Abstract
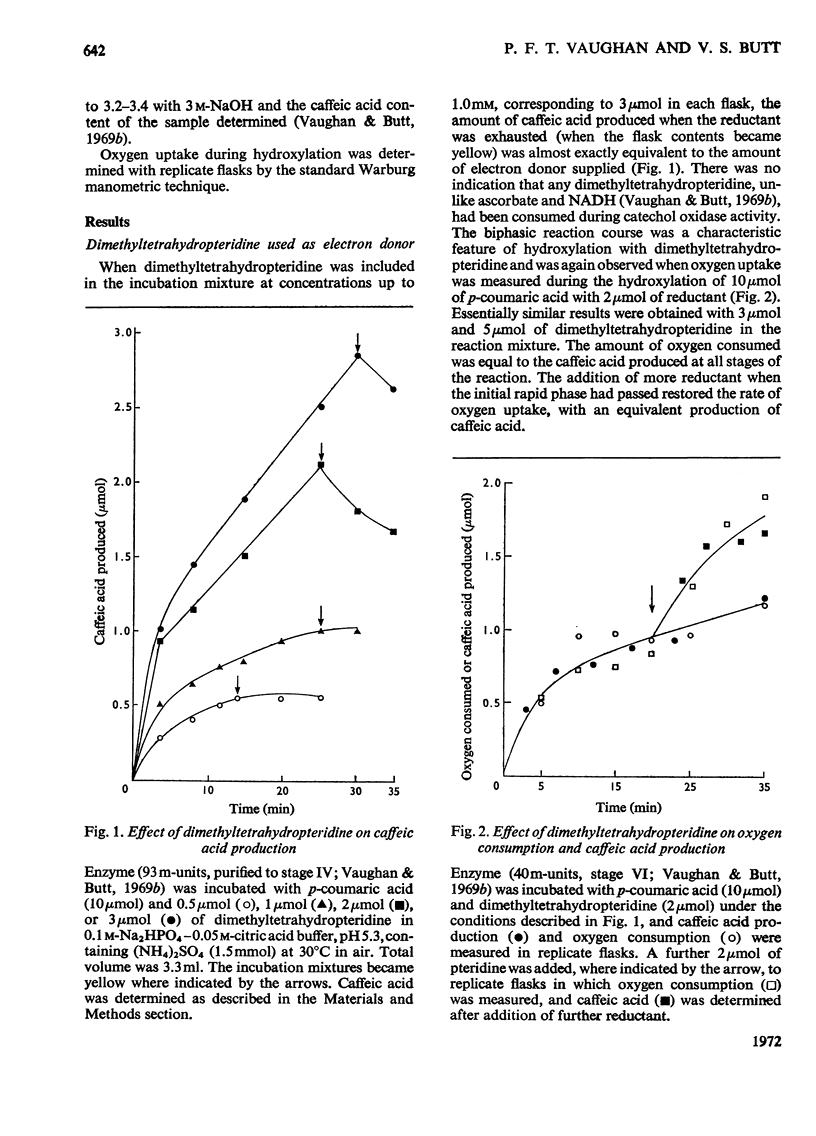
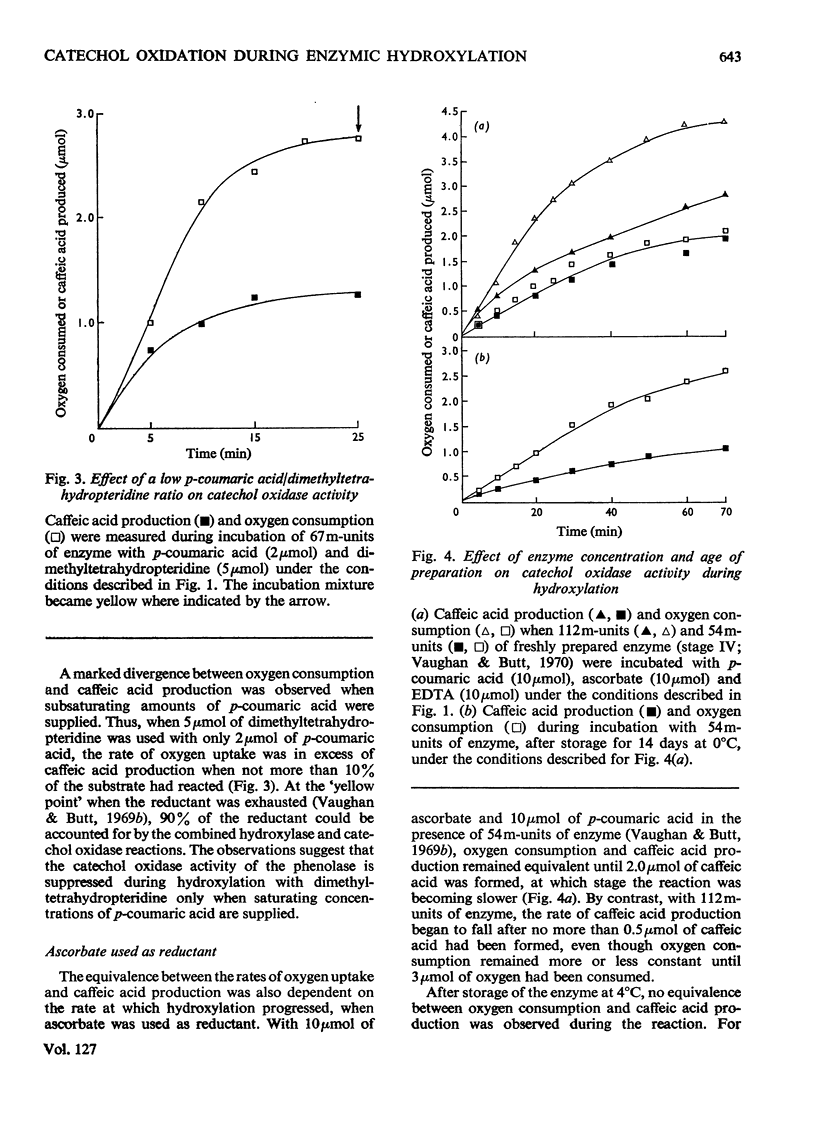
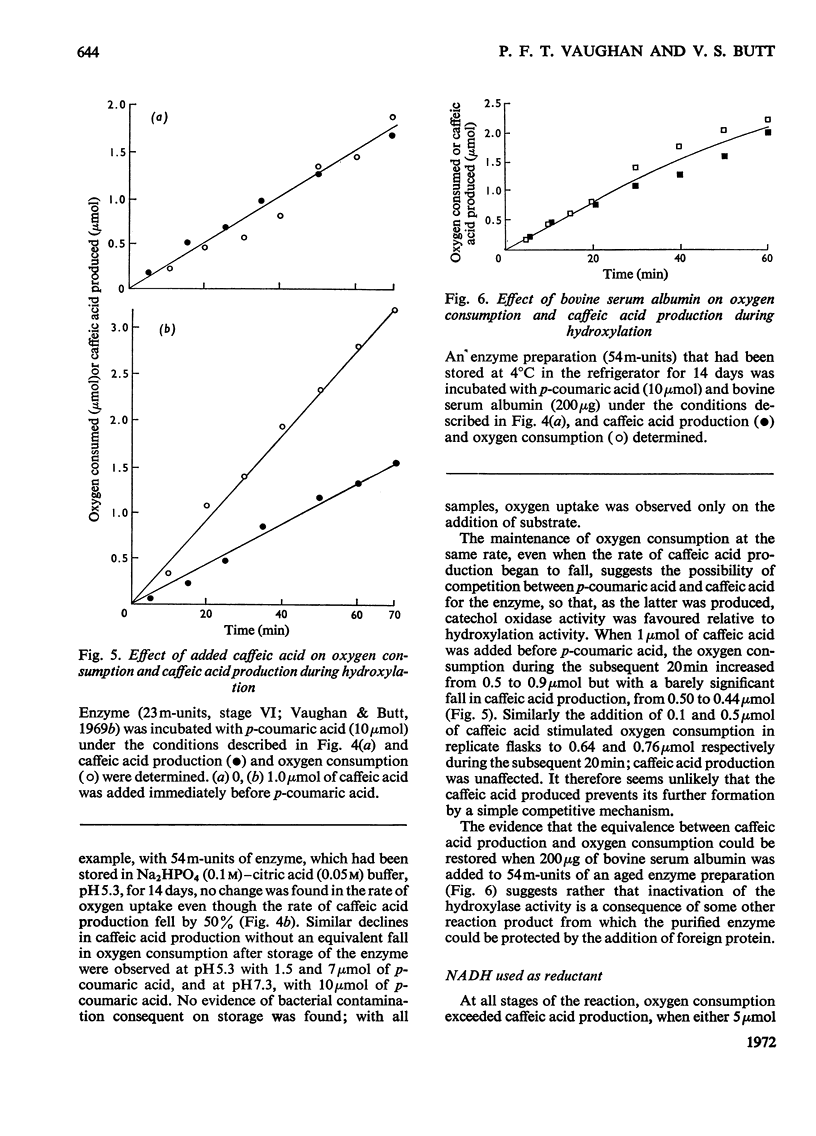
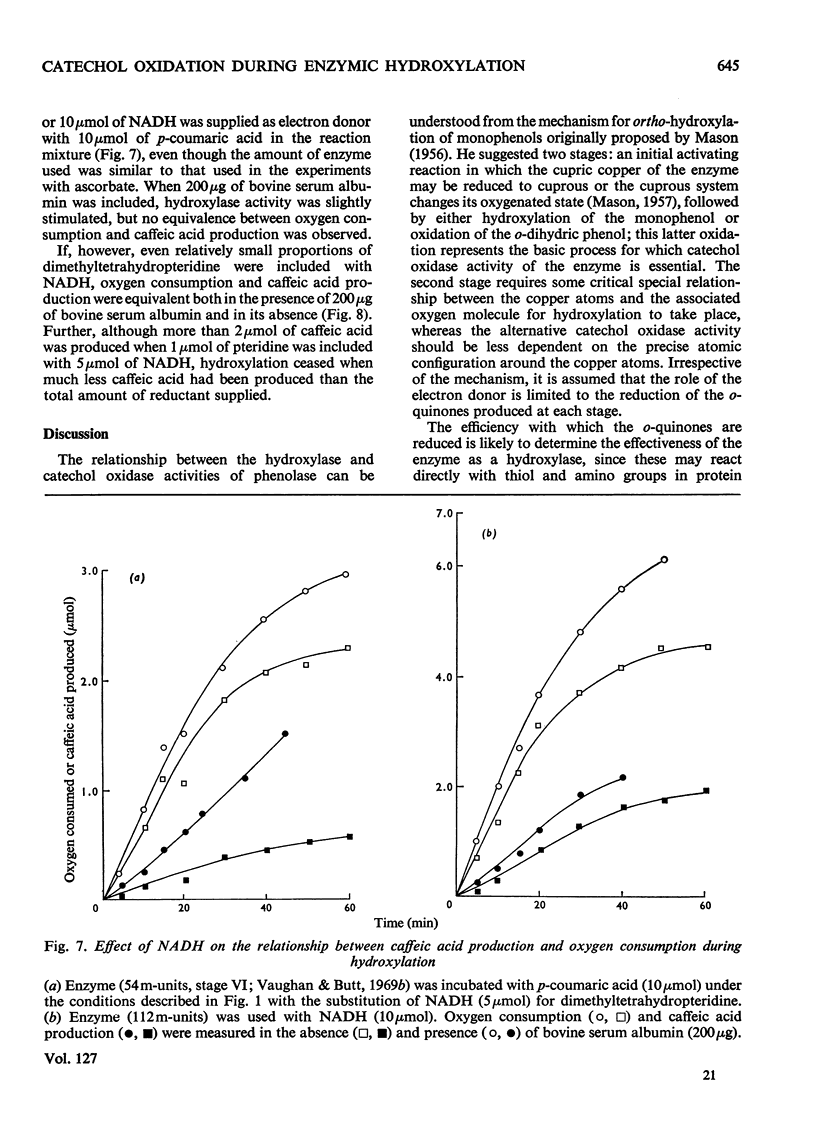
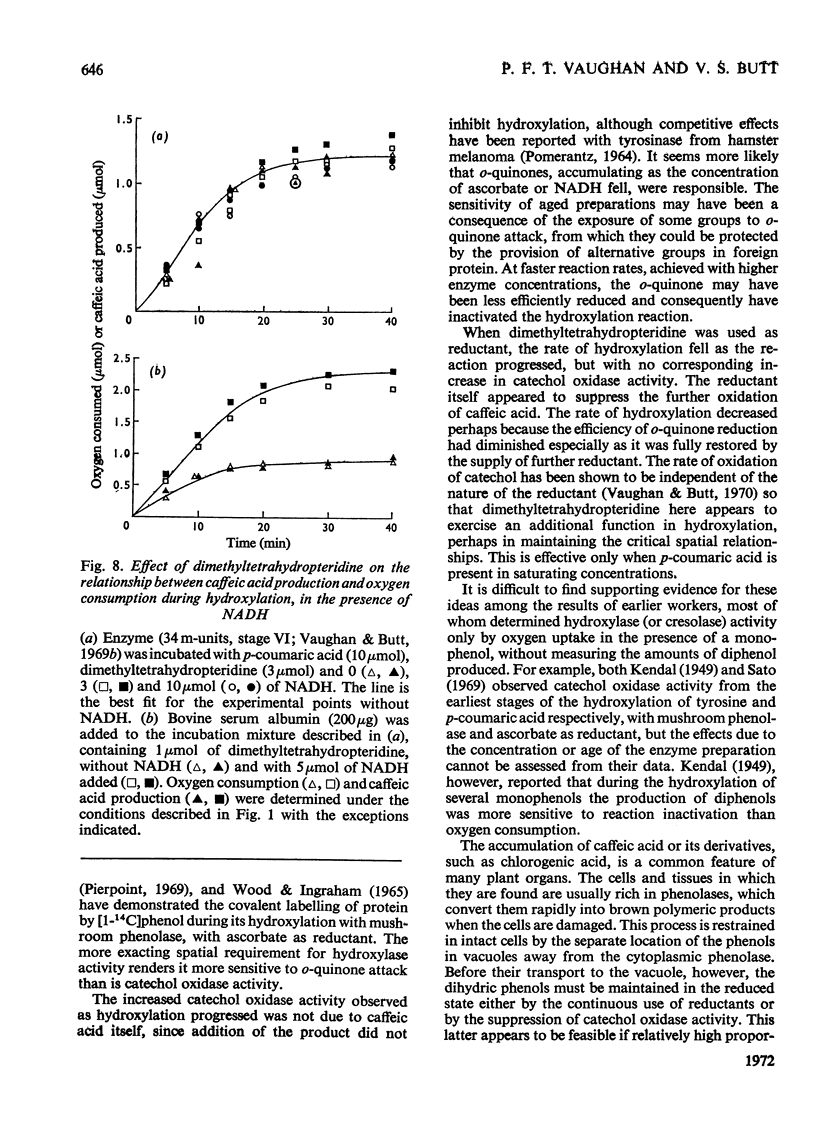
1. The conditions under which oxygen consumption in excess of that required for the hydroxylation of p-coumaric acid to caffeic acid, catalysed by spinach-beet phenolase, can be suppressed, have been examined. 2. With dimethyltetrahydropteridine as electron donor, oxygen uptake was exactly equivalent to the caffeic acid produced, provided that p-coumaric acid was in excess, but with excess of reductant, oxygen uptake caused by the further oxidation of caffeic acid was also observed. 3. With equal concentrations of ascorbate and p-coumaric acid, equivalent oxygen uptake and caffeic acid production was found only in the first stages of the reaction, whereas with NADH substituted for ascorbate, oxygen uptake was in excess throughout. 