Abstract
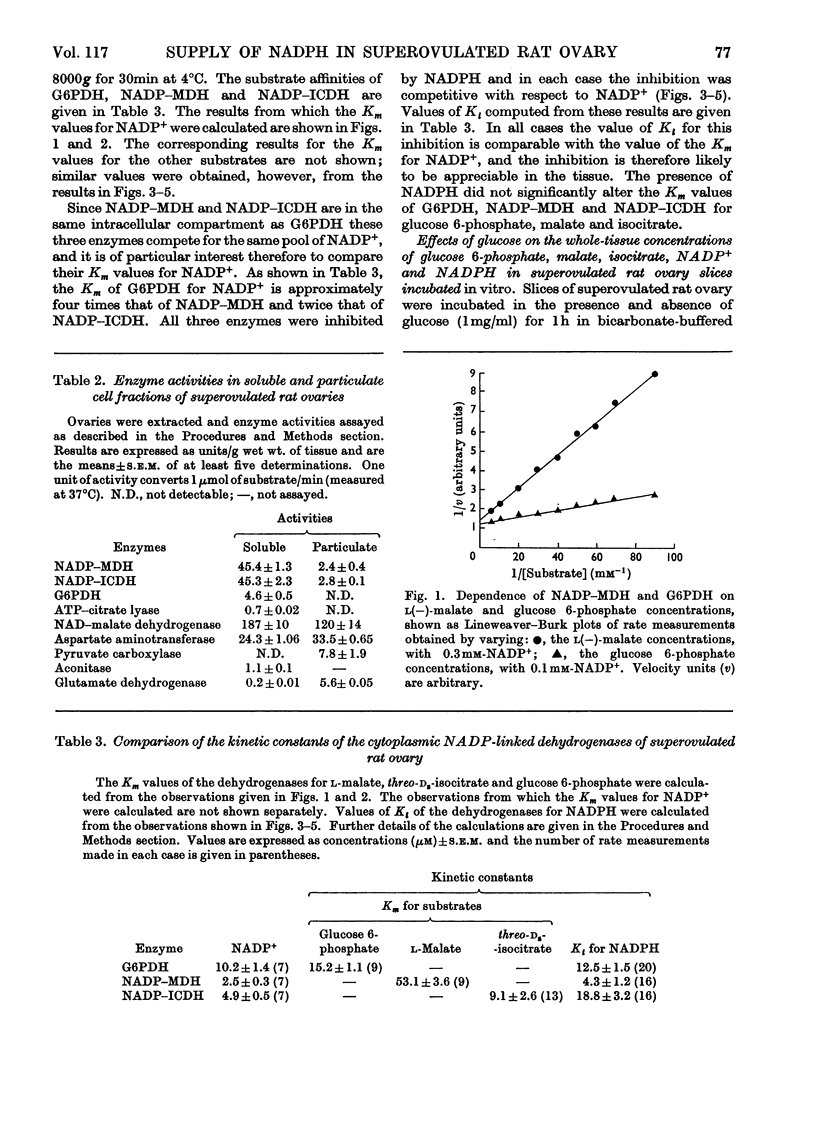
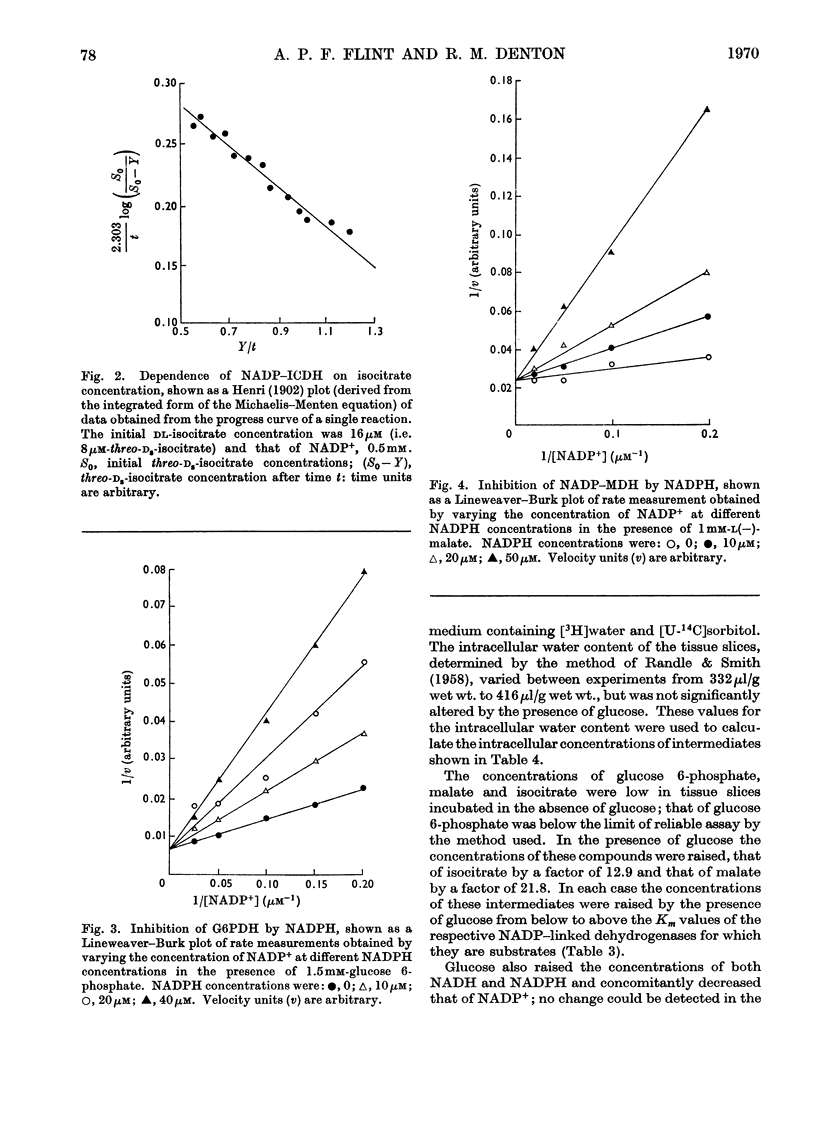
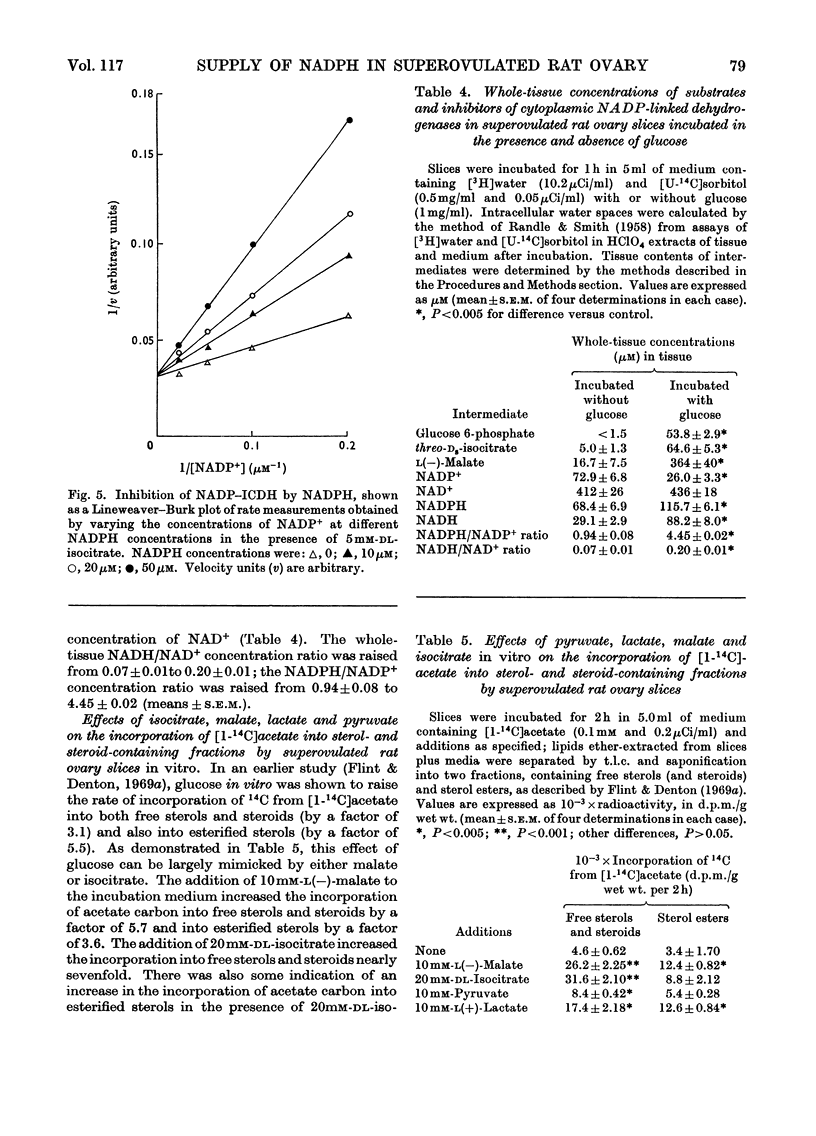
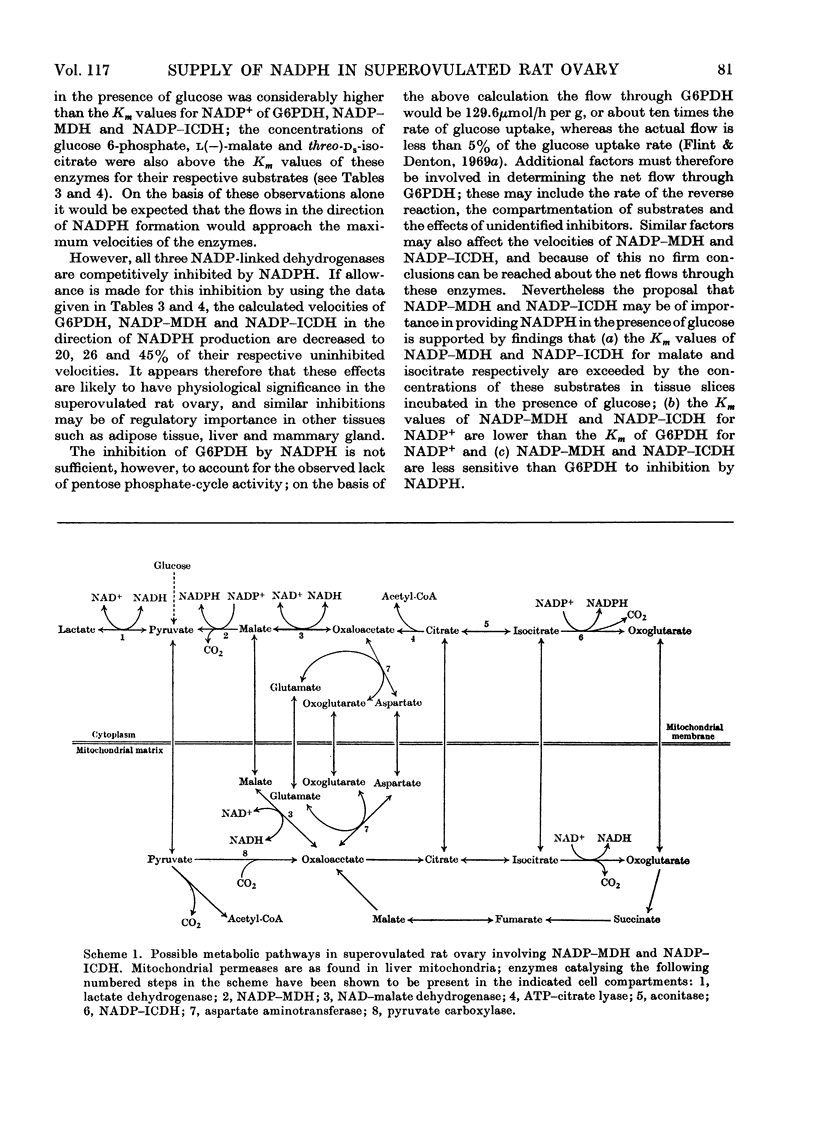
1. Superovulated rat ovary was found to contain high activities of NADP–malate dehydrogenase and NADP–isocitrate dehydrogenase. The activity of each enzyme was approximately four times that of glucose 6-phosphate dehydrogenase and equalled or exceeded the activities reported to be present in other mammalian tissues. Fractionation of a whole tissue homogenate of superovulated rat ovary indicated that both enzymes were exclusively cytoplasmic. The tissue was also found to contain pyruvate carboxylase (exclusively mitochondrial), NAD–malate dehydrogenase and aspartate aminotransferase (both mitochondrial and cytoplasmic) and ATP–citrate lyase (exclusively cytoplasmic). 2. The kinetic properties of glucose 6-phosphate dehydrogenase, NADP–malate dehydrogenase and NADP–isocitrate dehydrogenase were determined and compared with the whole-tissue concentrations of their substrates and NADPH; NADPH is a competitive inhibitor of all three enzymes. The concentrations of glucose 6-phosphate, malate and isocitrate in incubated tissue slices were raised at least tenfold by the addition of glucose to the incubation medium, from the values below to values above the respective Km values of the dehydrogenases. Glucose doubled the tissue concentration of NADPH. 3. Steroidogenesis from acetate is stimulated by glucose in slices of superovulated rat ovary incubated in vitro. It was found that this stimulatory effect of glucose can be mimicked by malate, isocitrate, lactate and pyruvate. 