Abstract
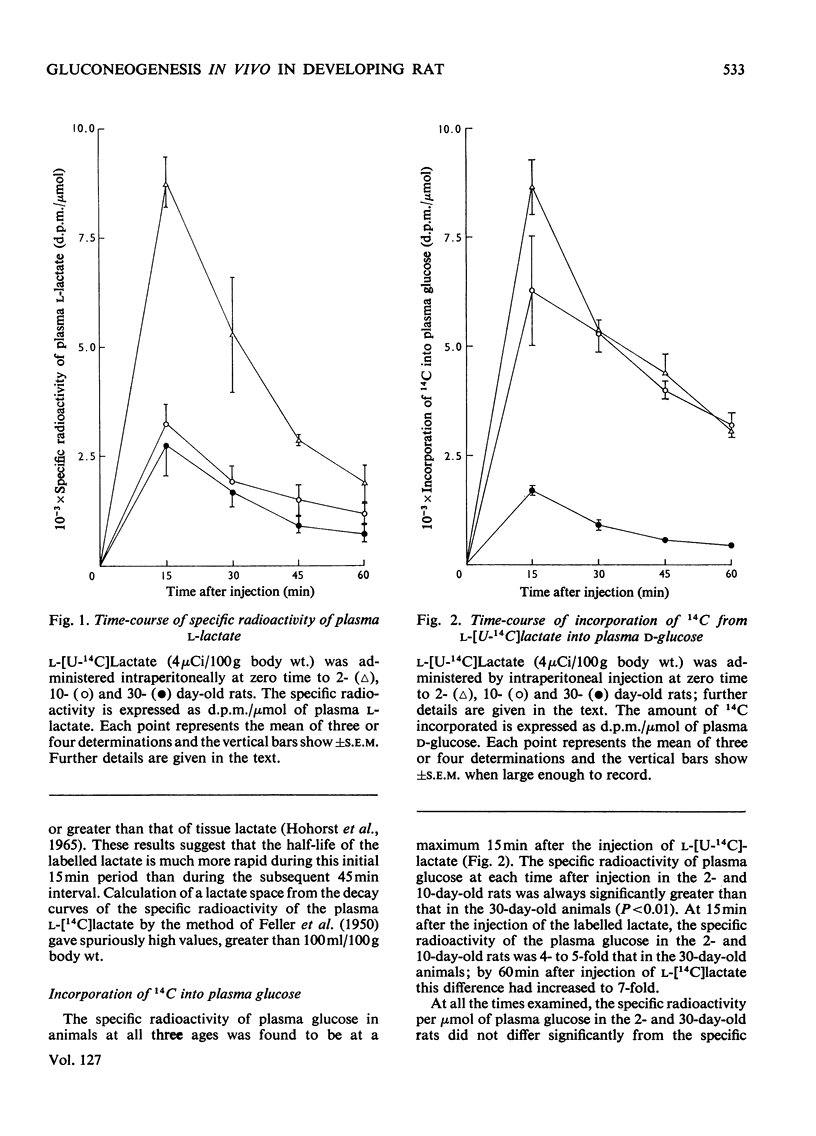
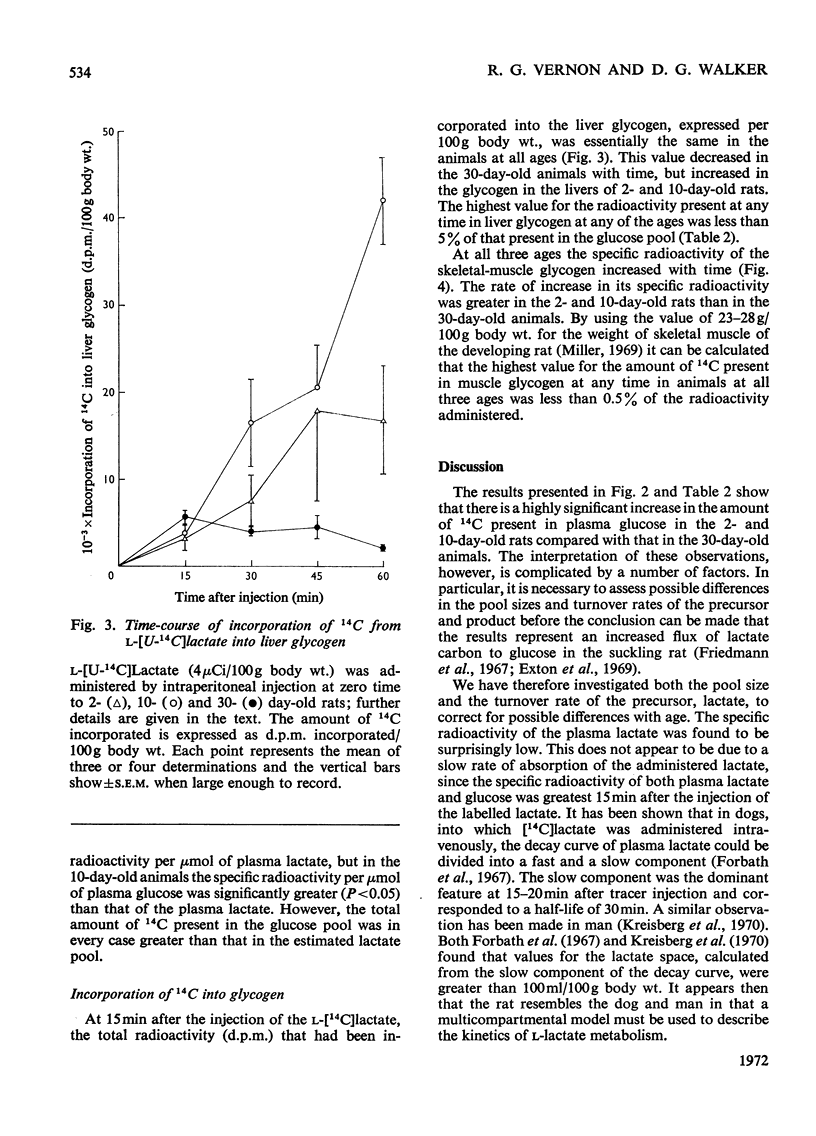
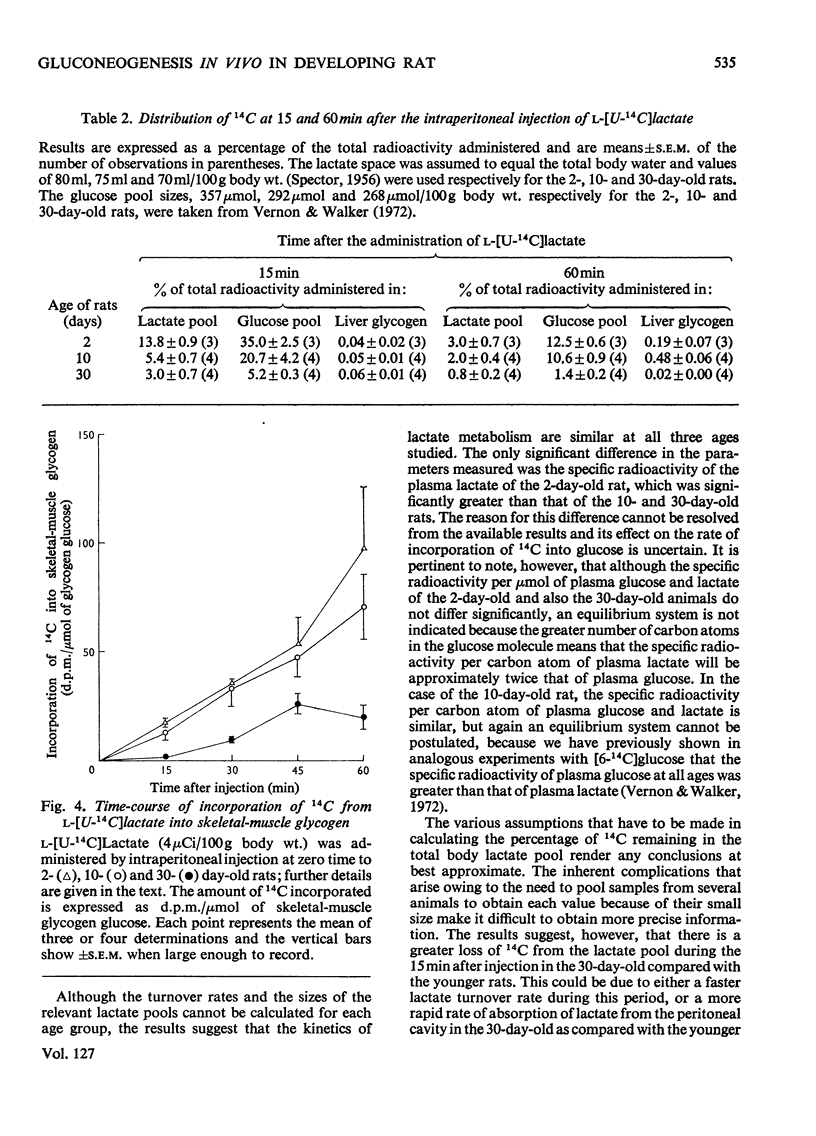
1. The specific radioactivity of plasma l-lactate and the incorporation of 14C into plasma d-glucose, liver glycogen and skeletal-muscle glycogen were measured as a function of time after the intraperitoneal injection of l-[U-14C]lactate into 2-, 10- and 30-day-old rats. 2. Between 15 and 60min after the injection of the l-[U-14C]lactate, the specific radioactivity of plasma lactate decreased with a half-life of 20–33min in animals at all three ages. 3. At all times after injection examined, the specific radioactivity of plasma glucose of the 2- and 10-day-old rats was at least fourfold greater than that of the 30-day-old rats. 4. Although 14C was incorporated into liver glycogen the amount incorporated was always less than 5% of that present in plasma glucose. 5. The results are discussed with reference to the factors that may influence the rate of incorporation of 14C into plasma glucose, and it is concluded that the rate of gluconeogenesis in the 2- and 10-day-old suckling rat is at least twice that of the weaned 30-day-old animal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLARD F. J., OLIVER I. T. Glycogen metabolism in embryonic chick and neonatal rat liver. Biochim Biophys Acta. 1963 Jun 4;71:578–588. doi: 10.1016/0006-3002(63)91130-2. [DOI] [PubMed] [Google Scholar]
- Drahota Z., Hahn P., Honová E. Acetoacetate formation by livers of young and adult rats. Biol Neonat. 1965;9(1):124–131. doi: 10.1159/000239984. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Corbin J. G., Park C. R. Control of gluconeogenesis in liver. IV. Differential effects of fatty acids and glucagon on ketogenesis and gluconeogenesis in the perfused rat liver. J Biol Chem. 1969 Aug 10;244(15):4095–4102. [PubMed] [Google Scholar]
