibition by synergistic enhanced chemo-/chemodynamic/photothermal/starvation therapy. European journal of pharmaceutical Sciences. Official Journal of the European Federation For Pharmaceutical Sciences. 2023;180:106319. doi: 10.1016/j.ejps.2022.106319. [DOI] [PubMed] [Google Scholar]
- 197.Gregory J.V., et al. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat. Commun. 2020;11(1):5687. doi: 10.1038/s41467-020-19225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bhattamisra S.K., et al. Nose to brain delivery of rotigotine loaded chitosan nanoparticles in human SH-SY5Y neuroblastoma cells and animal model of Parkinson's disease. Int. J. Pharm. 2020;579:119148. doi: 10.1016/j.ijpharm.2020.119148. [DOI] [PubMed] [Google Scholar]
- 199.Zhang H., et al. Development of curcumin-loaded zein nanoparticles for transport across the blood-brain barrier and inhibition of glioblastoma cell growth. Biomater. Sci. 2021;9(21):7092–7103. doi: 10.1039/d0bm01536a. [DOI] [PubMed] [Google Scholar]
- 200.Xu Z., et al. Aggregation-induced emission nanoprobes Working in the NIR-II region: from material Design to fluorescence Imaging and phototherapy. Adv. Opt. Mater. 2021;9(20):2100859. [Google Scholar]
- 201.Lu X., et al. Development of L-carnosine functionalized iron oxide nanoparticles loaded with dexamethasone for simultaneous therapeutic potential of blood brain barrier crossing and ischemic stroke treatment. Drug Deliv. 2021;28(1):380–389. doi: 10.1080/10717544.2021.1883158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang S., et al. Macrophage-mediated porous magnetic Nanoparticles for multimodal Imaging and postoperative photothermal Therapy of gliomas. ACS Appl. Mater. Interfaces. 2021;13(48):56825–56837. doi: 10.1021/acsami.1c12406. [DOI] [PubMed] [Google Scholar]
- 203.Norouzi M., et al. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: a combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020;10(1):11292. doi: 10.1038/s41598-020-68017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xu R., et al. Rhynchophylline loaded-mPEG-PLGA nanoparticles Coated with tween-80 for preliminary Study in Alzheimer's disease. Int J Nanomedicine. 2020;15:1149–1160. doi: 10.2147/IJN.S236922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hou K., et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer's disease. Nat. Commun. 2020;11(1):4790. doi: 10.1038/s41467-020-18525-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhu J., et al. Angiopep-2 modified lipid-coated mesoporous silica nanoparticles for glioma targeting therapy overcoming BBB. Biochem. Biophys. Res. Commun. 2021;534:902–907. doi: 10.1016/j.bbrc.2020.10.076. [DOI] [PubMed] [Google Scholar]
- 207.Pucci C., et al. Hybrid magnetic nanovectors promote selective glioblastoma cell death through a combined effect of lysosomal membrane permeabilization and chemotherapy. ACS Appl. Mater. Interfaces. 2020;12(26):29037–29055. doi: 10.1021/acsami.0c05556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Janjua T.I., et al. Facile synthesis of lactoferrin conjugated ultra small large pore silica nanoparticles for the treatment of glioblastoma. Nanoscale. 2021;13(40):16909–16922. doi: 10.1039/d1nr03553c. [DOI] [PubMed] [Google Scholar]
- 209.Rizzuto M.A., et al. H-Ferritin nanoparticle-mediated delivery of antibodies across a BBB in vitro model for treatment of brain malignancies. Biomater. Sci. 2021;9(6):2032–2042. doi: 10.1039/d0bm01726d. [DOI] [PubMed] [Google Scholar]
- 210.Yuan X., et al. A diselenide bond-containing ROS-responsive ruthenium nanoplatform delivers nerve growth factor for Alzheimer's disease management by repairing and promoting neuron regeneration. J. Mater. Chem. B. 2021;9(37):7835–7847. doi: 10.1039/d1tb01290h. [DOI] [PubMed] [Google Scholar]
- 211.Shi X., et al. iRGD and TGN co-modified PAMAM for multi-targeted delivery of ATO to gliomas. Biochem. Biophys. Res. Commun. 2020;527(1):117–123. doi: 10.1016/j.bbrc.2020.04.064. [DOI] [PubMed] [Google Scholar]