4. It is concluded that NADP–malate dehydrogenase or NADP–isocitrate dehydrogenase or both may play an important role in the formation of NADPH in the superovulated rat ovary. It is suggested that the stimulatory effect of glucose on steroidogenesis from acetate results from an increased rate of NADPH formation through one or both dehydrogenases, brought about by the increases in the concentrations of malate, isocitrate or both. Possible pathways involving the two enzymes are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG D. T., GREEP R. O. Effect of gonadotrophic hormones on glucose metabolism by luteinized rat ovaries. Endocrinology. 1962 May;70:701–710. doi: 10.1210/endo-70-5-701. [DOI] [PubMed] [Google Scholar]
- Brown J., McLean P., Greenbaum A. L. Influence of thyroxine and luteinizing hormone on some enzymes concerned with lopogenesis in adipose tissue, testis and adrenal gland. Biochem J. 1966 Oct;101(1):197–203. doi: 10.1042/bj1010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. B., Robinson B. H. Penetration of the mitochondrial membrane by tricarboxylic acid anions. Biochem Soc Symp. 1968;27:123–133. [PubMed] [Google Scholar]
- ESTABROOK R. W., MAITRA P. K. A fluorimetric method for the quantitative microanalysis of adenine and pyridine nucleotides. Anal Biochem. 1962 May;3:369–382. doi: 10.1016/0003-2697(62)90065-9. [DOI] [PubMed] [Google Scholar]
- Eckstein B., Villee C. A. Effect of estradiol on enzymes of carbohydrate metabolism in rat uterus. Endocrinology. 1966 Feb;78(2):409–411. doi: 10.1210/endo-78-2-409. [DOI] [PubMed] [Google Scholar]
