Abstract
1. Two forms of hepatic pyruvate kinase, designated type L and type M, were distinguished on the basis of kinetic, chromatographic, electrophoretic and immunological criteria. They were partially purified and their properties compared with each other and with the purified enzyme from skeletal muscle. 2. In contrast with type L, the type M enzyme showed no marked evidence of co-operative interactions with phosphoenolpyruvate and was not stimulated by fructose diphosphate. 3. The activity profiles of type L and type M enzymes were determined in developing rat liver by utilizing differences in the kinetic properties of the two forms. The high activity of type M enzyme in the early foetal rat decreased in late gestation and immediately after birth to reach a low value, which remained essentially constant for the remainder of the developmental period. The activity of type L enzyme, in contrast, was low in the early foetal and neonatal liver but increased markedly at the onset of weaning. 4. Possible roles of the two forms of hepatic pyruvate kinase in the control of glycolysis and gluconeogenesis are discussed.
Full text
PDF

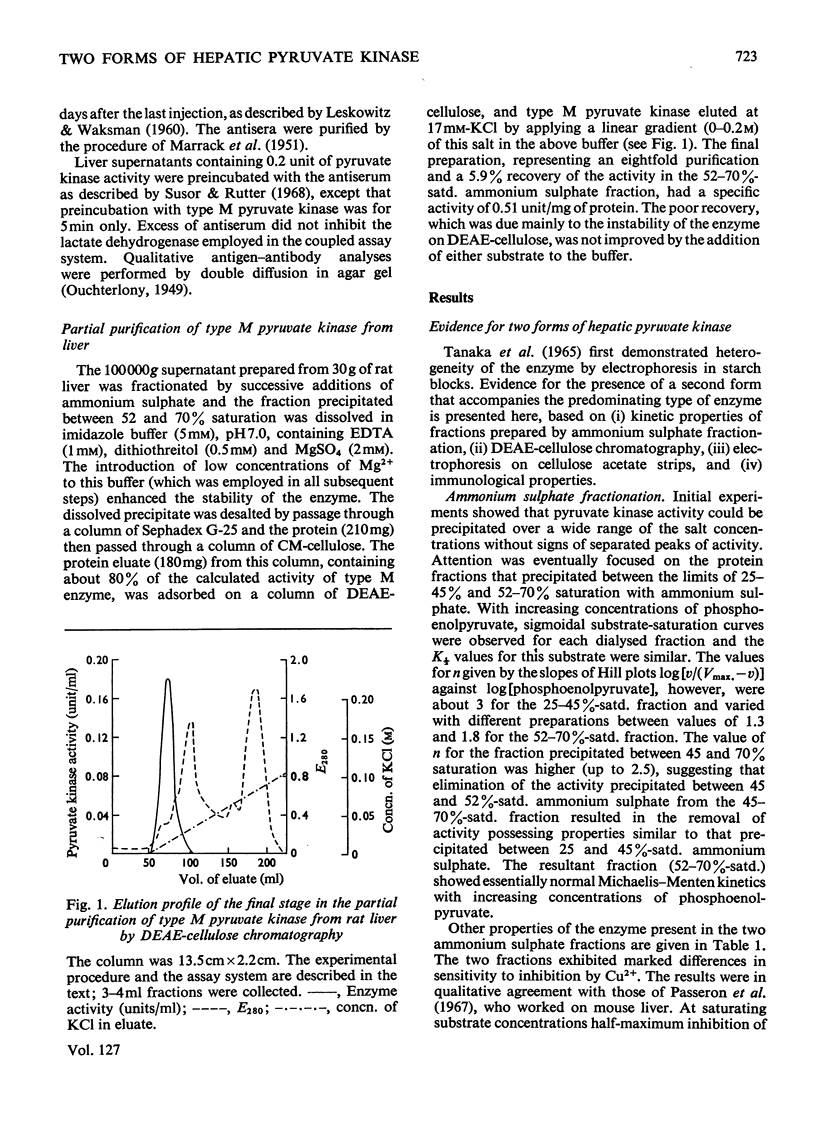
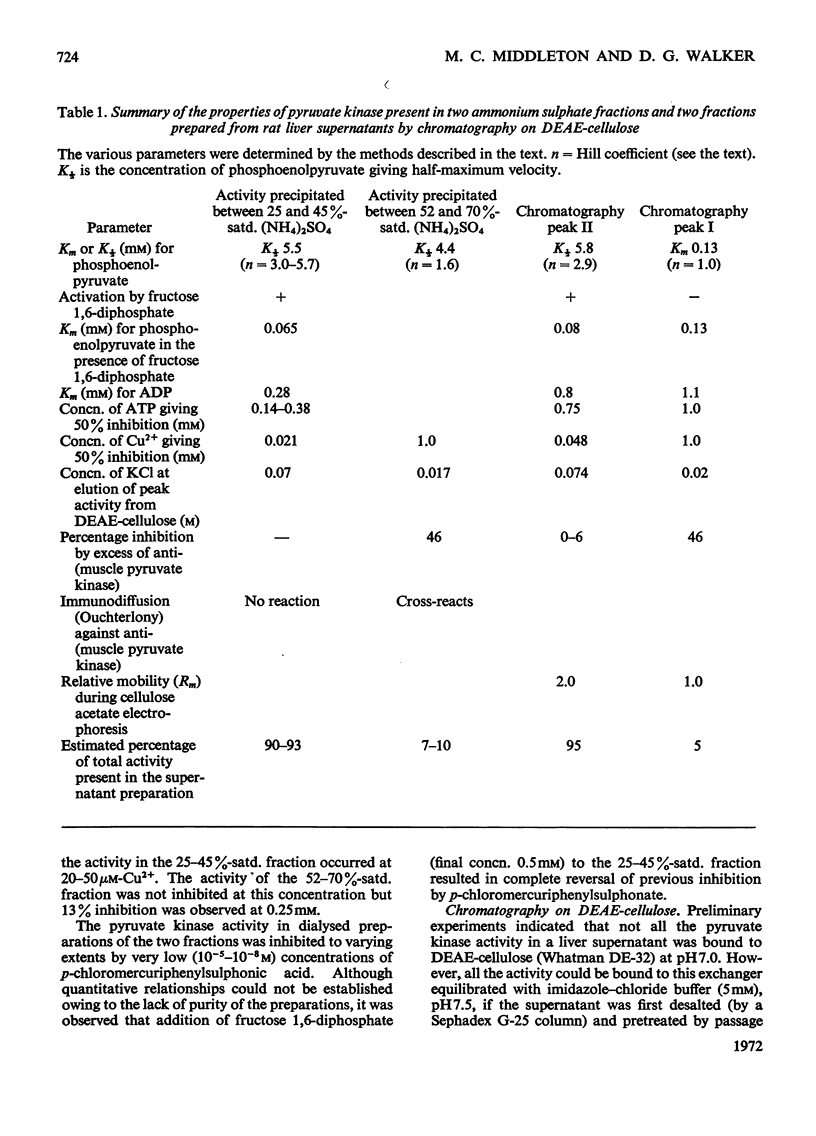
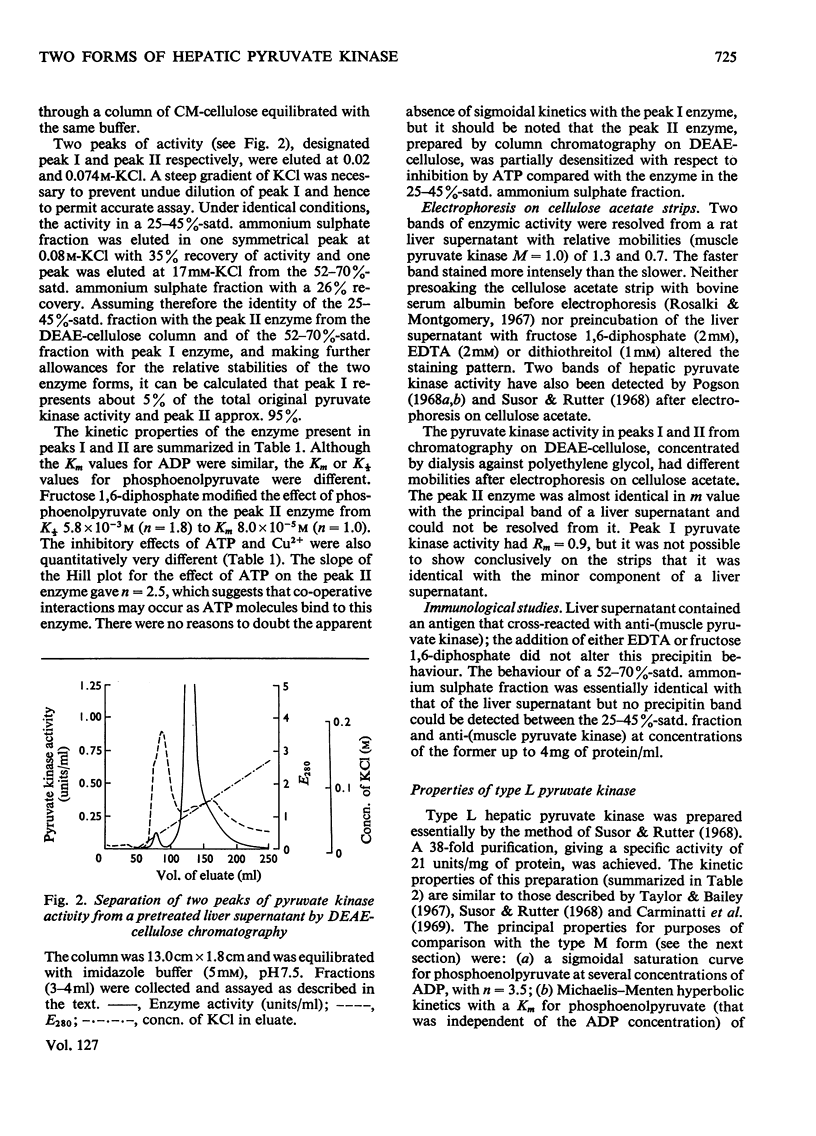
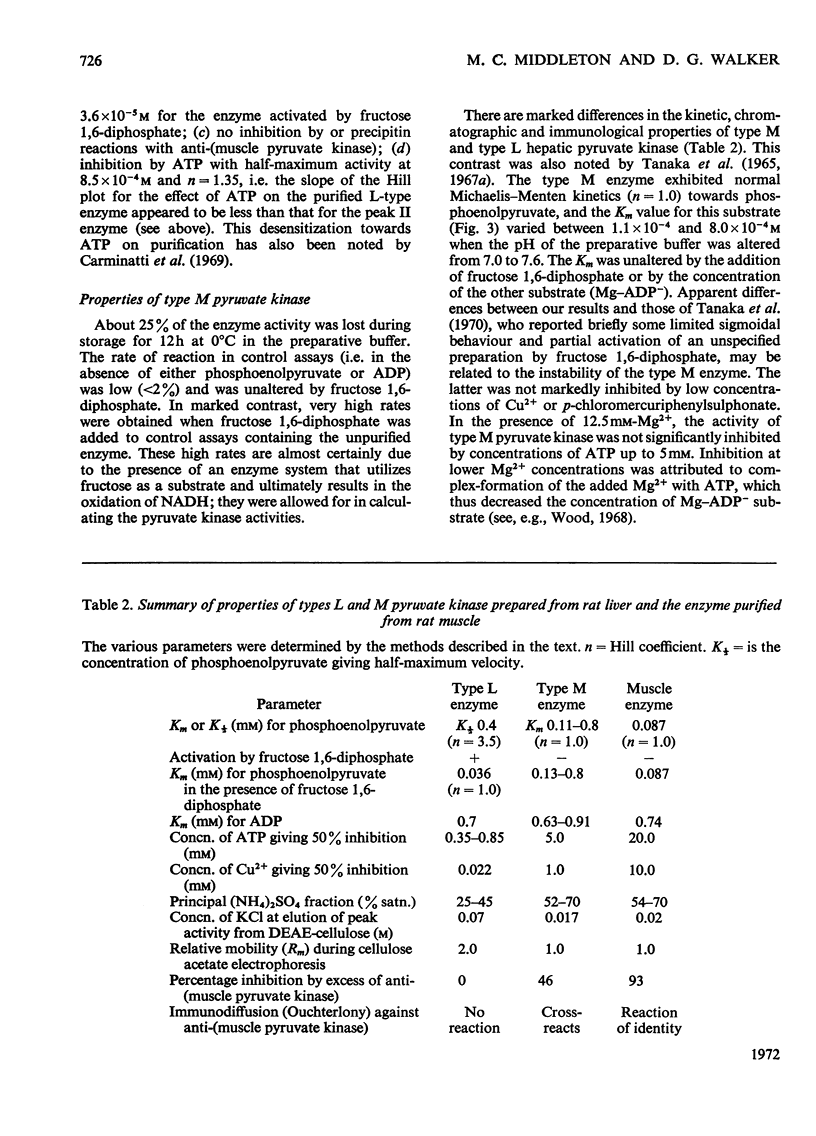


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURCH H. B., LOWRY O. H., KUHLMAN A. M., SKERJANCE J., DIAMANT E. J., LOWRY S. R., VON DIPPE P. Changes in patterns of enzymes of carbohydrate metabolism in the developing rat liver. J Biol Chem. 1963 Jul;238:2267–2273. [PubMed] [Google Scholar]