4. When ascorbate was used, the period of the reaction over which this equivalence was found was decreased at high reaction rates and not observed at all with aged enzyme preparations; equivalence was restored by adding bovine serum albumin to these aged preparations. 5. Equivalence between oxygen consumption and caffeic acid production was observed with NADH, if small quantities of dimethyltetrahydropteridine were also added. 6. It is concluded that hydroxylation proceeds without the concomitant production of caffeic acid only if the enzyme is stabilized for hydroxylation by p-coumaric acid and the reductant, and is protected from attack by o-quinones.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTT V. S., HALLAWAY M. The catalysis of ascorbate oxidation by ionic copper and its complexes. Arch Biochem Biophys. 1961 Jan;92:24–32. doi: 10.1016/0003-9861(61)90213-2. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Myers J. Comparative studies of cytochrome c redox reactions by photochemical lamellar preparations obtained from blue-green, red and green algae, and spinach chloroplasts. Arch Biochem Biophys. 1966 Mar;113(3):738–741. doi: 10.1016/0003-9861(66)90256-6. [DOI] [PubMed] [Google Scholar]
- Kendal L. P. The action of tyrosinase on monophenols. Biochem J. 1949;44(4):442–454. doi: 10.1042/bj0440442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON H. S. Mechanisms of oxygen metabolism. Adv Enzymol Relat Subj Biochem. 1957;19:79–233. doi: 10.1002/9780470122648.ch2. [DOI] [PubMed] [Google Scholar]
- MASON H. S. Structures and functions of the phenolase complex. Nature. 1956 Jan 14;177(4498):79–81. doi: 10.1038/177079a0. [DOI] [PubMed] [Google Scholar]
- Pierpoint W. S. o-Quinones formed in plant extracts. Their reaction with bovine serum albumin. Biochem J. 1969 May;112(5):619–629. doi: 10.1042/bj1120619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz S. H. Tyrosine hydroxylation catalyzed by mammalian tyrosinase: an improved method of assay. Biochem Biophys Res Commun. 1964 Jun 1;16(2):188–194. doi: 10.1016/0006-291x(64)90359-6. [DOI] [PubMed] [Google Scholar]
- Vaughan P. F., Butt V. S. The action of o-dihydric phenols in the hydroxylation of p-coumaric acid by a phenolase from leaves of spinach beet (Beta vulgaris L.). Biochem J. 1970 Aug;119(1):89–94. doi: 10.1042/bj1190089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. F., Butt V. S. The hydroxylation of p-coumaric acid by an enzyme from leaves of spinach beet (Beta vulgaris L.). Biochem J. 1969 Jun;113(1):109–115. doi: 10.1042/bj1130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD B. J., INGRAHAM L. L. LABELLED TYROSINASE FROM LABELLED SUBSTRATE. Nature. 1965 Jan 16;205:291–292. doi: 10.1038/205291a0. [DOI] [PubMed] [Google Scholar]


