Abstract
Contrast-induced encephalopathy (CIE) is a rare complication associated with the use of iodine-based contrast agents and can be severe in some cases. In such cases, symptoms of encephalopathy, seizures, and neurological deficits appear shortly after contrast administration. This case report discusses a 90-year-old woman who developed severe CIE after iodine contrast agent administration. The patient underwent contrast enhanced computed tomography (CT) and left lower extremity angioplasty 2 days later. The patient's level of consciousness decreased the day after angioplasty; CT and magnetic resonance imaging (MRI) scans suggested CIE. Although the patient was treated with dialysis, but passed away 2 days after onset. Head CT at the time of onset showed extensive high-density area in the cerebral sulci. However, the distribution was different from typical subarachnoid hemorrhage due to ruptured aneurysm; subsequent MRI showed no evidence of subarachnoid hemorrhage. Therefore, CIE was suspected, rather than hemorrhage. A head dual-energy (DE)-CT, which can non-invasively assess the presence of intracranial iodine, was planned for diagnosing CIE. Although her poor condition made it difficult to performed prior to the death, so postmortem DE-CT was performed and confirmed the presence of iodine intracranially. This case suggests considering CIE in patients who develop impaired consciousness after contrast agents use, even when the contrast agents are not directly injected into cerebral blood vessels. In suspected CIE cases, DE-CT is useful for distinguishing iodine from hemorrhage.
Keywords: Case report, Dual-energy computed tomography, Contrast medium, Contrast-induced encephalopathy, Iodine
Introduction
Contrast-induced encephalopathy (CIE) is a rare complication of imaging using an iodine contrast agent [1]. Although the actual mechanism of CIE remains unknown, the most likely hypothesis is that repeated injections of hyperosmotic contrast agents into the same vessel disrupt the blood-brain barrier, allowing contrast agents to leak into the brain parenchyma, cortex, or subarachnoid space, causing the chemical toxicity of the contrast agents to directly affect the brain and produce symptoms [[2], [3], [4]]. The clinical presentation of CIE varies and includes encephalopathy, seizures, motor and sensory disturbances, visual disturbances, and focal neurological deficits [1,[5], [6], [7]]. Symptoms of neurological dysfunction present within minutes to hours after contrast agent administration, and most patients recover fully within 48-72 h [1,5,6,8].
Dual-energy computed tomography (DE-CT) is an imaging technique that acquires data from 2 different X-ray energies and uses the fact that the mass attenuation coefficient varies with the material and X-ray energy. Virtual non-contrast CT (VNC—CT) and iodine maps can be created from DE-CT data, and these are useful for differentiating hemorrhage from iodine [9]. VNC can remove elevated absorption values due to iodine contrast agent leakage, and iodine maps are useful for quantitative analysis of iodine distribution. DE-CT is useful for differentiating intracranial hemorrhage from iodine contrast leakage.
Herein, we report a case in which a patient developed CIE after contrast-enhanced CT and lower extremity angiography and iodine was detected in the subarachnoid space using postmortem DE-CT.
Case report
A 90-year-old woman was referred to our vascular surgery department for the treatment of critical left leg ischemia due to arteriosclerosis obliterans of the lower extremity. Her medical history included type 2 diabetes mellitus, diabetic nephropathy, hypertension, and hypercholesterolemia. The patient had been undergoing hemodialysis for end-stage renal failure and had no relevant family history. Day 1: contrast CT was performed using 100 mL of an iodine contrast agent (300 mg/mL; Omnipaque 300; GE Healthcare, Hino, Japan) for vascular evaluation. Day 2: the patient was admitted to our hospital after undergoing hemodialysis scheduled by the previous hospital. Day 3: left lower extremity angioplasty was performed using 150 mL of an iodine contrast agent (320 mg/mL; Visipaque 320; GE Healthcare, Hino, Japan), and amputation of the left first toe was performed for gangrene of the left first toe. All procedures were completed without apparent complications.