- Bailey E., Stirpe F., Taylor C. B. Regulation of rat liver pyruvate kinase. The effect of preincubation, pH, copper ions, fructose 1,6-diphosphate and dietary changes on enzyme activity. Biochem J. 1968 Jul;108(3):427–436. doi: 10.1042/bj1080427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey E., Taylor C. B., Bartley W. Effect of dietary carbohydrates on hepatic lipogenesis in the rat. Nature. 1968 Feb 3;217(5127):471–472. doi: 10.1038/217471b0. [DOI] [PubMed] [Google Scholar]
- Bartley W., Dean B., Taylor C. B., Bailey E. The effect on some enzymes of rat tissue of diets low in fat content. Biochem J. 1967 May;103(2):550–555. doi: 10.1042/bj1030550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J. O., Koler R. D., Bigley R. H. Kinetic differences between human red cell and leucocyte pyruvate kinase. Nature. 1965 Oct 9;208(5006):194–195. doi: 10.1038/208194a0. [DOI] [PubMed] [Google Scholar]
- Carminatti H., Jiménez de Asúa L., Recondo E., Passeron S., Rozengurt E. Some kinetic properties of liver pyruvate kinase (type L). J Biol Chem. 1968 Jun 10;243(11):3051–3056. [PubMed] [Google Scholar]
- Eggleston L. V., Krebs H. A. Strain differences in the activities of rat liver enzymes. Biochem J. 1969 Oct;114(4):877–879. doi: 10.1042/bj1140877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- Jiménez de Asúa L., Rozengurt E., Devalle J. J., Carminatti H. Some kinetic differences between the M isoenzymes of pyruvate kinase from liver and muscle. Biochim Biophys Acta. 1971 May 12;235(2):326–334. doi: 10.1016/0005-2744(71)90211-7. [DOI] [PubMed] [Google Scholar]
- LESKOWITZ S., WAKSMAN B. H. Studies on immunization. 1. The effect of route of injection of bovine serum albumin in Freund adjuvant on production of circulating antibody and delayed hypersensitivity. J Immunol. 1960 Jan;84:58–72. [PubMed] [Google Scholar]
