Abstract
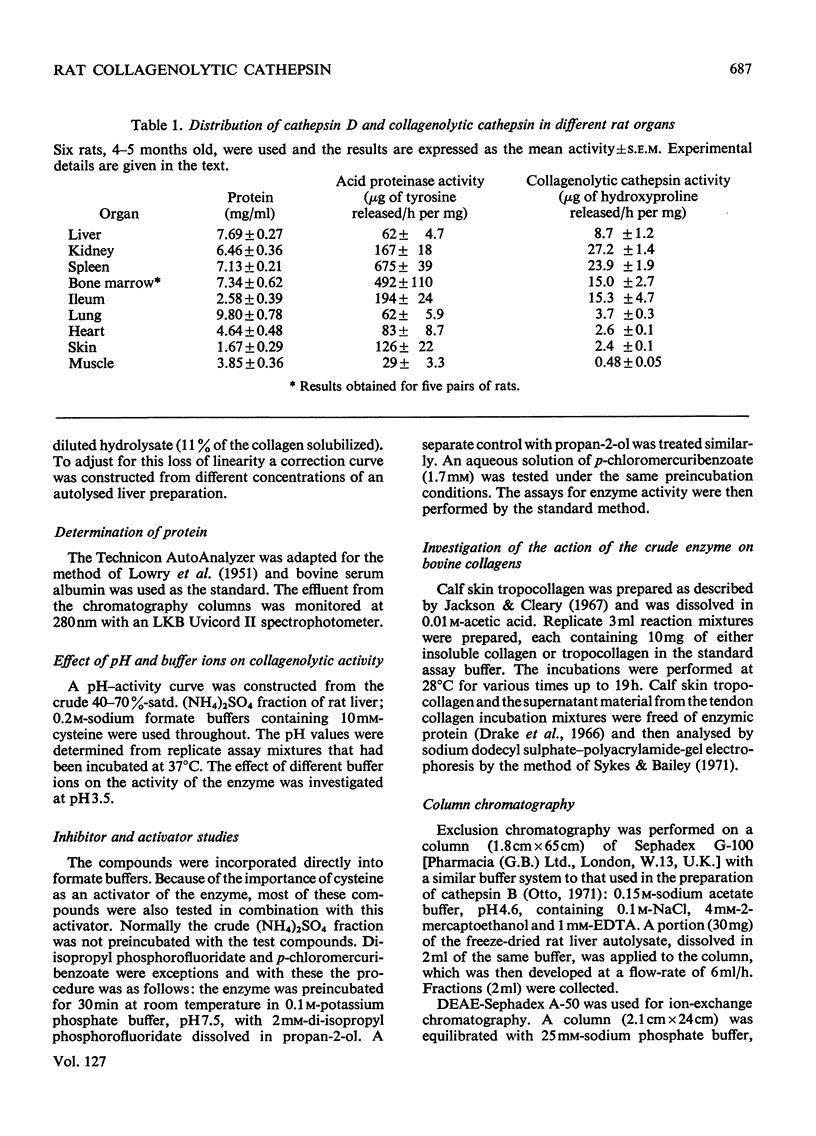
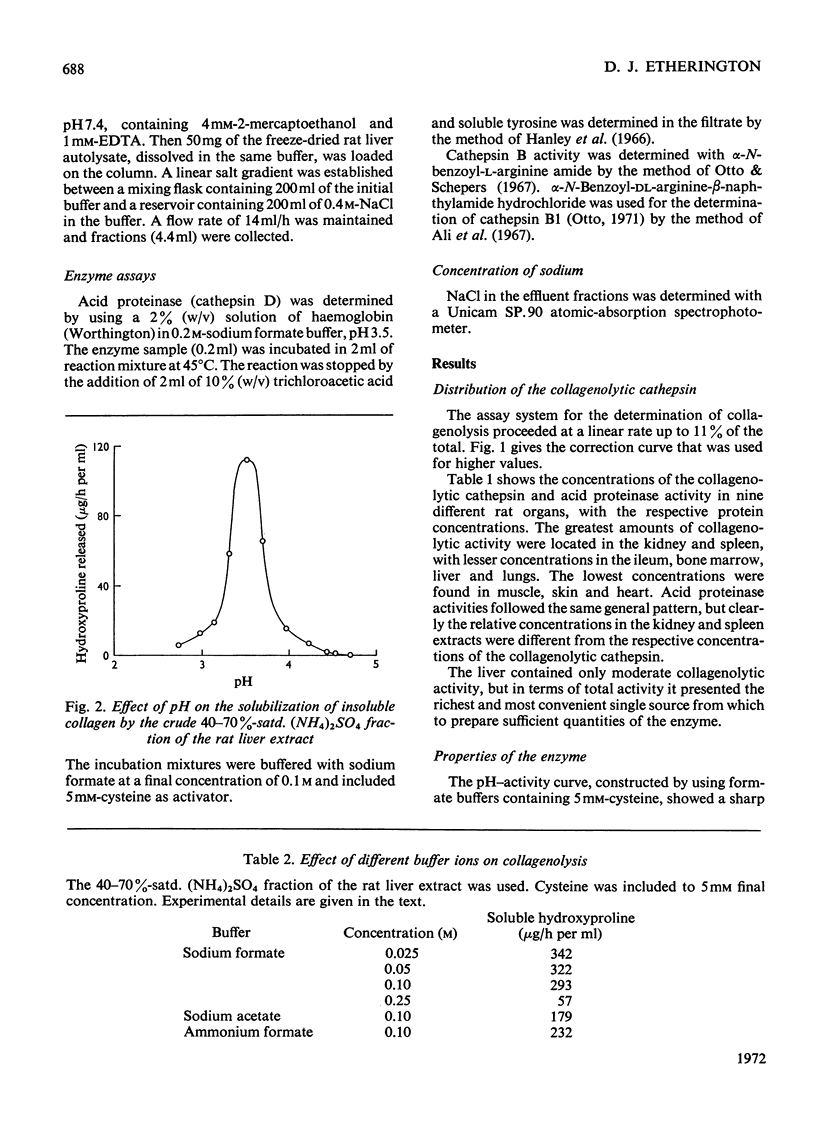
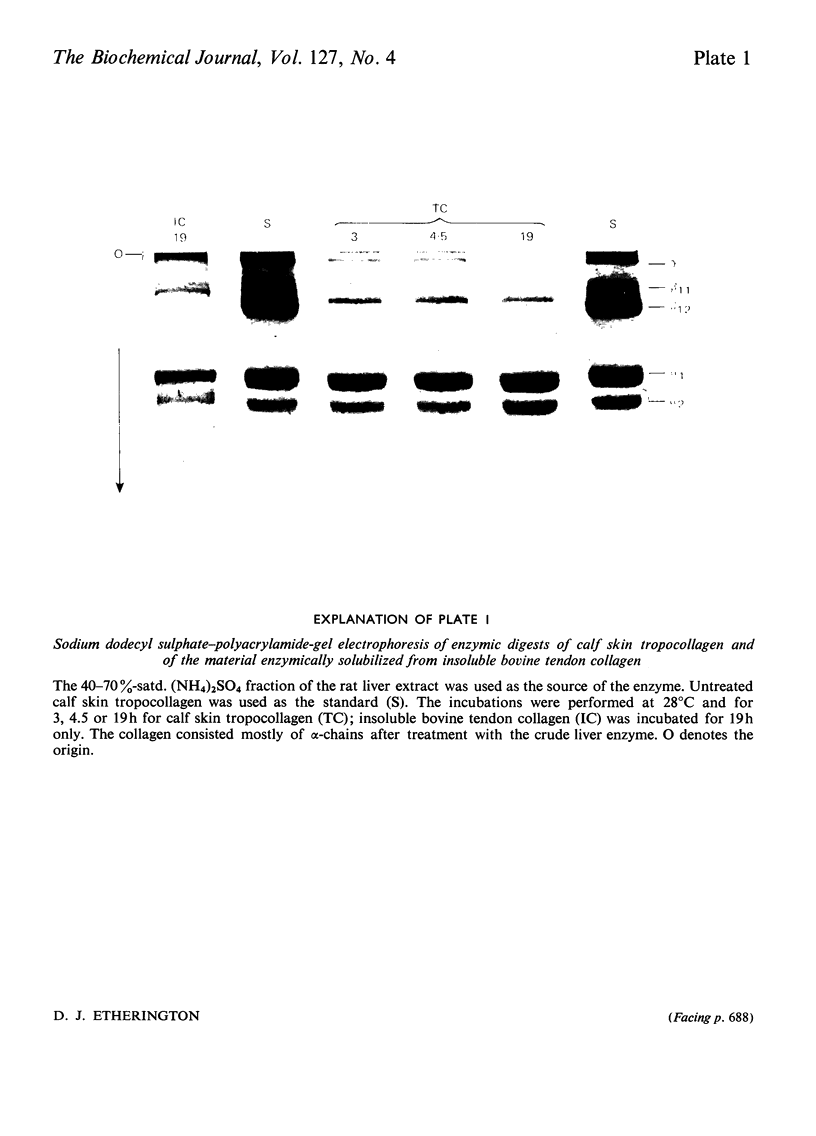
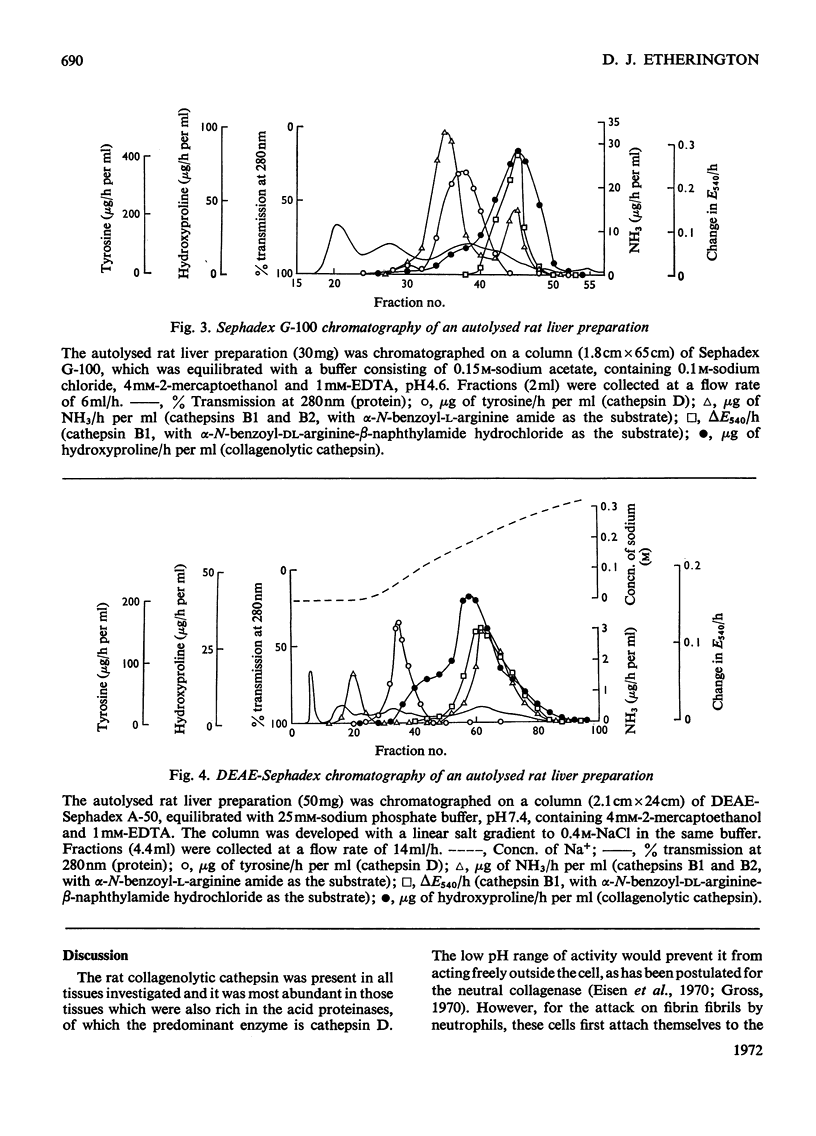
1. An enzyme present in rat liver extracts degraded insoluble collagen maximally at pH3.5. Collagenolytic activity was more abundant in kidney, spleen and bone marrow and was also present in decreasing concentrations in ileum, lung, heart, skin and muscle. 2. The crude collagenolytic cathepsin was activated by cysteine and dithiothreitol, but not by 2-mercaptoethanol. Iodoacetamide, p-chloromercuribenzoate and 7-amino-1-chloro-3-l-tosylamidoheptan-2-one hydrochloride inhibited the enzyme. Zn2+, Fe3+ and Hg2+ ions were strongly inhibitory, but Ca2+, Co2+, Mg2+ and Fe2+ ions had little or no effect. EDTA was an activator of the enzyme. Inhibitors of cathepsin B were found to enhance collagenolysis, but phenylpyruvic acid, a cathepsin D inhibitor, inhibited the enzyme. Di-isopropyl phosphorofluoridate had no effect. 3. Collagenolysis at pH3.5 and 28°C was restricted to cleavage of the telopeptide region in insoluble collagen, and the material that was solubilized consisted mostly of α-chains. 4. The collagenolytic cathepsin was separated from cathepsins B2 and D by fractionation on Sephadex G-100 and a partial separation from cathepsin B1 was obtained by chromatography on DEAE-Sephadex. 