- FELLER D. D., STRISOWER E. H., CHAIKOFF I. L. Turnover and oxidation of body glucose in normal and alloxan-diabetic rats. J Biol Chem. 1950 Dec;187(2):571–588. [PubMed] [Google Scholar]
- Forbath N., Kenshole A. B., Hetenyi G., Jr Turnover of lactic acid in normal and diabetic dogs calculated by two tracer methods. Am J Physiol. 1967 May;212(5):1179–1184. doi: 10.1152/ajplegacy.1967.212.5.1179. [DOI] [PubMed] [Google Scholar]
- Friedmann B., Goodman E. H., Jr, Weinhouse S. Dietary and hormonal effects on gluconeogenesis in the rat. J Biol Chem. 1965 Oct;240(10):3729–3735. [PubMed] [Google Scholar]
- Friedmann B., Goodman E. H., Jr, Weinhouse S. Effects of insulin and fatty acids on gluconeogenesis in the rat. J Biol Chem. 1967 Aug 25;242(16):3620–3627. [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H., Krebs H. A. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971 Mar;122(1):13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohorst H. J., Arese P., Bartels H., Stratmann D., Talke H. L(+) lactic acid and the steady state of cellular red/ox-systems. Ann N Y Acad Sci. 1965 Jul 31;119(3):974–994. doi: 10.1111/j.1749-6632.1965.tb47456.x. [DOI] [PubMed] [Google Scholar]
- KREBS H. THE CROONIAN LECTURE, 1963. GLUCONEOGENESIS. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:545–564. doi: 10.1098/rspb.1964.0019. [DOI] [PubMed] [Google Scholar]
- Kreisberg R. A., Pennington L. F., Boshell B. R. Lactate turnover and gluconeogenesis in normal and obese humans. Effect of starvation. Diabetes. 1970 Jan;19(1):53–63. doi: 10.2337/diab.19.1.53. [DOI] [PubMed] [Google Scholar]