- 212.Janjua T.I., et al. Efficient delivery of Temozolomide using ultrasmall large-pore silica nanoparticles for glioblastoma. J Control Release. 2023;357:161–174. doi: 10.1016/j.jconrel.2023.03.040. [DOI] [PubMed] [Google Scholar]
- 213.Li Y., et al. Yb(3+), Er(3+) codoped cerium oxide upconversion nanoparticles Enhanced the enzymelike catalytic Activity and antioxidative Activity for Parkinson's disease treatment. ACS Appl. Mater. Interfaces. 2021;13(12):13968–13977. doi: 10.1021/acsami.1c00157. [DOI] [PubMed] [Google Scholar]
- 214.Li Z., et al. A flower-like brain targeted selenium nanocluster Lowers the chlorogenic acid Dose for ameliorating cognitive Impairment in APP/PS1 mice. J agric food chem. 2023;71(6):2883–2897. doi: 10.1021/acs.jafc.2c06809. [DOI] [PubMed] [Google Scholar]
- 215.Pulvirenti L., et al. Synthesis of MIL-modified Fe(3)O(4) magnetic Nanoparticles for enhancing Uptake and Efficiency of Temozolomide in glioblastoma treatment. Int. J. Mol. Sci. 2022;23(5) doi: 10.3390/ijms23052874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ugur Yilmaz C., et al. Targeted delivery of lacosamide-conjugated gold nanoparticles into the brain in temporal lobe epilepsy in rats. Life Sci. 2020;257:118081. doi: 10.1016/j.lfs.2020.118081. [DOI] [PubMed] [Google Scholar]
- 217.Farhangi S., et al. Peptide mediated targeted delivery of gold nanoparticles into the demyelination site ameliorates myelin impairment and gliosis. Nanomedicine. 2023;47:102609. doi: 10.1016/j.nano.2022.102609. [DOI] [PubMed] [Google Scholar]
- 218.Wilson B., et al. Albumin nanoparticles coated with polysorbate 80 for the targeted delivery of antiepileptic drug levetiracetam into the brain. Drug Deliv Transl Res. 2020;10(6):1853–1861. doi: 10.1007/s13346-020-00831-3. [DOI] [PubMed] [Google Scholar]
- 219.Thirumurugan S., et al. Angiopep-2-conjugated FeTi@Au core-shell nanoparticles for tumor targeted dual-mode magnetic resonance imaging and hyperthermic glioma therapy. Nanomedicine. 2023;50:102673. doi: 10.1016/j.nano.2023.102673. [DOI] [PubMed] [Google Scholar]
- 220.Li B., et al. Synchronous disintegration of ferroptosis defense Axis via engineered exosome-conjugated magnetic nanoparticles for glioblastoma therapy. Adv. Sci. 2022;9(17) doi: 10.1002/advs.202105451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li Y., et al. Targeted delivery of DNA topoisomerase inhibitor SN38 to intracranial tumors of glioblastoma using sub-5 ultrafine iron oxide nanoparticles. Adv Healthc Mater. 2022;11(14) doi: 10.1002/adhm.202102816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li X., et al. Cerium oxide nanoparticles with antioxidative neurorestoration for ischemic stroke. Biomaterials. 2022;291:121904. doi: 10.1016/j.biomaterials.2022.121904. [DOI] [PubMed] [Google Scholar]
- 223.Shirvalilou S., et al. Enhancement radiation-induced apoptosis in C6 glioma tumor-bearing rats via pH-responsive magnetic graphene oxide nanocarrier. J. Photochem. Photobiol., B. 2020;205:111827. doi: 10.1016/j.jphotobiol.2020.111827. [DOI] [PubMed] [Google Scholar]
- 224.Pan C.Y., et al. Zinc oxide nanoparticles modulate the gene expression of ZnT1 and ZIP8 to manipulate zinc homeostasis and stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0232729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Caban-Toktas S., et al. Combination of Paclitaxel and R-flurbiprofen loaded PLGA nanoparticles suppresses glioblastoma growth on systemic administration. Int J Pharm. 2020;578:119076. doi: 10.1016/j.ijpharm.2020.119076. [DOI] [PubMed] [Google Scholar]
- 226.Liu N., et al. Dual-targeted magnetic mesoporous silica nanoparticles reduce brain amyloid-β burden via depolymerization and intestinal metabolism. Theranostics. 2022;12(15):6646–6664. doi: 10.7150/thno.76574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jahansooz F., et al. Dopamine-loaded poly (butyl cyanoacrylate) nanoparticles reverse behavioral deficits in Parkinson's animal models. Ther. Deliv. 2020;11(6):387–399. doi: 10.4155/tde-2020-0026. [DOI] [PubMed] [Google Scholar]