5. The function of the collagenolytic cathepsin in the catabolism of collagen is discussed in relation to the action of the other lysosomal proteinases and the neutral collagenase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y., Evans L., Stainthorpe E., Lack C. H. Characterization of cathepsins in cartilage. Biochem J. 1967 Nov;105(2):549–557. doi: 10.1042/bj1050549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. Y. The degradation of cartilage matrix by an intracellular protease. Biochem J. 1964 Dec;93(3):611–618. doi: 10.1042/bj0930611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A. J. Effects of lysosomal collagenolytic enzymes, anti-inflammatory drugs and other substances on some properties of insoluble collagen. Biochem J. 1969 Jul;113(3):457–463. doi: 10.1042/bj1130457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Lysosomal acid proteinase of rabbit liver. Biochem J. 1967 Aug;104(2):601–608. doi: 10.1042/bj1040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin S., Delaunay A. Biochimie de l'inflammation. IX. Etude chimique et physiologique de deux cathepsines isolées de foyers inflammatoires. Ann Inst Pasteur (Paris) 1971 Jan;120(1):50–61. [PubMed] [Google Scholar]
- Bazin S., Delaunay A. Caractères de cathepsines collagénolytiques présentes dans les tissus enflammés du rat. Ann Inst Pasteur (Paris) 1966 Feb;110(2):192–204. [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Weston P. D. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971 Jun;123(1):1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake M. P., Davison P. F., Bump S., Schmitt F. O. Action of proteolytic enzymes on tropocollagen and insoluble collagen. Biochemistry. 1966 Jan;5(1):301–312. doi: 10.1021/bi00865a039. [DOI] [PubMed] [Google Scholar]
- Eisen A. Z., Bauer E. A., Jeffrey J. J. Animal and human collagenases. J Invest Dermatol. 1970 Dec;55(6):359–373. doi: 10.1111/1523-1747.ep12260483. [DOI] [PubMed] [Google Scholar]
- Eisen A. Z., Bauer E. A., Jeffrey J. J. Human skin collagenase. The role of serum alpha-globulins in the control of activity in vivo and in vitro. Proc Natl Acad Sci U S A. 1971 Jan;68(1):248–251. doi: 10.1073/pnas.68.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLAND D. M., WYNN C. H. The degradation of acidsoluble collagen by rat-liver preparations. Biochem J. 1962 Nov;85:276–282. doi: 10.1042/bj0850276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell H. B. Role of biological membranes in some skeletal reactions. Ann Rheum Dis. 1969 May;28(3):213–227. doi: 10.1136/ard.28.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
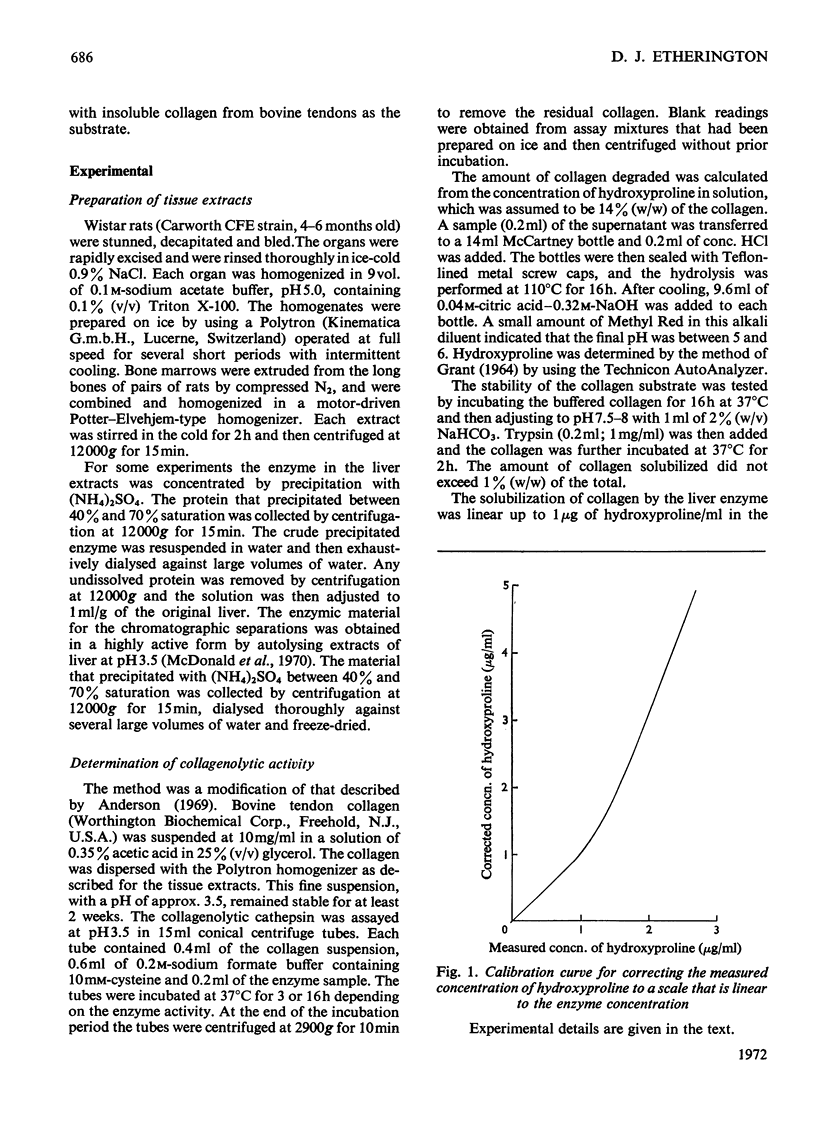
