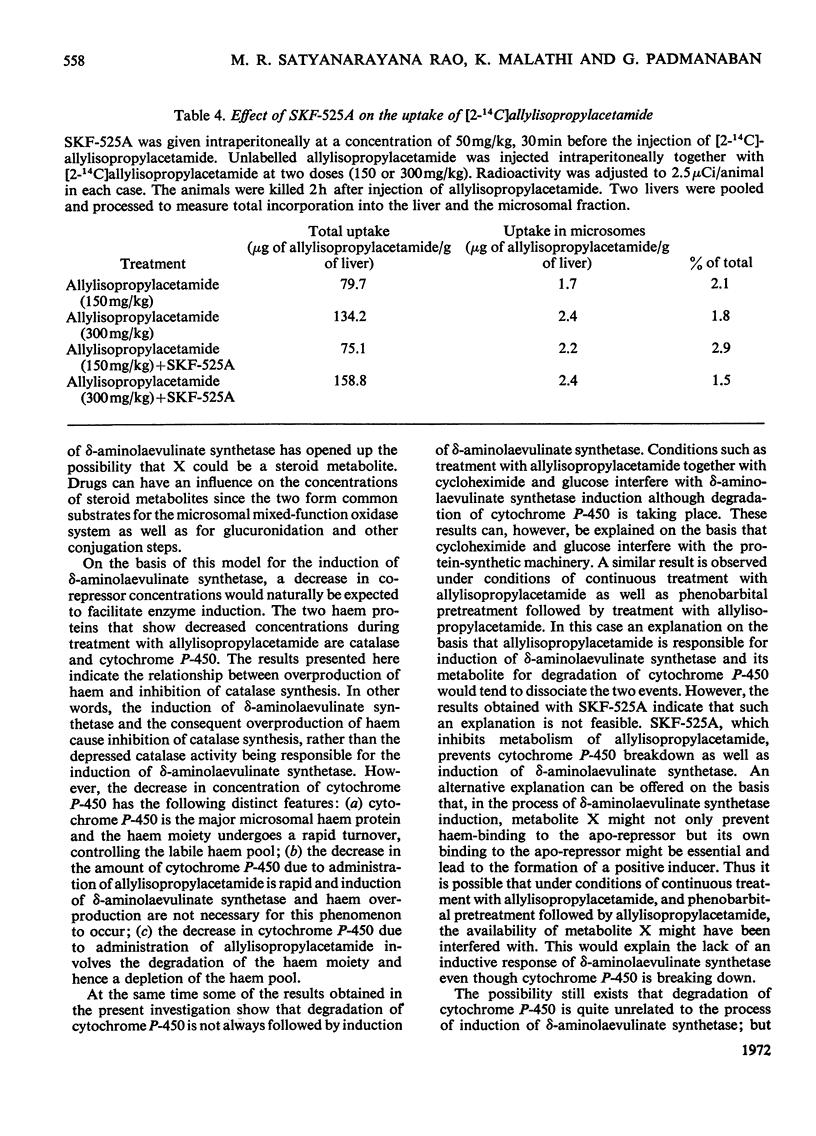
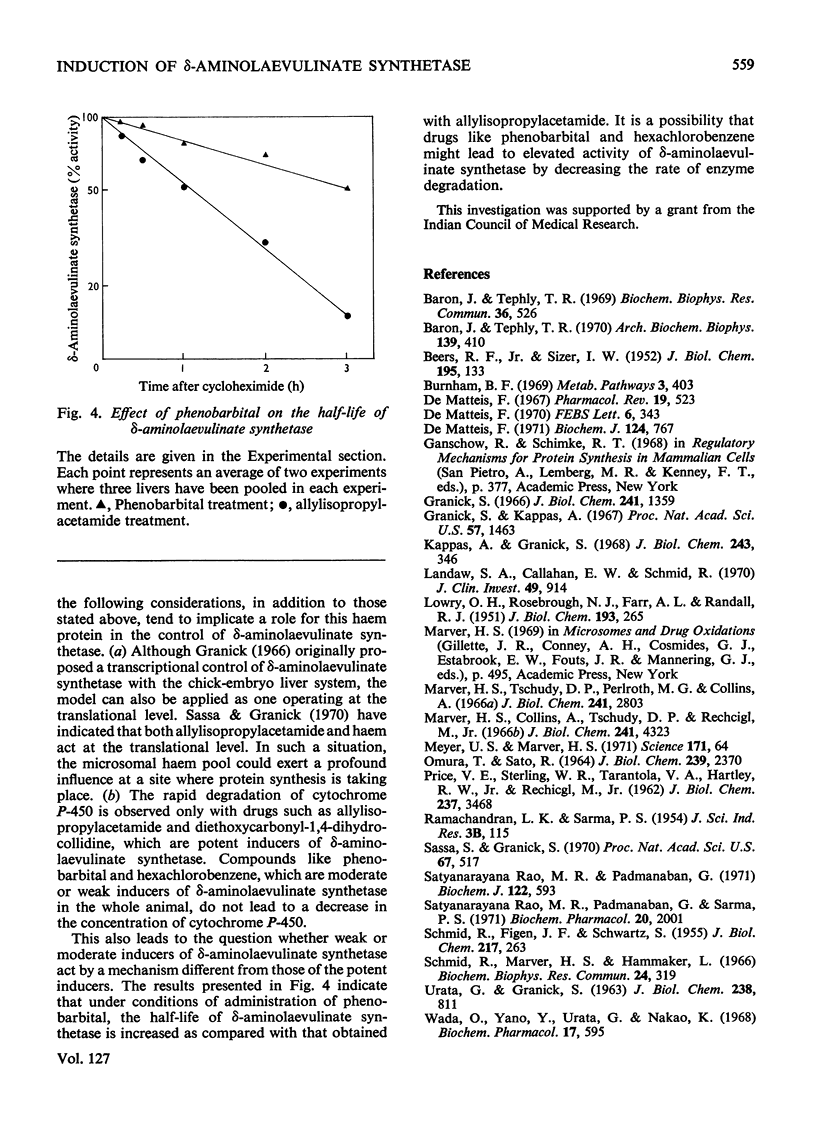
Abstract
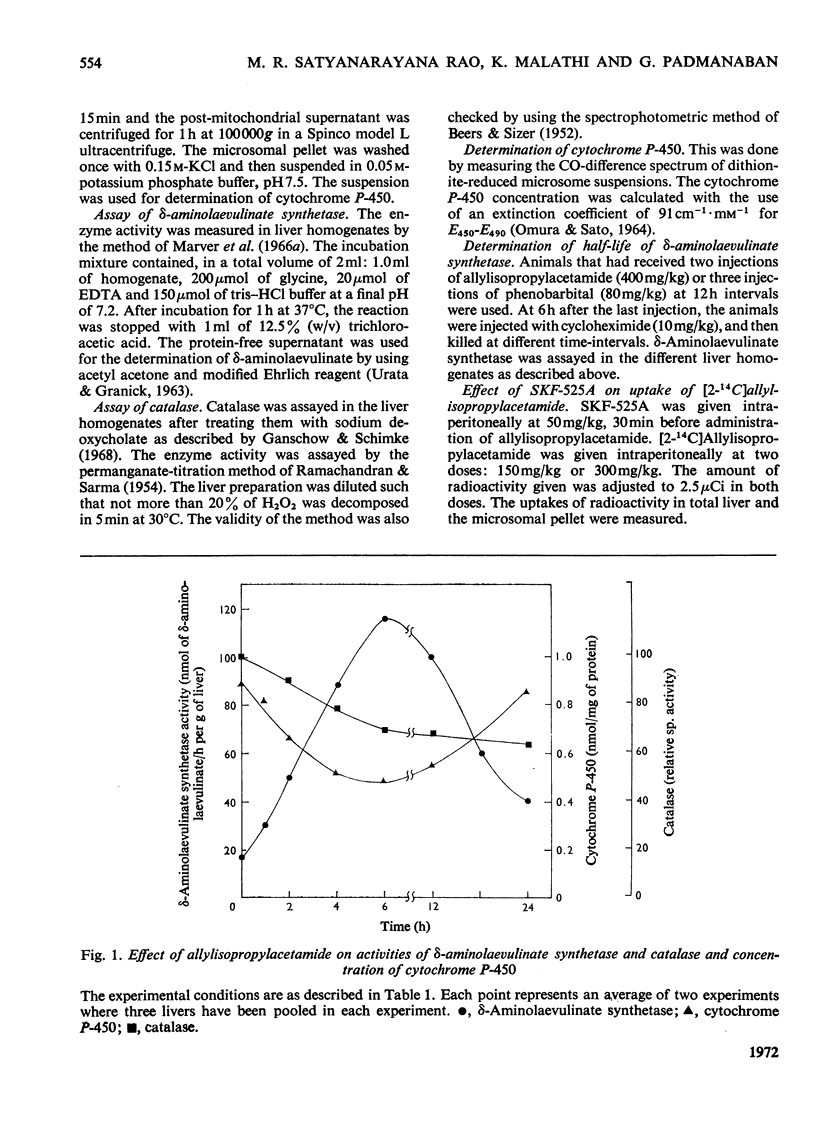
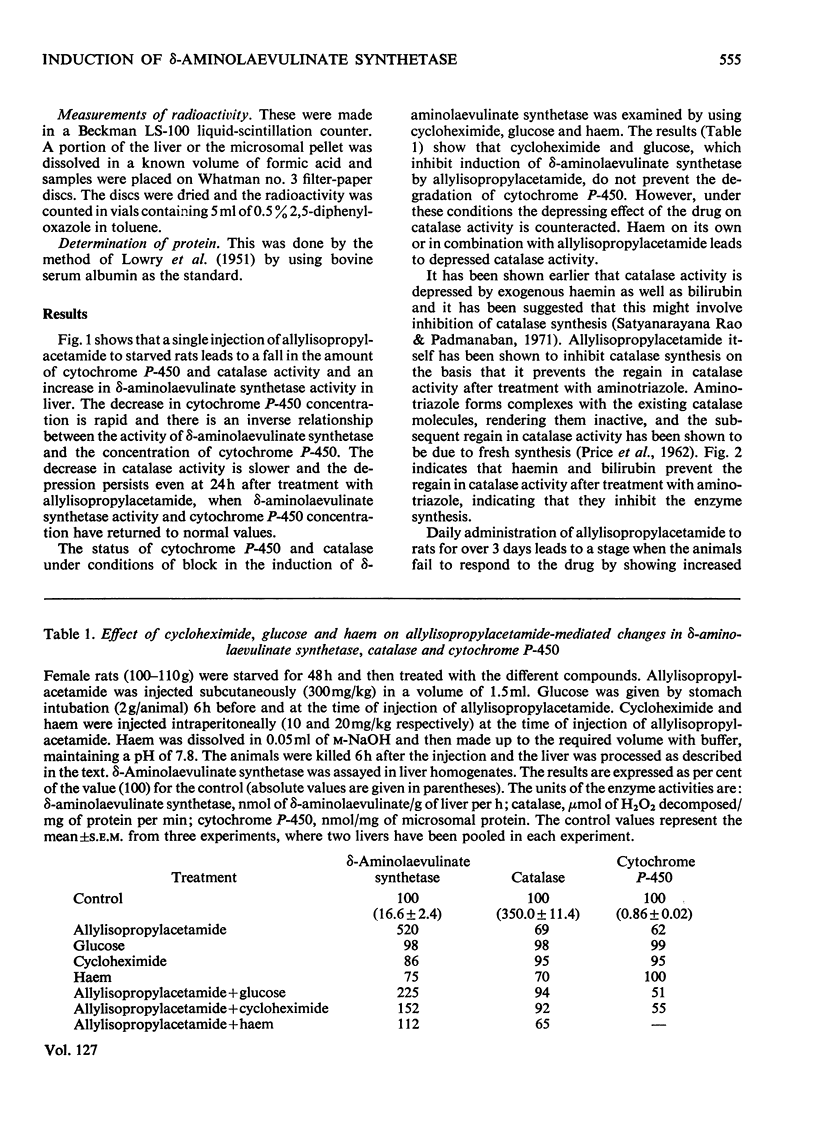
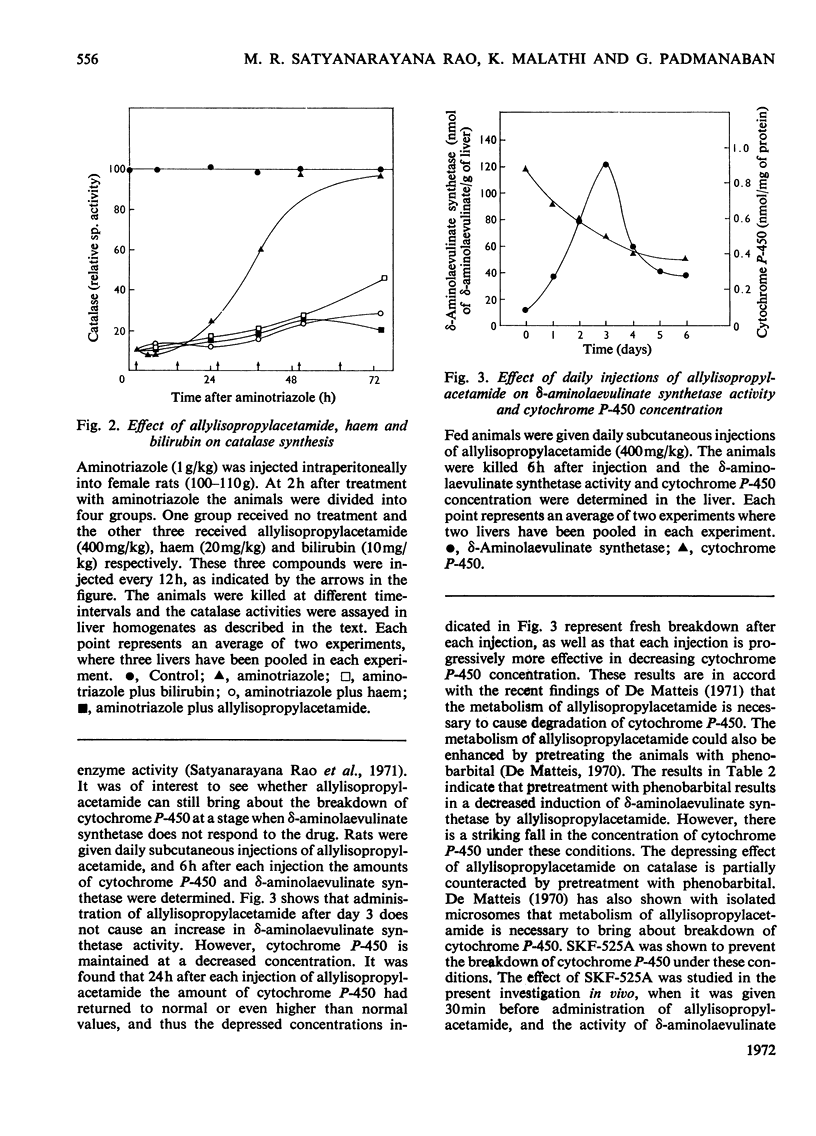
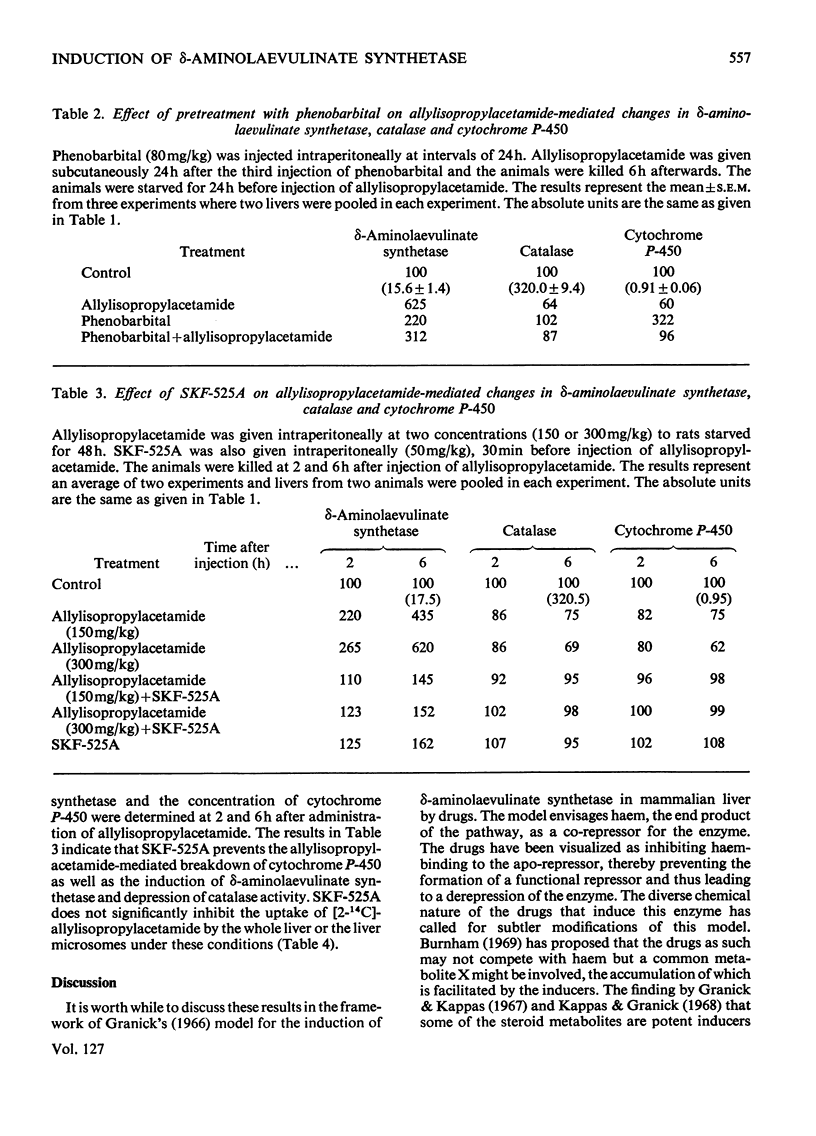
The porphyrinogenic drug 2-allyl-2-isopropylacetamide causes the degradation of microsomal cytochrome P-450 and inhibits the synthesis of catalase in rat liver. The inhibition of catalase synthesis follows the induction of δ-aminolaevulinate synthetase and the consequent overproduction of haem. The allylisopropylacetamide-mediated breakdown of cytochrome P-450 is a rapid event and has a reciprocal relationship to the pattern of δ-aminolaevulinate synthetase induction. Breakdown of cytochrome P-450 appears to be one of the conditions leading to the `derepression' of δ-aminolaevulinate synthetase.