- 228.Dana P., et al. Inhibiting metastasis and improving chemosensitivity via chitosan-coated selenium nanoparticles for brain cancer therapy. Nanomaterials. 2022;12(15) doi: 10.3390/nano12152606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Liyanage W., et al. Dendrimer-siRNA Conjugates for targeted intracellular Delivery in glioblastoma animal models. ACS Appl. Mater. Interfaces. 2022;14(41):46290–46303. doi: 10.1021/acsami.2c13129. [DOI] [PubMed] [Google Scholar]
- 230.Wang L., et al. Intranasal delivery of temozolomide-conjugated gold nanoparticles functionalized with anti-EphA3 for glioblastoma targeting. Mol. Pharm. 2021;18(3):915–927. doi: 10.1021/acs.molpharmaceut.0c00911. [DOI] [PubMed] [Google Scholar]
- 231.Song P., et al. Angiopep-2-Modified carboxymethyl chitosan-Based pH/reduction dual-stimuli-responsive Nanogels for enhanced targeting glioblastoma. Biomacromolecules. 2021;22(7):2921–2934. doi: 10.1021/acs.biomac.1c00314. [DOI] [PubMed] [Google Scholar]
- 232.Norouzi M., et al. Salinomycin-loaded iron oxide Nanoparticles for glioblastoma therapy. Nanomaterials. 2020;10(3) doi: 10.3390/nano10030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Costachi A., et al. The potential of helianthin loaded into magnetic nanoparticles to induce cytotoxicity in glioblastoma cells. Curr Health Sci J. 2021;47(3):412–419. doi: 10.12865/CHSJ.47.03.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Alghamri M.S., et al. Systemic delivery of an adjuvant CXCR4-CXCL12 signaling inhibitor encapsulated in synthetic protein nanoparticles for glioma immunotherapy. ACS Nano. 2022;16(6):8729–8750. doi: 10.1021/acsnano.1c07492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ung C., et al. Doxorubicin-loaded gold Nanoarchitectures as a therapeutic Strategy against diffuse intrinsic pontine glioma. Cancers. 2021;13(6) doi: 10.3390/cancers13061278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Israel L.L., et al. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J Control Release. 2020;320:45–62. doi: 10.1016/j.jconrel.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Huang Y., et al. Superparamagnetic iron oxide nanoparticles modified with tween 80 Pass through the intact blood-brain barrier in rats under magnetic field. ACS Appl. Mater. Interfaces. 2016;8(18):11336–11341. doi: 10.1021/acsami.6b02838. [DOI] [PubMed] [Google Scholar]
- 238.Chen J., et al. Ultrasmall iron oxide nanoparticles with MRgFUS for enhanced magnetic resonance imaging of orthotopic glioblastoma. J. Mater. Chem. B. 2024;12(20):4833–4842. doi: 10.1039/d3tb02966b. [DOI] [PubMed] [Google Scholar]
- 239.Norouzi M., et al. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: a combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020;10(1):11292. doi: 10.1038/s41598-020-68017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang J., et al. Applications of gold nanoparticles in brain diseases across the blood-brain barrier. Curr. Med. Chem. 2022;29(39):6063–6083. doi: 10.2174/0929867329666220527121943. [DOI] [PubMed] [Google Scholar]
- 241.Sela H., et al. Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB) J Nanobiotechnology. 2015;13:71. doi: 10.1186/s12951-015-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ribeiro T.C., et al. Exploiting mesoporous silica, silver and gold nanoparticles for neurodegenerative diseases treatment. Int. J. Pharm. 2022;624:121978. doi: 10.1016/j.ijpharm.2022.121978. [DOI] [PubMed] [Google Scholar]
- 243.Prades R., et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials. 2012;33(29):7194–7205. doi: 10.1016/j.biomaterials.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 244.Cheng Y., et al. Blood-brain barrier permeable gold nanoparticles: an efficient delivery platform for enhanced malignant glioma therapy and imaging. Small. 2014;10(24):5137–5150. doi: 10.1002/smll.201400654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nigro A., et al. Dealing with skin and blood-brain barriers: the unconventional challenges of mesoporous silica nanoparticles. Pharmaceutics. 2018;10(4) doi: 10.3390/pharmaceutics10040250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Janjua T.I., et al. Silica nanoparticles: a review of their safety and current strategies to overcome biological barriers. Adv. Drug Deliv. Rev. 2023;203:115115. doi: 10.1016/j.addr.2023.115115. [DOI] [PubMed] [Google Scholar]