- GRANT R. A. ESTIMATION OF HYDROXYPROLINE BY THE AUTOANALYSER. J Clin Pathol. 1964 Nov;17:685–686. doi: 10.1136/jcp.17.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKNESS R. D., MORALEE B. E. The time-course and route of loss of collagen from the rat's uterus during post-partum involution. J Physiol. 1956 Jun 28;132(3):502–508. doi: 10.1113/jphysiol.1956.sp005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley W. B., Boyer S. H., Naughton M. A. Electrophoretic and functional heterogeneity of pepsinogen in several species. Nature. 1966 Mar 5;209(5027):996–1002. doi: 10.1038/209996a0. [DOI] [PubMed] [Google Scholar]
- Hirayama C., Hiroshige K., Masuya T. Hepatic collagenolytic activity in rats after carbon tetrachloride poisoning. Biochem J. 1969 Dec;115(4):843–847. doi: 10.1042/bj1150843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. S., Cleary E. G. The determination of collagen and elastin. Methods Biochem Anal. 1967;15:25–76. doi: 10.1002/9780470110331.ch2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leibovich S. J., Weiss J. B. Failure of human rheumatoid synovial collagenase to degrade either normal or rheumatoid arthritic polymeric collagen. Biochim Biophys Acta. 1971 Oct;251(1):109–118. doi: 10.1016/0005-2795(71)90067-5. [DOI] [PubMed] [Google Scholar]
- McDonald J. K., Zeitman B. B., Ellis S. Leucine naphthylamide: an inappropriate [corrected] substrate for the histochemical detection of cathepsins B and B'. Nature. 1970 Mar 14;225(5237):1048–1049. doi: 10.1038/2251048a0. [DOI] [PubMed] [Google Scholar]