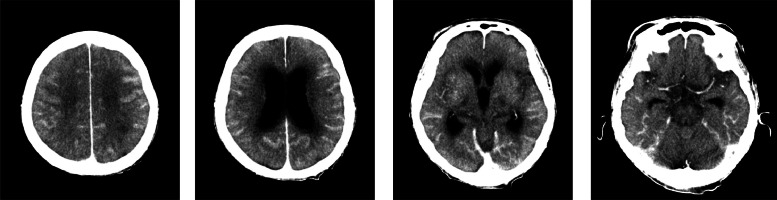
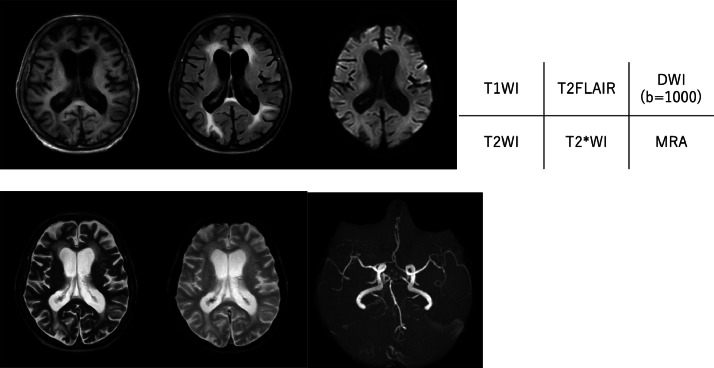
Day 4: the patient's consciousness level decreased (Glasgow Coma Scale 6 [E1V1M4]). CT of the head was performed to identify the cause (Fig. 1). There was extensive high-density area in the cerebral sulcus along the cerebral hemispheres. There were no high-density areas in the suprasellar cistern or sylvian fissure, which are often seen in subarachnoid hemorrhage due to aneurysmal rupture, and the high-density area was not suggestive of a typical subarachnoid hemorrhage. Intracerebral vessels also showed high density, suggesting residual contrast agents. Therefore, we suspected that the high-density area represented migrated contrast agents in the subarachnoid space rather than bleeding and performed magnetic resonance imaging (MRI) of the head (Fig. 2). The MRI did not reveal any abnormal signal in the subarachnoid space, and none of the sequences suggested the presence of hemorrhage. Magnetic resonance angiography revealed no aneurysms.
Fig. 1.
Computed tomography images of the head obtained on the day after angiography when the level of consciousness had decreased. There was extensive high-density area in the cerebral sulcus along the cerebral hemispheres. There were no high-density areas in the suprasellar cistern or sylvian fissure, which is often seen in subarachnoid hemorrhage due to aneurysmal rupture.
Fig. 2.
Magnetic resonance images of the head. No abnormal signal suggesting subarachnoid hemorrhage is seen, and no aneurysm is seen on the magnetic resonance angiogram.
CIE was diagnosed based on these imaging findings. The patient was treated conservatively with hemodialysis owing to the advanced age and poor general condition. DE-CT, which can non-invasively assess the presence of iodine, was scheduled as a follow-up CT examination to confirm CIE. However, the patient's health deteriorated during hemodialysis. Day 6: the patient died; therefore, follow-up DE-CT evaluation was not possible.