- Linder-Horowitz M. Changes in glutaminase activities of rat liver and kidney during pre- and post-natal development. Biochem J. 1969 Aug;114(1):65–70. doi: 10.1042/bj1140065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente P., Marco R., Sols A. Regulation of liver pyruvate kinase and the phosphoenolpyruvate crossroads. Eur J Biochem. 1970 Mar 1;13(1):45–54. doi: 10.1111/j.1432-1033.1970.tb00897.x. [DOI] [PubMed] [Google Scholar]
- MARRACK J. R., HOCH H., JOHNS R. G. S. The valency of antibodies. Br J Exp Pathol. 1951 Jun;32(3):212–230. [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Gevers W. Control of glycolysis and gluconeogenesis in liver and kidney cortex. Vitam Horm. 1967;25:1–87. doi: 10.1016/s0083-6729(08)60033-3. [DOI] [PubMed] [Google Scholar]
- OLIVER I. T., BLUMER W. F., WITHAM I. J. FREE RIBOSOMES DURING MATURATION OF RAT LIVER. Comp Biochem Physiol. 1963 Sep;10:33–38. doi: 10.1016/0010-406x(63)90100-2. [DOI] [PubMed] [Google Scholar]
- Passeron S., Jiménez de Asua L., Carminatti H. Fructose 1,6-diphosphate, a reactivator of Cu++-inhibited pyruvate kinase from liver. Biochem Biophys Res Commun. 1967 Apr 7;27(1):33–38. doi: 10.1016/s0006-291x(67)80035-4. [DOI] [PubMed] [Google Scholar]
- Pogson C. I. Adipose-tissue pyruvate kinase. Properties and interconversion of two active forms. Biochem J. 1968 Nov;110(1):67–77. doi: 10.1042/bj1100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson C. I. Two interconvertible forms of pyruvate kinase in adipose tissue. Biochem Biophys Res Commun. 1968 Feb 15;30(3):297–302. doi: 10.1016/0006-291x(68)90450-6. [DOI] [PubMed] [Google Scholar]
- REYNARD A. M., HASS L. F., JACOBSEN D. D., BOYER P. D. The correlation of reaction kinetics and substrate binding with the mechanism of pyruvate kinase. J Biol Chem. 1961 Aug;236:2277–2283. [PubMed] [Google Scholar]
