Abstract
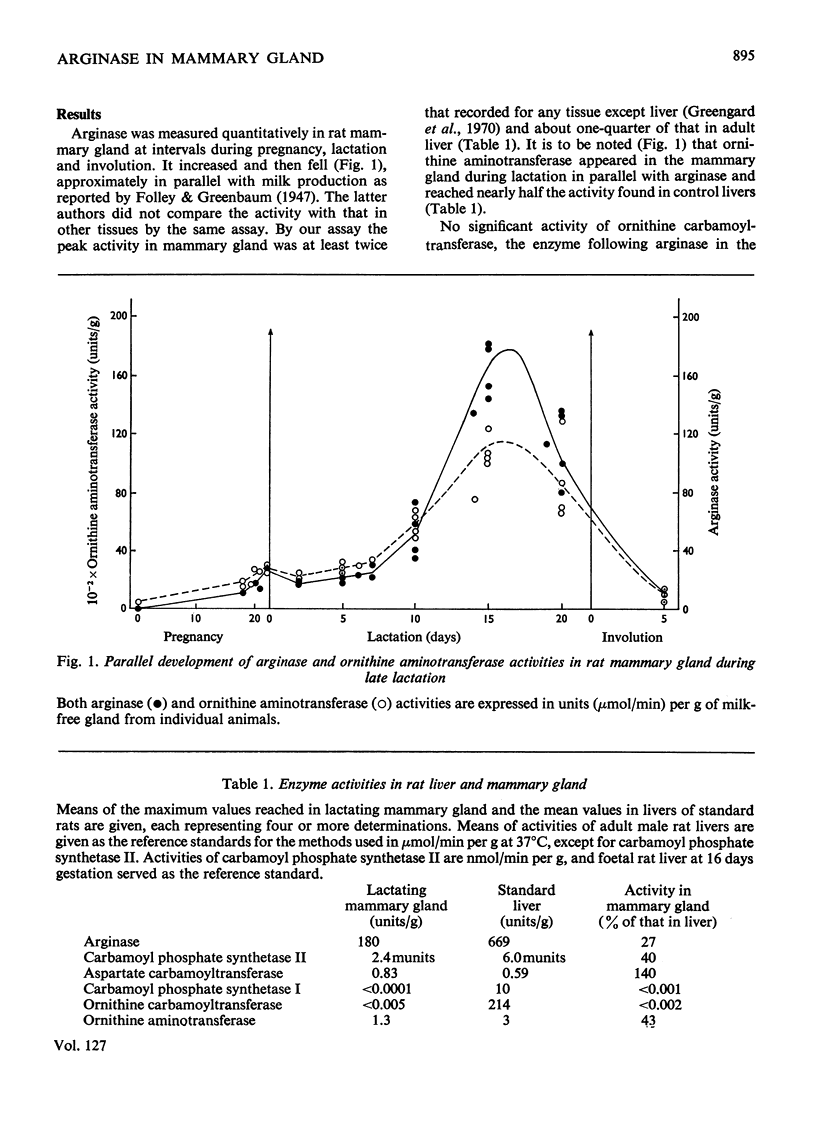
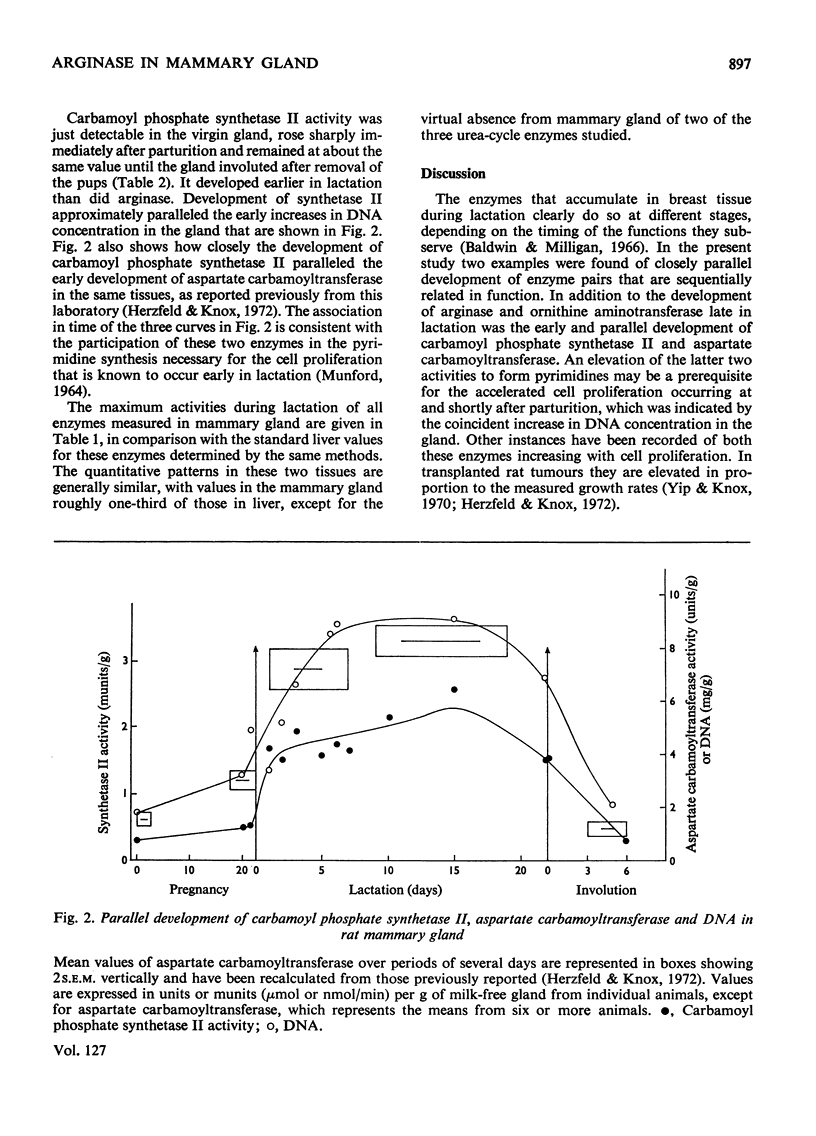
The potential for a considerable formation of ornithine exists in lactating mammary gland because of its arginase content. Late in lactation arginase reaches an activity in the gland higher than that present in any rat tissue except liver. Occurrence of the urea cycle can be excluded since two enzymes for the further reaction of ornithine in the cycle, carbamoyl phosphate synthetase I and ornithine carbamoyltransferase, are both absent from this tissue. Instead, carbamoyl phosphate synthetase II appears early in lactation, associated with accumulation of aspartate carbamoyltransferase and DNA, consistent with the proposed role of these enzymes in pyrimidine synthesis. The facts require another physiological role for arginase apart from its known function in the urea cycle. Significant activity of ornithine aminotransferase develops in mammary gland in close parallel with the arginase. By this reaction, ornithine can be converted into glutamic semialdehyde and subsequently into proline. The enzymic composition of the lactating mammary gland is therefore appropriate for the major conversion of arginine into proline that is known to occur in the intact gland.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. L., Milligan L. P. Enzymatic changes associated with the initiation and maintenance of lactation in the rat. J Biol Chem. 1966 May 10;241(9):2058–2066. [PubMed] [Google Scholar]
- Folley S. J., Greenbaum A. L. Changes in the arginase and alkaline phosphatase contents of the mammary gland and liver of the rat during pregnancy, lactation and mammary involution. Biochem J. 1947;41(2):261–269. doi: 10.1042/bj0410261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBAUM A. L., SLATER T. F. Studies on the particulate components of rat mammary gland. II. Changes in the levels of the nucleic acids of the mammary glands of rats during pregnancy, lactation and mammary involution. Biochem J. 1957 May;66(1):155–161. doi: 10.1042/bj0660155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard O., Sahib M. K., Knox W. E. Developmental formation and distribution of arginase in rat tissues. Arch Biochem Biophys. 1970 Apr;137(2):477–482. doi: 10.1016/0003-9861(70)90465-0. [DOI] [PubMed] [Google Scholar]