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Baron J., Tephly T. R. Further studies on the relationship of the stimulatory effects of phenobarbital and 3,4-benzpyrene on hepatic heme synthesis to their effects on hepatic microsomal drug oxidations. Arch Biochem Biophys. 1970 Aug;139(2):410–420. doi: 10.1016/0003-9861(70)90494-7. [DOI] [PubMed] [Google Scholar]
- Baron J., Tephly T. R. The role of heme synthesis during the induction of hepatic microsomal cytochrome P-450 and drug metabolism produced by benzpyrene. Biochem Biophys Res Commun. 1969 Aug 15;36(4):526–532. doi: 10.1016/0006-291x(69)90336-2. [DOI] [PubMed] [Google Scholar]
- De Matteis F. Loss of haem in rat liver caused by the porphyrogenic agent 2-allyl-2-isopropylacetamide. Biochem J. 1971 Oct;124(4):767–777. doi: 10.1042/bj1240767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. Rapid loss of cytochrome P-450 and haem caused in the liver microsomes by the porphyrogenic agent 2-allyl-2-isopropylacetamide. FEBS Lett. 1970 Feb 25;6(4):343–345. doi: 10.1016/0014-5793(70)80094-1. [DOI] [PubMed] [Google Scholar]
- Granick S., Kappas A. Steroid control of porphyrin and heme biosynthesis: a new biological function of steroid hormone metabolites. Proc Natl Acad Sci U S A. 1967 May;57(5):1463–1467. doi: 10.1073/pnas.57.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Kappas A., Granick S. Steroid induction of porphyrin synthesis in liver cell culture. II. The effects of heme, uridine diphosphate glucuronic acid, and inhibitors of nucleic acid and protein synthesis on the induction process. J Biol Chem. 1968 Jan 25;243(2):346–351. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landaw S. A., Callahan E. W., Jr, Schmid R. Catabolism of heme in vivo: comparison of the simultaneous production of bilirubin and carbon monoxide. J Clin Invest. 