- 247.Liu D., et al. In vitro and in vivo studies on the transport of PEGylated silica nanoparticles across the blood-brain barrier. ACS Appl. Mater. Interfaces. 2014;6(3):2131–2136. doi: 10.1021/am405219u. [DOI] [PubMed] [Google Scholar]
- 248.Song Y., et al. In vitro study of receptor-mediated silica nanoparticles delivery across blood-brain barrier. ACS Appl. Mater. Interfaces. 2017;9(24):20410–20416. doi: 10.1021/acsami.7b03504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kravanja G., et al. Chitosan-based (Nano)materials for novel biomedical applications. Molecules. 2019;24(10) doi: 10.3390/molecules24101960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hassan D.M., et al. Chitosan-coated nanostructured lipid carriers for effective brain delivery of Tanshinone IIA in Parkinson’s disease: interplay between nuclear factor-kappa β and cathepsin B. Drug Deliv. Transl. Res. 2023;14(2):400–417. doi: 10.1007/s13346-023-01407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sardoiwala M.N., Karmakar S., Choudhury S.R. Chitosan nanocarrier for FTY720 enhanced delivery retards Parkinson's disease via PP2A-EzH2 signaling in vitro and ex vivo. Carbohydr. Polym. 2021;254:117435. doi: 10.1016/j.carbpol.2020.117435. [DOI] [PubMed] [Google Scholar]
- 252.Danhier F., et al. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 253.Guo W., et al. Transferrin modified PEG-PLA-resveratrol conjugates: In vitro and in vivo studies for glioma. Eur. J. Pharmacol. 2013;718(1):41–47. doi: 10.1016/j.ejphar.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 254.Zhao H., et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. doi: 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bose A., et al. Nanomicelles: types, properties and applications in drug delivery. IET Nanobiotechnol. 2021;15(1):19–27. doi: 10.1049/nbt2.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li L., et al. Application of nanomicelles in enhancing bioavailability and biological efficacy of bioactive nutrients. Polymers. 2022;14(16) doi: 10.3390/polym14163278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhang Z.A., et al. Novel brain-targeted nanomicelles for anti-glioma therapy mediated by the ApoE-enriched protein corona in vivo. J Nanobiotechnology. 2021;19(1):453. doi: 10.1186/s12951-021-01097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Amano A., et al. Peripheral administration of nanomicelle-encapsulated anti-Aβ oligomer fragment antibody reduces various toxic Aβ species in the brain. J Nanobiotechnology. 2023;21(1):36. doi: 10.1186/s12951-023-01772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Tang L., et al. A simple self-assembly nanomicelle based on brain tumor-targeting peptide-mediated siRNA delivery for glioma immunotherapy via intranasal administration. Acta Biomater. 2023;155:521–537. doi: 10.1016/j.actbio.2022.11.013. [DOI] [PubMed] [Google Scholar]
- 260.Mogharbel B.F., et al. Biodegradable nanoparticles loaded with levodopa and curcumin for treatment of Parkinson's disease. Molecules. 2022;27(9) doi: 10.3390/molecules27092811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bagherian A., et al. Anti-glioblastoma effects of nanomicelle-curcumin plus erlotinib. Food Funct. 2021;12(21):10926–10937. doi: 10.1039/d1fo01611c. [DOI] [PubMed] [Google Scholar]
- 262.Ou Z., et al. Interpreting the therapeutic efficiency of multifunctional hybrid nanostructure against glioblastoma. ACS Omega. 2023;8(13):12259–12267. doi: 10.1021/acsomega.2c08265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Han M., et al. Efficient antiglioblastoma therapy in mice through doxorubicin-loaded nanomicelles modified using a novel brain-targeted RVG-15 peptide. J. Drug Target. 2021;29(9):1016–1028. doi: 10.1080/1061186X.2021.1912053. [DOI] [PubMed] [Google Scholar]