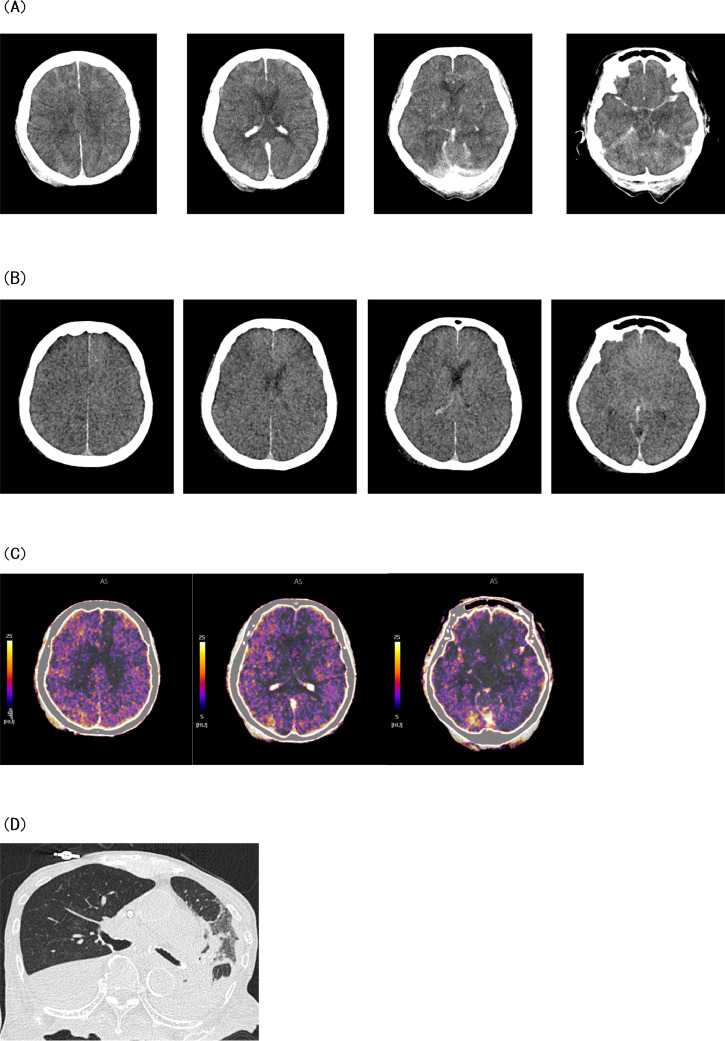
A postmortem DE-CT scan was planned to prove the presence of iodine in the brain as conclusive evidence of CIE. We explained to the family that the presence of iodine in the brain can be proven by DE-CT and suggested that a postmortem DE-CT scan be performed. With the family's consent, a DE-CT scan was performed. DE-CT data were acquired using a 320-detector row DE-CT scanner (Aquilion ONE; Canon, Nasu, Japan). VNC—CT images and iodine maps were generated using a dedicated workstation (Vitrea, Canon, Nasu, Japan). Postmortem CT of the head revealed persisting high sulcal density (Fig. 3A); however, the density was lower than that observed in the initial CT. The VNC—CT images revealed no high-density area in the sulcus (Fig. 3B), and the iodine map demonstrated iodine distribution in the same area (Fig. 3C). These postmortem DE-CT findings confirmed the presence of iodine in the brain. CT of the trunk performed at the same time revealed aspiration pneumonia (Fig. 3D).
Fig. 3.
Postmortem dual-energy computed tomography images. (A) The computed tomography image of the head reveals the persisting high-density area in the cerebral sulcus, although its density has decreased compared with the initial computed tomography image. (B) On virtual non-contrast computed tomography images generated using dual-energy computed tomography data, the high-density area in the cerebral sulcus seems faded. (C) Iodine distribution on the iodine map is consistent with the high-density zone on the plain computed tomography image. (D) The computed tomography image of the trunk shows aspiration pneumonia.
Discussion
In most cases, CIE occurs when contrast agents flow directly into cerebral blood vessels during cerebral or coronary angiography, and only few reports of CIE secondary to using intravenous contrast agents exist [8,10]. In the present case, contrast-enhanced CT was performed 2 days before angioplasty, and hemodialysis was performed the following day. CIE developed the day after the lower extremity angioplasty. Several papers suggest that chronic hypertension, diabetes mellitus, renal insufficiency, and previous reactions to contrast media may be associated with poor prognosis of CIE [1,5,11]. For example, renal impairment would be expected to limit the clearance of contrast agents and increase the risk of iodine accumulation. However, why these factors pose a risk is unclear: in part, because the pathogenesis of CIE is not clear. Our patient had renal failure that required hemodialysis, hypertension, and diabetes mellitus; therefore, the risk of CIE is expected to be high. However, despite the presence of these complications, confirming CIE was difficult at the onset.