1970 May;49(5):914–925. doi: 10.1172/JCI106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marver H. S., Collins A., Tschudy D. P., Rechcigl M., Jr Delta-aminolevulinic acid synthetase. II. Induction in rat liver. J Biol Chem. 1966 Oct 10;241(19):4323–4329. [PubMed] [Google Scholar]
- Marver H. S., Tschudy D. P., Perlroth M. G., Collins A. Delta-aminolevulinic acid synthetase. I. Studies in liver homogenates. J Biol Chem. 1966 Jun 25;241(12):2803–2809. [PubMed] [Google Scholar]
- Meyer U. A., Marver H. S. Chemically induced porphyria: increased microsomal heme turnover after treatment with allylisopropylacetamide. Science. 1971 Jan 8;171(3966):64–66. doi: 10.1126/science.171.3966.64. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- PRICE V. E., STERLING W. R., TARANTOLA V. A., HARTLEY R. W., Jr, RECHCIGL M., Jr The kinetics of catalase synthesis and destruction in vivo. J Biol Chem. 1962 Nov;237:3468–3475. [PubMed] [Google Scholar]
- Rao M. R., Padmanaban G., Sarma P. S. Porphyrin synthesis in drug induced hepatic porphyria. Biochem Pharmacol. 1971 Aug;20(8):2001–2007. doi: 10.1016/0006-2952(71)90399-6. [DOI] [PubMed] [Google Scholar]
- SCHMID R., FIGEN J. F., SCHWARTZ S. Experimental porphyria. IV. Studies of liver catalase and other heme enzymes in sedormid porphyria. J Biol Chem. 1955 Nov;217(1):263–274. [PubMed] [Google Scholar]
- Sassa S., Granick S. Induction of -aminolevulinic acid synthetase in chick embryo liver cells in cluture. Proc Natl Acad Sci U S A. 1970 Oct;67(2):517–522. doi: 10.1073/pnas.67.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana Rao M. R., Padmanaban G. delta-aminolaevulate synthetase induction and catalase repression by porphyrinogenic drugs. Biochem J. 1971 May;122(4):593–595. doi: 10.1042/bj1220593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R., Marver H. S., Hammaker L. Enhanced formation of rapidly labelled bilirubin by phenobarbital: hepatic microsomal cytochromes as a possible source. Biochem Biophys Res Commun. 1966 Aug 12;24(3):319–328. doi: 10.1016/0006-291x(66)90158-6. [DOI] [PubMed] [Google Scholar]
- URATA G., GRANICK S. Biosynthesis of alpha-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963 Feb;238:811–820. [PubMed] [Google Scholar]
- Wada O., Yano Y., Urata G., Nakao K. Behavior of hepatic microsomal cytochromes after treatment of mice with drugs known to disturb porphyrin metabolism in liver. Biochem Pharmacol. 1968 Apr;17(4):595–603. doi: 10.1016/0006-2952(68)90275-x. [DOI] [PubMed] [Google Scholar]
- de Matteis F. Disturbances of liver porphyrin metabolism caused by drugs. Pharmacol Rev. 1967 Dec;19(4):523–557. [PubMed] [Google Scholar]