- Rosalki S. B., Montgomery A. Loss of cathodic isoenzymes during cellulose acetate enzyme electrophoresis. Clin Chim Acta. 1967 Jun;16(3):440–441. doi: 10.1016/0009-8981(67)90312-9. [DOI] [PubMed] [Google Scholar]
- Schoner W., Haag U., Seubert W. On the mechanism of gluconeogenesis and its regulation. VI. Regulation of carbohydrate metabolism by cortisol independent of the de novo synthesis of enzymes in rat liver. Hoppe Seylers Z Physiol Chem. 1970 Sep;351(9):1071–1088. doi: 10.1515/bchm2.1970.351.2.1071. [DOI] [PubMed] [Google Scholar]
- Seubert W., Henning H. V., Schoner W., L'age M. Effects of cortisol on the levels of metabolites and enzymes controlling glucose production from pyruvate. Adv Enzyme Regul. 1968;6:153–187. doi: 10.1016/0065-2571(68)90012-5. [DOI] [PubMed] [Google Scholar]
- Susor W. A., Rutter W. J. Some distinctive properties of pyruvate kinase purified from rat liver. Biochem Biophys Res Commun. 1968 Jan 11;30(1):14–20. doi: 10.1016/0006-291x(68)90705-5. [DOI] [PubMed] [Google Scholar]
- Szepesi B., Freedland R. A. Dietary regulation of pyruvate kinase synthesis in rat liver. J Nutr. 1968 Aug;95(4):591–602. doi: 10.1093/jn/95.4.591. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Harano Y., Morimura H., Mori R. Evidence for the presence of two types of pyruvate kinase in rat liver. Biochem Biophys Res Commun. 1965 Oct 8;21(1):55–60. doi: 10.1016/0006-291x(65)90425-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Harano Y., Sue F., Morimura H. Crystallization, characterization and metabolic regulation of two types of pyruvate kinase isolated from rat tissues. J Biochem. 1967 Jul;62(1):71–91. doi: 10.1093/oxfordjournals.jbchem.a128639. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Sue F., Morimura H. Feed-forward activation and feed-back inhibition of pyruvate kinase type L of rat liver. Biochem Biophys Res Commun. 1967 Nov 17;29(3):444–449. doi: 10.1016/0006-291x(67)90477-9. [DOI] [PubMed] [Google Scholar]