- FRIEDEN C. Glutamic dehydrogenase. I. The effect of coenzyme on the sedimentation velocity and kinetic behavior. J Biol Chem. 1959 Apr;234(4):809–814. [PubMed] [Google Scholar]
- Flint A. P., Denton R. M. Glucose metabolism in the superovulated rat ovary in vitro. Effects of luteinizing hormone and the role of glucose metabolism in steroidogenesis. Biochem J. 1969 Apr;112(2):243–254. doi: 10.1042/bj1120243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A. P., Denton R. M. Metabolism of endogenous sterol ester by the superovulated rat ovary in vitro. Biochem J. 1970 Jan;116(1):79–82. doi: 10.1042/bj1160079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASER L., BROWN D. H. Purification and properties of d-glucose-6-phosphate dehydrogenase. J Biol Chem. 1955 Sep;216(1):67–79. [PubMed] [Google Scholar]
- Heldt H. W., Greif N., Klingenberg M., Scholz R., Panten U., Grunst J., Bücher T. On the problem of acid-labile triphosphopyridine nucleotide in biological material. J Biol Chem. 1965 Dec;240(12):4659–4661. [PubMed] [Google Scholar]
- KARMEN A. A note on the spectrometric assay of glutamic-oxalacetic transaminase in human blood serum. J Clin Invest. 1955 Jan;34(1):131–133. [PubMed] [Google Scholar]
- Kidwell W. R., Balogh K., Jr, Wiest W. G. Effects of luteinizing hormones on glucose-6-phosphate and 20-alpha-hydroxysteroid dehydrogenase activities in superovulated rat ovaries. Endocrinology. 1966 Aug;79(2):352–361. doi: 10.1210/endo-79-2-352. [DOI] [PubMed] [Google Scholar]
- Kornacker M. S., Ball E. G. Citrate cleavage in adipose tissue. Proc Natl Acad Sci U S A. 1965 Sep;54(3):899–904. doi: 10.1073/pnas.54.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWENSTEIN J. M. The pathway of hydrogen in biosyntheses. I. Experiments with glucose-1-H3 and lactate-2-H3. J Biol Chem. 1961 May;236:1213–1216. [PubMed] [Google Scholar]