- Miller S. A. Nutrition in the neonatal development of protein metabolism. Fed Proc. 1970 Jul-Aug;29(4):1497–1502. [PubMed] [Google Scholar]
- Page M. A., Krebs H. A., Williamson D. H. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem J. 1971 Jan;121(1):49–53. doi: 10.1042/bj1210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. G., Eaton S. W., Walker D. G. Carbohydrate formation from various precursors in noenatal rat liver. Biochem J. 1968 Dec;110(4):725–731. doi: 10.1042/bj1100725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. G., Walker D. G. Changes in activity of some enzymes involved in glucose utilization and formation in developing rat liver. Biochem J. 1968 Jan;106(2):321–329. doi: 10.1042/bj1060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. G., Walker D. G. Glucose metabolism in the developing rat. Studies in vivo. Biochem J. 1972 Apr;127(3):521–529. doi: 10.1042/bj1270521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. G., Walker D. G. Glycerol metabolism in the neonatal rat. Biochem J. 1970 Jul;118(3):531–536. doi: 10.1042/bj1180531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. G., Holland G. The development of hepatic glucokinase in the neonatal rat. Biochem J. 1965 Dec;97(3):845–854. doi: 10.1042/bj0970845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung D., Oliver I. T. Gluconeogenesis from amino acids in neonatal rat liver. Biochem J. 1967 Jun;103(3):744–748. doi: 10.1042/bj1030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzoli A., Turkenkopf I. J., Mueller V. L. Gluconeogenesis in developing rat kidney cortex. Biochem J. 1969 Jan;111(2):181–185. doi: 10.1042/bj1110181. [DOI] [PMC free article] [PubMed] [Google Scholar]