- Hager S. E., Jones M. E. Initial steps in pyrimidine synthesis in Ehrlich ascites carcinoma in vitro. II. The synthesis of carbamyl phosphate by a soluble, glutamine-dependent carbamyl phosphate synthetase. J Biol Chem. 1967 Dec 25;242(24):5667–5673. [PubMed] [Google Scholar]
- Herzfeld A., Knox W. E. The properties, developmental formation, and estrogen induction of ornithine aminotransferase in rat tissues. J Biol Chem. 1968 Jun 25;243(12):3327–3332. [PubMed] [Google Scholar]
- Mepham T. B., Linzell J. L. A quantitative assessment of the contribution of individual plasma amino acids to the synthesis of milk proteins by the goat mammary gland. Biochem J. 1966 Oct;101(1):76–83. doi: 10.1042/bj1010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mepham T. B., Linzell J. L. Urea formation by the lactating goat mammary gland. Nature. 1967 Apr 29;214(5087):507–508. doi: 10.1038/214507b0. [DOI] [PubMed] [Google Scholar]
- PEISACH J., STRECKER H. J. The interconversion of glutamic acid and proline. V. The reduction of delta 1-pyrroline-5-carboxylic acid to proline. J Biol Chem. 1962 Jul;237:2255–2260. [PubMed] [Google Scholar]
- Raghupathi Reddy S. R., Campbell J. W. Arginine metabolism in insects. Role of arginase in proline formation during silkmoth development. Biochem J. 1969 Nov;115(3):495–503. doi: 10.1042/bj1150495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T. Adaptive characteristics of urea cycle enzymes in the rat. J Biol Chem. 1962 Feb;237:459–468. [PubMed] [Google Scholar]
- Smith A. D., Benziman M., Strecker H. J. The formation of ornithine from proline in animal tissues. Biochem J. 1967 Aug;104(2):557–563. doi: 10.1042/bj1040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip M. C., Knox W. E. Glutamine-dependent carbamyl phosphate synthetase. Properties and distribution in normal and neoplastic rat tissues. J Biol Chem. 1970 May 10;245(9):2199–2204. [PubMed] [Google Scholar]