- 264.Liu L., et al. Phenylboronic ester-bridged chitosan/myricetin Nanomicelle for Penetrating the endothelial Barrier and regulating macrophage Polarization and Inflammation against ischemic diseases. ACS Biomater. Sci. Eng. 2023;9(7):4311–4327. doi: 10.1021/acsbiomaterials.3c00414. [DOI] [PubMed] [Google Scholar]
- 265.Xie J., et al. Dual-sensitive nanomicelles enhancing systemic Delivery of therapeutically active antibodies Specifically into the brain. ACS Nano. 2020;14(6):6729–6742. doi: 10.1021/acsnano.9b09991. [DOI] [PubMed] [Google Scholar]
- 266.Yang Y., et al. Enhanced nose-to-brain delivery of siRNA using hyaluronan-enveloped nanomicelles for glioma therapy. J Control Release. 2022;342:66–80. doi: 10.1016/j.jconrel.2021.12.034. [DOI] [PubMed] [Google Scholar]
- 267.Prakash R., et al. NLRP3 inflammasome-targeting Nanomicelles for preventing ischemia-reperfusion-induced inflammatory injury. ACS Nano. 2023;17(9):8680–8693. doi: 10.1021/acsnano.3c01760. [DOI] [PubMed] [Google Scholar]
- 268.Familtseva A., Jeremic N., Tyagi S.C. Exosomes: cell-created drug delivery systems. Mol. Cell. Biochem. 2019;459(1–2):1–6. doi: 10.1007/s11010-019-03545-4. [DOI] [PubMed] [Google Scholar]
- 269.Kimiz-Gebologlu I., Oncel S.S. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533–543. doi: 10.1016/j.jconrel.2022.05.027. [DOI] [PubMed] [Google Scholar]
- 270.Isaac R., et al. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33(9):1744–1762. doi: 10.1016/j.cmet.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ha D., Yang N., Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm. Sin. B. 2016;6(4):287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Xu M., et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11(18):8926–8944. doi: 10.7150/thno.62330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Rehman F.U., et al. Exosomes based strategies for brain drug delivery. Biomaterials. 2023;293:121949. doi: 10.1016/j.biomaterials.2022.121949. [DOI] [PubMed] [Google Scholar]
- 274.Chen H.X., et al. Exosomes derived from mesenchymal stem cells repair a Parkinson's disease model by inducing autophagy. Cell Death Dis. 2020;11(4):288. doi: 10.1038/s41419-020-2473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Morad G., et al. Tumor-Derived extracellular vesicles Breach the intact blood-brain Barrier via transcytosis. ACS Nano. 2019;13(12):13853–13865. doi: 10.1021/acsnano.9b04397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Dong X. Current strategies for brain drug delivery. Theranostics. 2018;8(6):1481–1493. doi: 10.7150/thno.21254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wu T., et al. Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Adv Mater. 2022;34(15) doi: 10.1002/adma.202110364. [DOI] [PubMed] [Google Scholar]
- 278.Zhang C., et al. Doxorubicin-loaded nanoparticle coated with endothelial cells-derived exosomes for immunogenic chemotherapy of glioblastoma. Bioeng Transl Med. 2021;6(3) doi: 10.1002/btm2.10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.He R., et al. Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ROS-mediated mitochondrial apoptosis. Mater Sci Eng C Mater Biol Appl. 2020;117:111314. doi: 10.1016/j.msec.2020.111314. [DOI] [PubMed] [Google Scholar]
- 280.Shan S., et al. Functionalized macrophage exosomes with panobinostat and ppm1d-siRNA for diffuse intrinsic pontine gliomas therapy. Adv. Sci. 2022;9(21) doi: 10.1002/advs.202200353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zhan Q., et al. Blood exosomes-based targeted delivery of cPLA2 siRNA and metformin to modulate glioblastoma energy metabolism for tailoring personalized therapy. Neuro Oncol. 2022;24(11):1871–1883. doi: 10.1093/neuonc/noac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wang J., et al. Inflammatory tumor microenvironment responsive neutrophil exosomes-based drug delivery system for targeted glioma therapy. Biomaterials. 2021;273:120784. doi: 10.1016/j.biomaterials.2021.120784. [DOI] [PubMed] [Google Scholar]