The diagnosis of CIE requires the exclusion of cerebrovascular diseases such as subarachnoid hemorrhage. In the present case, the high-density area on the initial CT image was not suggestive of typical subarachnoid hemorrhage; the subsequent MRI revealed no abnormal signals in the subarachnoid space, thus excluding subarachnoid hemorrhage. Postmortem DE-CT revealed the presence of iodine in the subarachnoid space, confirming the diagnosis of CIE. DE-CT is useful for differentiating intracranial hemorrhage from iodine contrast leakage. It has many advantages over MRI, including shorter acquisition time, lower cost, and lack of contraindications in patients with metallic implants [9]. In our facility, we do not perform DE-CT during routine head CT examinations, because we have only a limited number of CT scanners capable of performing DE-CT. The decision to collect DE-CT data must be made before the examination. Technically, there is no drawback to performing a postmortem CT scan, as long as the family's consent can be obtained. Because the patient cannot lift his or her arms, there are more artifacts in a CT scan of the trunk, but this is not a problem with a head CT scan, and there are no artifacts due to body movement. There have been previous reports of the use of DE-CT to differentiate hemorrhage from iodine contrast agent [9,12]. Most of the reports are cases in which contrast agent was administered directly to the cerebral arteries. In a report by Phan et al., intracranial leakage of contrast agents was examined using DE-CT after intraarterial or intravenous administration, but no cases of intracranial leakage of contrast agent after intravenous administration were demonstrated [9]. To our knowledge, this is the first report of a patient who developed severe CIE without direct administration of contrast to the cerebral arteries and in whom the presence of iodine was confirmed by DE-CT, albeit postmortem.
In the present case, diagnosing CIE at the onset was difficult, and DE-CT could not be performed as an initial examination. If patients with multiple risk factors for CIE develop symptoms such as encephalopathy, convulsions, and neurological deficits immediately after iodine contrast agent administration, confirming the presence of the contrast agent using DE-CT may enable early diagnosis of CIE.
CIE is often mild and improves within a few days of follow-up [1,5,6,8]. In the present case, postmortem whole-body CT revealed aspiration pneumonia. Although the clinical course suggested that CIE might have contributed to the patient's death, the extent to which the contrast agent contributed to the deterioration of the patient's general condition was unclear. Hemodialysis can effectively eliminate contrast agents from the blood, with approximately 80 % of the contrast agent being removed within 4 hours [[13], [14], [15]]. Contrast agents have a negative effect on renal function; however, even in cases of renal failure, contrast CT is routinely performed, followed by hemodialysis. The present patient developed severe CIE despite undergoing hemodialysis after administering the contrast agent. Hemodialysis is effective in removing iodine but does not always prevent CIE as in this case. We believe that contrast agents should be used with caution in patients with risk factors for CIE. Prior to any examination using contrast agents, a risk assessment should be performed and risk reduction, such as minimizing the amount of contrast agents, should be considered, even if the contrast agents is not directly flowing into the cerebral arteries.
Conclusion
We encountered a patient with a severe CIE that developed after contrast CT and lower extremity arteriography, and the patient died shortly. The possibility of CIE after contrast-enhanced CT or peripheral vascular therapy should be considered, especially in patients with risk factors for CIE. In cases of suspected CIE, DE-CT is diagnostically useful for differentiating iodine from hemorrhage. Appropriate monitoring is necessary after administration of contrast agents, and in high-risk patients, conversion to other imaging modalities that do not use contrast agents or risk reduction should be considered in advance.
Patient consent
The written informed consent was obtained from the patient's family for being included in the case and it is available upon request.