- LOWENSTEIN J. M. The pathway of hydrogen in biosyntheses. II. Extramitochondrial isocitrate dehydrogenase. J Biol Chem. 1961 May;236:1217–1219. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., ROCK M. K. The stability of pyridine nucleotides. J Biol Chem. 1961 Oct;236:2756–2759. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luzzatto L. Regulation of the activity of glucose-6-phosphate dehydrogenase by NADP+ and NADPH. Biochim Biophys Acta. 1967 Sep 12;146(1):18–25. doi: 10.1016/0005-2744(67)90069-1. [DOI] [PubMed] [Google Scholar]
- PANDE S. V., KHAN R. P., VENKITASUBRAMANIAN T. A. NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE-SPECIFIC DEHYDROGENASES IN RELATION TO LIPOGENESIS. Biochim Biophys Acta. 1964 Jun 15;84:239–250. doi: 10.1016/0926-6542(64)90053-8. [DOI] [PubMed] [Google Scholar]
- Péron F. G., Caldwell B. V. Further studies on corticosteroidogenesis. V. 11 Beta-hydroxylation of deoxycorticosterone by mitochondria incubated with malate, supernatant fraction and supernatant fraction+pyruvate+CO2. Biochim Biophys Acta. 1968 Oct 22;164(2):396–411. doi: 10.1016/0005-2760(68)90164-1. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., SMITH G. H. Regulation of glucose uptake by muscle. 2. The effects of insulin, anaerobiosis and cell poisons on the penetration of isolated rat diaphragm by sugars. Biochem J. 1958 Nov;70(3):501–508. doi: 10.1042/bj0700501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES E. D., HUGGINS C. Steroid influences on respiration, glycolysis, and levels of pyridine nucleotide-linked dehydrogenases of experimental mammary cancers. Cancer Res. 1960 Jul;20:963–971. [PubMed] [Google Scholar]