- 283.Liang S., Xu H., Ye B.C. Membrane-Decorated Exosomes for combination drug Delivery and improved glioma therapy. Langmuir. 2022;38(1):299–308. doi: 10.1021/acs.langmuir.1c02500. [DOI] [PubMed] [Google Scholar]
- 284.Wu J.Y., et al. Multifunctional exosome-mimetics for targeted anti-glioblastoma therapy by manipulating protein corona. J Nanobiotechnology. 2021;19(1):405. doi: 10.1186/s12951-021-01153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Rehman F.U., et al. Heme Oxygenase-1 targeting exosomes for temozolomide resistant glioblastoma synergistic therapy. J Control Release. 2022;345:696–708. doi: 10.1016/j.jconrel.2022.03.036. [DOI] [PubMed] [Google Scholar]
- 286.Wu X.Y., et al. Encapsulation of bryostatin-1 by targeted exosomes enhances remyelination and neuroprotection effects in the cuprizone-induced demyelinating animal model of multiple sclerosis. Biomater. Sci. 2022;10(3):714–727. doi: 10.1039/d1bm01142a. [DOI] [PubMed] [Google Scholar]
- 287.Pan J., et al. Brain microvascular endothelial cell derived exosomes potently ameliorate cognitive dysfunction by enhancing the clearance of Aβ through up-regulation of P-gp in mouse model of AD. Neurochem. Res. 2020;45(9):2161–2172. doi: 10.1007/s11064-020-03076-1. [DOI] [PubMed] [Google Scholar]
- 288.Thakur A., et al. Inhibition of glioma cells' proliferation by doxorubicin-loaded exosomes via microfluidics. Int J Nanomedicine. 2020;15:8331–8343. doi: 10.2147/IJN.S263956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wang R., et al. Tumor-derived exosomes reversing TMZ resistance by synergistic drug delivery for glioma-targeting treatment. Colloids Surf. B Biointerfaces. 2022;215:112505. doi: 10.1016/j.colsurfb.2022.112505. [DOI] [PubMed] [Google Scholar]
- 290.Hu Q., et al. Detection of urinary albumin using a "Turn-on" fluorescent probe with aggregation-induced emission characteristics. Chem. Asian J. 2021;16(10):1245–1252. doi: 10.1002/asia.202100180. [DOI] [PubMed] [Google Scholar]
- 291.Valipour E., et al. The anti-angiogenic effect of atorvastatin loaded exosomes on glioblastoma tumor cells: an in vitro 3D culture model. Microvasc. Res. 2022;143:104385. doi: 10.1016/j.mvr.2022.104385. [DOI] [PubMed] [Google Scholar]
- 292.Huo Q., et al. Biomimetic silibinin-loaded macrophage-derived exosomes induce dual inhibition of Aβ aggregation and astrocyte activation to alleviate cognitive impairment in a model of Alzheimer's disease. Mater Sci Eng C Mater Biol Appl. 2021;129:112365. doi: 10.1016/j.msec.2021.112365. [DOI] [PubMed] [Google Scholar]
- 293.Lee Y., et al. Brain-targeted exosome-mimetic cell membrane nanovesicles with therapeutic oligonucleotides elicit anti-tumor effects in glioblastoma animal models. Bioeng Transl Med. 2023;8(2) doi: 10.1002/btm2.10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Mobahat M., et al. Curcumin-loaded human endometrial stem cells derived exosomes as an effective carrier to suppress alpha-synuclein aggregates in 6OHDA-induced Parkinson's disease mouse model. Cell Tissue Bank. 2023;24(1):75–91. doi: 10.1007/s10561-022-10008-6. [DOI] [PubMed] [Google Scholar]
- 295.Qian C., et al. Neural stem cell-derived exosomes transfer miR-124-3p into cells to inhibit glioma growth by targeting FLOT2. Int. J. Oncol. 2022;61(4) doi: 10.3892/ijo.2022.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zhan Y., et al. Focused ultrasound combined with miR-1208-equipped exosomes inhibits malignant progression of glioma. Br. J. Cancer. 2023;129(7):1083–1094. doi: 10.1038/s41416-023-02393-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Liu X., et al. Kill two birds with one stone: engineered exosome-mediated delivery of cholesterol modified YY1-siRNA enhances chemoradiotherapy sensitivity of glioblastoma. Front. Pharmacol. 