Abstract
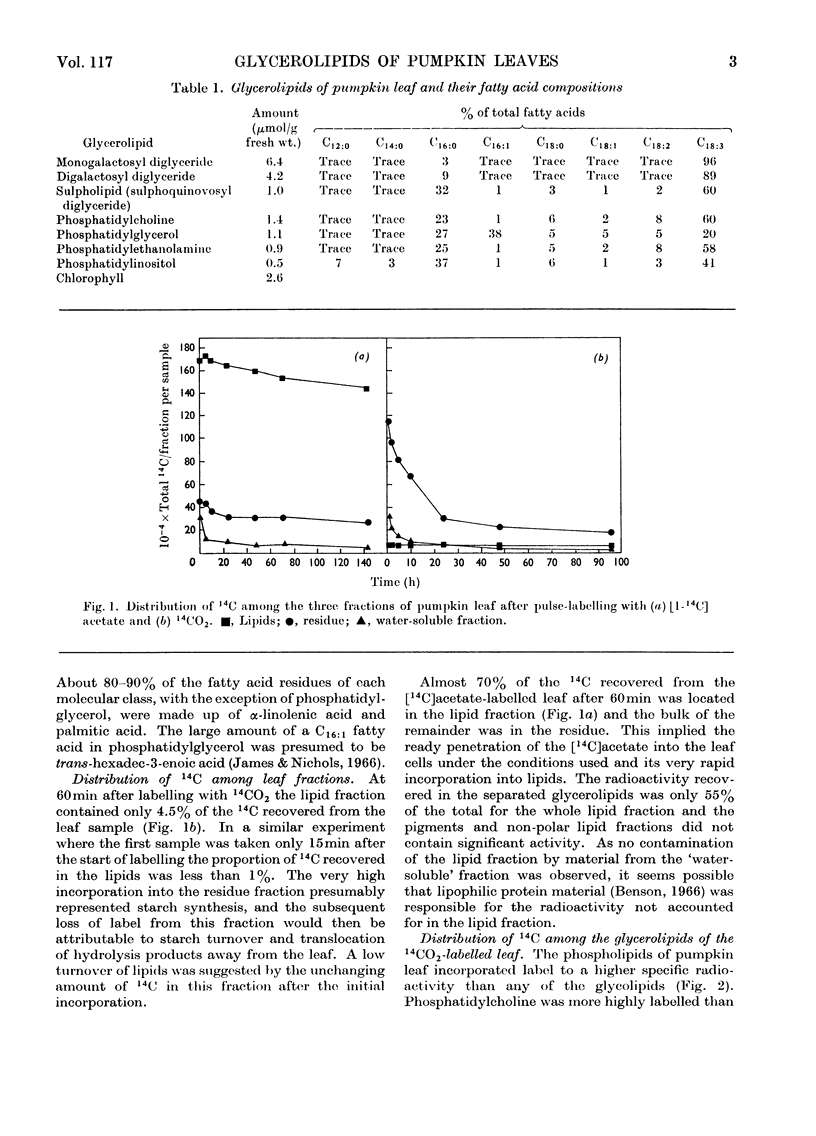
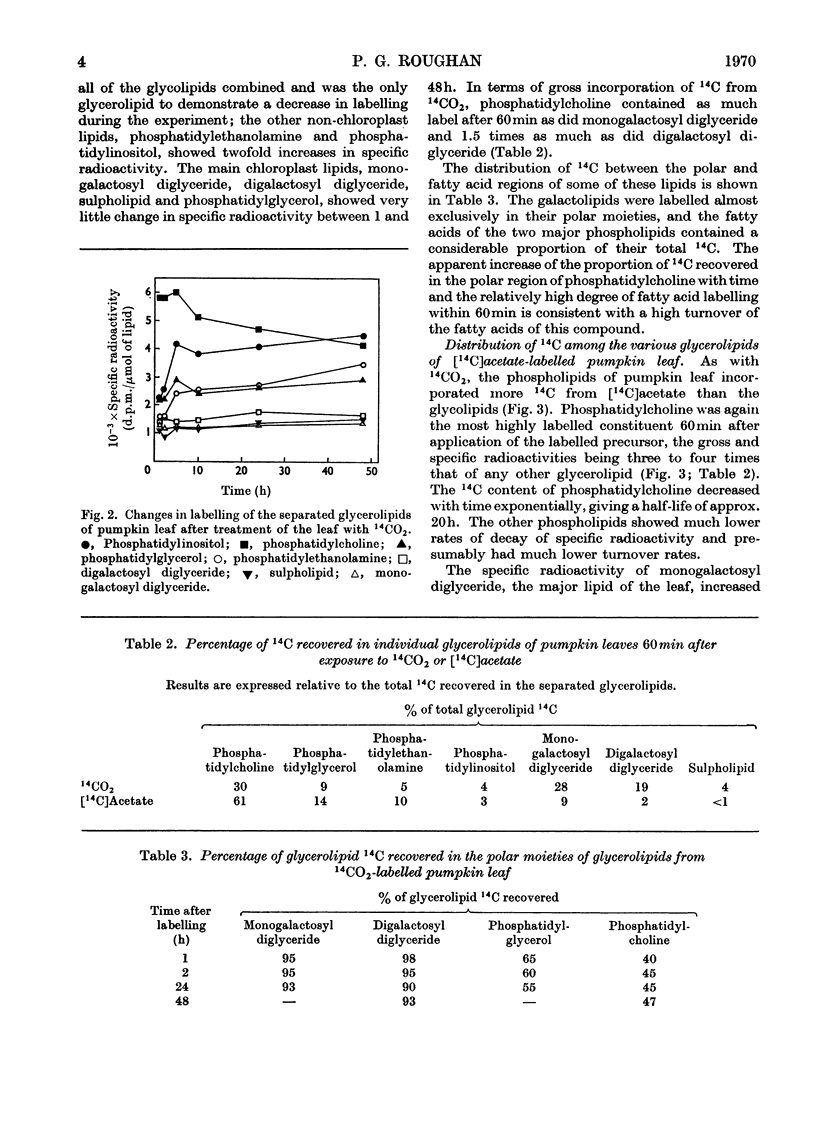
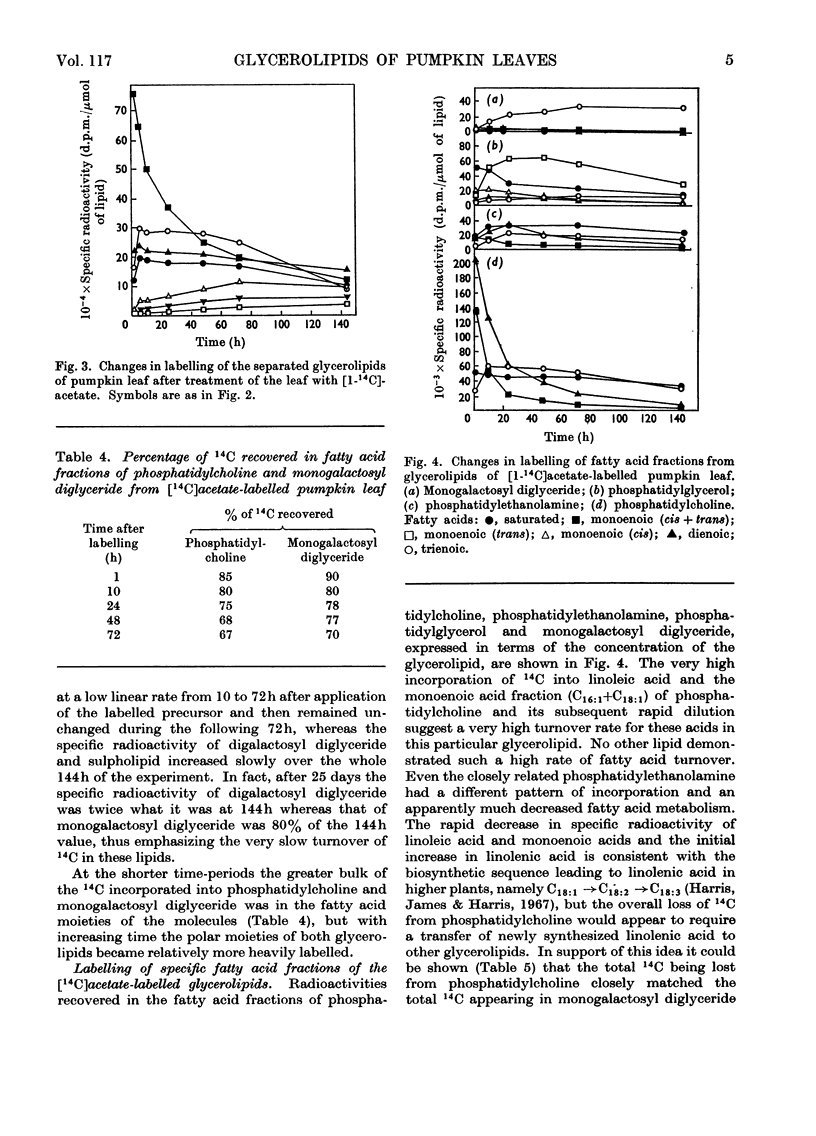
Between 1 and 5% of the 14C recovered from pumpkin leaves within 15–60min after pulse-labelling with 14CO2 was in the lipids. The specific radioactivity of the phospholipids was higher than that of the glycolipids. Phosphatidylcholine had five times the specific radioactivity of monogalactosyl diglyceride, and the specific radioactivity of neither galactolipid changed significantly between 1 and 48h after labelling. It therefore seemed unlikely that the galactose moieties of the galactolipids were involved in the transport of assimilated compounds across the chloroplast membrane. Within 60min of the application of [1-14C]acetate to the surfaces of mature, intact pumpkin leaves 70% of the recovered 14C was in the lipid fraction. Of the separated glycerolipids, phosphatidylcholine had by far the highest specific radioactivity at the shorter time-intervals, and the glycolipids again had the lowest specific radioactivities. Phosphatidylcholine was the only lipid to show a significant turnover of radiocarbon as judged by the decrease in specific radioactivity with time. From a comparison of the changes with time of the labelling of fatty acid fractions from phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol and monogalactosyl diglyceride, it is suggested that the primary site of linolenic acid biosynthesis in leaf cells is within the phosphatidylcholine molecule.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson A. A. On the orientation of lipids in chloroplast and cell membranes. J Am Oil Chem Soc. 1966 May;43(5):265–270. doi: 10.1007/BF02609671. [DOI] [PubMed] [Google Scholar]