Footnotes
Acknowledgments: We would like to thank Editage (www.editage.jp) for English language editing.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhang Y., Zhang J., Yuan S., Shu H. Contrast-induced encephalopathy and permanent neurological deficit following cerebral angiography: a case report and review of the literature. Front Cell Neurosci. 2022;16 doi: 10.3389/fncel.2022.1070357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawasaki T., Hayase M., Miyakoshi A., Taki J., Nakamura T., Hatano T. Two cases of symptomatic contrast-induced encephalopathy after coil embolization of unruptured cerebral aneurysm. JNET. 2015;9:96–102. doi: 10.5797/JNET.CR.2015-0002. [DOI] [Google Scholar]
- 3.Renú A., Amaro S., Laredo C., Román L.S., Llull L., Lopez A., et al. Relevance of blood-brain barrier disruption after endovascular treatment of ischemic stroke: ddual-energy computed tomographic study. Stroke. 2015;46:673–679. doi: 10.1161/STROKEAHA.114.008147. [DOI] [PubMed] [Google Scholar]
- 4.Iwata T., Mori T., Tajiri H., Miyazaki Y., Nakazaki M. Repeated injection of contrast medium inducing dysfunction of the blood-brain barrier: ccase report. Neurol Med Chir (Tokyo) 2013;53:34–36. doi: 10.2176/nmc.53.34. [DOI] [PubMed] [Google Scholar]
- 5.Liu M.R., Jiang H., Li X.L., Yang P. Case report and literature review on low-osmolar, non-ionic iodine-based contrast-induced encephalopathy. Clin Interv Aging. 2020;15:2277–2289. doi: 10.2147/CIA.S280931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meijer F.J.A., Steens S.C.A., Tuladhar A.M., van Dijk E.D., Boogaarts H.D. Contrast-induced encephalopathy-neuroimaging findings and clinical relevance. Neuroradiology. 2022;64:1265–1268. doi: 10.1007/s00234-022-02930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawtharani S., Horanieh E., Ali B., Housheimy M., Darwish H. Contrast induced encephalopathy: case report and review of the literature. J Neurol Neuromedicine. 2024;8:1–4. doi: 10.29245/2572.942X/2024/1.1294. [DOI] [Google Scholar]
- 8.Capasso R., Caranci F., Conforti R., Rinaldi F.O., Pinto A. Contrast-induced encephalopathy after abdominal CT examination. Acta Neurol Belg. 2021;121:1325–1326. doi: 10.1007/s13760-021-01690-6. [DOI] [PubMed] [Google Scholar]
- 9.Phan C.M., Yoo A.J., Hirsch J.A., Nogueira R.G., Gupta R. Differentiation of hemorrhage from iodinated contrast in different intracranial compartments using dual-energy head CT. AJNR Am J Neuroradiol. 2012;33:1088–1094. doi: 10.3174/ajnr.A2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao L.D., Zhu X.L., Yang R.L., Zhang M.M. Cardiorespiratory arrest after iso-osmolar iodinated contrast injection: a case report of contrast-induced encephalopathy following contrast-enhanced computed-tomography. Medicine. 2021;100:e24035. doi: 10.1097/MD.0000000000024035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gollol Raju N.S.G., Joshi D., Daggubati R., Movahed A. Contrast induced neurotoxicity following coronary angiogram with iohexol in an end stage renal disease patient. World J Clin Cases. 2015;3:942–945. doi: 10.12998/wjcc.v3.i11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K., Jiang L., Ruan J., Xia W., Huang H., Niu G., et al. The role of dual energy CT in evaluating hemorrhagic complications at different stages after thrombectomy. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.583411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis P.W., Krisanapan P., Tangpanithandee S., Thongprayoon C., Miao J., Hassanein M., et al. Contrast-induced encephalopathy in patients with chronic kidney disease and end-stage kidney disease: a systematic review and meta-analysis. Medicines (Basel) 2023;10:46. doi: 10.3390/medicines10080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsubara N., Izumi T., Miyachi S., Ota K., Wakabayashi T. Contrast-induced encephalopathy following embolization of intracranial aneurysms in hemodialysis patients. Neurol Med Chir (Tokyo) 2017;57:641–648. doi: 10.2176/nmc.oa.2017-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorusso V., Taroni P., Alvino S., Spinazzi A. Pharmacokinetics and safety of iomeprol in healthy volunteers and in patients with renal impairment or end-stage renal disease requiring hemodialysis. Invest Radiol. 2001;36:309–316. doi: 10.1097/00004424-200106000-00002. [DOI] [PubMed] [Google Scholar]