2022;13:975291. doi: 10.3389/fphar.2022.975291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Nooshabadi V.T., et al. Impact of atorvastatin loaded exosome as an anti-glioblastoma carrier to induce apoptosis of U87 cancer cells in 3D culture model. Biochem Biophys Rep. 2020;23:100792. doi: 10.1016/j.bbrep.2020.100792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Liao W., et al. Application and advances of biomimetic membrane materials in central nervous system disorders. J Nanobiotechnology. 2024;22(1):280. doi: 10.1186/s12951-024-02548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Cao Z., et al. Biomimetic macrophage membrane-camouflaged nanoparticles induce Ferroptosis by promoting mitochondrial Damage in glioblastoma. ACS Nano. 2023;17(23):23746–23760. doi: 10.1021/acsnano.3c07555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Luo D., et al. A dynamic DNA nanosponge for triggered amplification of gene-photodynamic modulation. Chem. Sci. 2022;13(18):5155–5163. doi: 10.1039/d2sc00459c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tang L., et al. Blood-brain barrier-Penetrating and lesion-targeting nanoplatforms Inspired by the pathophysiological Features for synergistic ischemic stroke therapy. Adv Mater. 2024;36(21) doi: 10.1002/adma.202312897. [DOI] [PubMed] [Google Scholar]
- 303.Song M., et al. Efficient delivery of lomitapide using hybrid membrane-coated tetrahedral DNA nanostructures for glioblastoma therapy. Adv Mater. 2024;36(24) doi: 10.1002/adma.202311760. [DOI] [PubMed] [Google Scholar]
- 304.Wang R., et al. Lipoprotein-biomimetic nanostructure enables tumor-targeted penetration delivery for enhanced photo-gene therapy towards glioma. Bioact. Mater. 2022;13:286–299. doi: 10.1016/j.bioactmat.2021.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Xu R., et al. All-in-One theranostic platforms: deep-red AIE Nanocrystals to target dual-Organelles for efficient photodynamic therapy. ACS Nano. 2022;16(12):20151–20162. doi: 10.1021/acsnano.2c04465. [DOI] [PubMed] [Google Scholar]
- 306.Gu J., et al. Erythrocyte membrane-coated nanocarriers modified by TGN for Alzheimer's disease. J Control Release. 2024;366:448–459. doi: 10.1016/j.jconrel.2023.12.030. [DOI] [PubMed] [Google Scholar]
- 307.Shan T., et al. Effective glioblastoma immune sonodynamic treatment mediated by macrophage cell membrane cloaked biomimetic nanomedicines. J Control Release. 2024;370:866–878. doi: 10.1016/j.jconrel.2024.04.043. [DOI] [PubMed] [Google Scholar]
- 308.Zhao P., et al. Biomimetic calcium carbonate nanoparticles delivered IL-12 mRNA for targeted glioblastoma sono-immunotherapy by ultrasound-induced necroptosis. J Nanobiotechnology. 2022;20(1):525. doi: 10.1186/s12951-022-01731-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhang L., et al. A trojan-horse-like biomimetic nano-NK to Elicit an immunostimulatory tumor Microenvironment for enhanced GBM chemo-immunotherapy. Small. 2023;19(44) doi: 10.1002/smll.202301439. [DOI] [PubMed] [Google Scholar]
- 310.Zou Y., et al. Cancer cell-mitochondria hybrid membrane coated Gboxin loaded nanomedicines for glioblastoma treatment. Nat. Commun. 2023;14(1):4557. doi: 10.1038/s41467-023-40280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sun Y., et al. An antisense oligonucleotide-loaded blood-brain barrier penetrable nanoparticle mediating Recruitment of endogenous neural stem Cells for the Treatment of Parkinson's disease. ACS Nano. 2023;17(5):4414–4432. doi: 10.1021/acsnano.2c09752. [DOI] [PubMed] [Google Scholar]
- 312.Han Y., et al. Macrophage membrane-coated nanocarriers Co-Modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer's disease mice. Bioact. Mater. 2021;6(2):529–542. doi: 10.1016/j.bioactmat.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zhu Y., et al. Multifunctional ginsenoside Rg3-based liposomes for glioma targeting therapy. J. Contr. Release. 2021;330:641–657. doi: 10.1016/j.jconrel.2020.12.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.