- ERWIN J., BLOCH K. POLYUNSATURATED FATTY ACIDS IN SOME PHOTOSYNTHETIC MICROORGANISMS. Biochem Z. 1963;338:496–511. [PubMed] [Google Scholar]
- FERRARI R. A., BENSON A. A. The path of carbon in photosynthesis of the lipids. Arch Biochem Biophys. 1961 May;93:185–192. doi: 10.1016/0003-9861(61)90248-x. [DOI] [PubMed] [Google Scholar]
- Green D. E., Perdue J. F. Membranes as expressions of repeating units. Proc Natl Acad Sci U S A. 1966 May;55(5):1295–1302. doi: 10.1073/pnas.55.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurr M. I., Robinson M. P., James A. T. The mechanism of formation of polyunsaturated fatty acids by photosynthetic tissue. The tight coupling of oleate desaturation with phospholipid synthesis in Chlorella vulgaris. Eur J Biochem. 1969 May 1;9(1):70–78. doi: 10.1111/j.1432-1033.1969.tb00577.x. [DOI] [PubMed] [Google Scholar]
- JAMES A. T. The biosynthesis of long-chain saturated and unsaturated fatty acids in isolated plant leaves. Biochim Biophys Acta. 1963 Feb 19;70:9–19. doi: 10.1016/0006-3002(63)90714-5. [DOI] [PubMed] [Google Scholar]
- KATES M. Chromatographic and radioisotopic investigations of the lipid components of runner bean leaves. Biochim Biophys Acta. 1960 Jul 1;41:315–328. doi: 10.1016/0006-3002(60)90015-9. [DOI] [PubMed] [Google Scholar]
- Katayama M., Benson A. A. Alpha-linoleate and photosynthetic activity in Chlorella protothecoides. Plant Physiol. 1967 Mar;42(3):308–313. doi: 10.1104/pp.42.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L. J. Separations of lipids by silver ion chromatography. J Lipid Res. 1966 Nov;7(6):717–732. [PubMed] [Google Scholar]