- Taylor C. B., Bailey E., Bartley W. Changes in hepatic lipigenesis during development of the rat. Biochem J. 1967 Nov;105(2):717–722. doi: 10.1042/bj1050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. G., Walker D. G. Changes in activity of some enzymes involved in glucose utilization and formation in developing rat liver. Biochem J. 1968 Jan;106(2):321–329. doi: 10.1042/bj1060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G., SINGHAL R. L., STAMM N. B., SRIVASTAVA S. K. HORMONAL INDUCTION AND SUPPRESSION OF LIVER ENZYME BIOSYNTHESIS. Fed Proc. 1965 May-Jun;24:745–754. [PubMed] [Google Scholar]
- Walker D. G., Holland G. The development of hepatic glucokinase in the neonatal rat. Biochem J. 1965 Dec;97(3):845–854. doi: 10.1042/bj0970845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Lea M. A., Stamm N. B. Sequential feedback inhibition and regulation of liver carbohydrate metabolism through control of enzyme activity. Adv Enzyme Regul. 1968;6:101–123. doi: 10.1016/0065-2571(68)90009-5. [DOI] [PubMed] [Google Scholar]
- Weber G., Singhal R. L., Stamm N. B., Lea M. A., Fisher E. A. Synchronous behavior pattern of key glycolytic enzymes: glucokinase, phosphofructokinase, and pyruvate kinase. Adv Enzyme Regul. 1966;4:59–81. doi: 10.1016/0065-2571(66)90007-0. [DOI] [PubMed] [Google Scholar]
- Wood T. The inhibition of pyruvate kinase by ATP. Biochem Biophys Res Commun. 1968 Jun 10;31(5):779–785. doi: 10.1016/0006-291x(68)90630-x. [DOI] [PubMed] [Google Scholar]
- Yudkin J., Krauss R. Dietary starch, dietary sucrose and hepatic pyruvate kinase in rat. Nature. 1967 Jul 1;215(5096):75–75. doi: 10.1038/215075a0. [DOI] [PubMed] [Google Scholar]