- NEUBERGER A., SLACK H. G. B. The metabolism of collagen from liver, bone, skin and tendon in the normal rat. Biochem J. 1953 Jan;53(1):47–52. doi: 10.1042/bj0530047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto K., Schepers P. Uber die katheptische Inaktivierung einiger Enzyme der Rattenleber, insbesondere der Glucokinase. Hoppe Seylers Z Physiol Chem. 1967 May;348(5):482–490. [PubMed] [Google Scholar]
- Parakkal P. F. Involvement of macrophages in collagen resorption. J Cell Biol. 1969 Apr;41(1):345–354. doi: 10.1083/jcb.41.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIDDLE J. M., BARNHART M. I. ULTRASTRUCTURAL STUDY OF FIBRIN DISSOLUTION VIA EMIGRATED POLYMORPHONUCLEAR NEUTROPHILS. Am J Pathol. 1964 Nov;45:805–823. [PMC free article] [PubMed] [Google Scholar]
- SCHAUB M. C. DEGRADATION OF YOUNG AND OLD COLLAGEN BY EXTRACTS OF VARIOUS ORGANS. Gerontologia. 1964;49:52–60. doi: 10.1159/000211236. [DOI] [PubMed] [Google Scholar]
- Sykes B. C., Bailey A. J. Molecular weight heterogeneity of the alpha-chain sub-units of collagen. Biochem Biophys Res Commun. 1971 Apr 16;43(2):340–345. doi: 10.1016/0006-291x(71)90758-3. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., BREWER T. H. FORMATION AND BREAKDOWN OF COLLAGEN AND ELASTIN IN THE HUMAN UTERUS DURING PREGNANCY AND POST-PARTUM INVOLUTION. Biochem J. 1963 Oct;89:75–82. doi: 10.1042/bj0890075. [DOI] [PMC free article] [PubMed] [Google Scholar]