- NICHOLS B. W. SEPARATION OF THE LIPIDS OF PHOTOSYNTHETIC TISSUES: IMPROVEMENTS IN ANALYSIS BY THIN-LAYER CHROMATOGRAPHY. Biochim Biophys Acta. 1963 Aug 27;70:417–422. doi: 10.1016/0006-3002(63)90771-6. [DOI] [PubMed] [Google Scholar]
- Nichols B. W. Fatty acid metabolism in the chloroplast lipids of green and blue-green algae. Lipids. 1968 Jul;3(4):354–360. doi: 10.1007/BF02530939. [DOI] [PubMed] [Google Scholar]
- Nichols B. W., James A. T., Breuer J. Interrelationships between fatty acid biosynthesis and acyl-lipid synthesis in Chlorella vulgaris. Biochem J. 1967 Aug;104(2):486–496. doi: 10.1042/bj1040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. W., Wood B. J. The occurrence and biosynthesis of gamma-linolenic acid in a blue-green alga,Spirulina platensis. Lipids. 1968 Jan;3(1):46–50. doi: 10.1007/BF02530968. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Batt R. D. Quantitative analysis of sulfolipid (sulfoquinovosyl diglyceride) and galactolipids (monogalactosyl and digalactosyl diglycerides) in plant tissues. Anal Biochem. 1968 Jan;22(1):74–88. doi: 10.1016/0003-2697(68)90261-3. [DOI] [PubMed] [Google Scholar]
- SNYDER F. RADIOASSAY OF THIN-LAYER CHROMATOGRAMS: A HIGH-RESOLUTION ZONAL SCRAPER FOR QUANTITATIVE C14 AND H3 SCANNING OF THIN-LAYER CHROMATOGRAMS. Anal Biochem. 1964 Oct;9:183–196. doi: 10.1016/0003-2697(64)90102-2. [DOI] [PubMed] [Google Scholar]
- STUMPF P. K., BARBER G. A. Fat metabolism in higher plants. IX. Enzymic synthesis of long chain fatty acids by avocado particles. J Biol Chem. 1957 Jul;227(1):407–417. [PubMed] [Google Scholar]
- STUMPF P. K., JAMES A. T. The biosynthesis of long-chain fatty acids by lettuce chloroplast preparations. Biochim Biophys Acta. 1963 Feb 19;70:20–32. doi: 10.1016/0006-3002(63)90715-7. [DOI] [PubMed] [Google Scholar]