- Rognstad R., Katz J. The balance of pyridine nucleotides and ATP in adipose tissue. Proc Natl Acad Sci U S A. 1966 May;55(5):1148–1156. doi: 10.1073/pnas.55.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVARD K., MARSH J. M., HOWELL D. S. PROGESTERONE BIOSYNTHESIS IN LUTEAL TISSUE: ROLE OF NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE AND NADP-LINKED DEHYDROGENASES. Endocrinology. 1963 Nov;73:554–563. doi: 10.1210/endo-73-5-554. [DOI] [PubMed] [Google Scholar]
- SRERE P. A. The citrate cleavage enzyme. I. Distribution and purification. J Biol Chem. 1959 Oct;234:2544–2547. [PubMed] [Google Scholar]
- Sanwal B. D., Smando R. Malic enzyme of Escherichia coli. Diversity of the effectors controlling enzyme activity. J Biol Chem. 1969 Apr 10;244(7):1817–1823. [PubMed] [Google Scholar]
- Schwert G. W. Use of integrated rate equations in estimating the kinetic constants of enzyme-catalyzed reactions. J Biol Chem. 1969 Mar 10;244(5):1278–1284. [PubMed] [Google Scholar]
- Simpson E. R., Estabrook R. W. Mitochondrial malic enzyme: the source of reduced nicotinamide adenine dinucleotide phosphate for steroid hydroxylation in bovine adrenal cortex mitochondria. Arch Biochem Biophys. 1969 Jan;129(1):384–395. doi: 10.1016/0003-9861(69)90190-8. [DOI] [PubMed] [Google Scholar]
- Stansfield D. A., Flint A. P. The entry of ascorbic acid into the corpus luteum in vivo and in vitro and the effect of luteinizing hormone. J Endocrinol. 1967 Sep;39(1):27–35. doi: 10.1677/joe.0.0390027. [DOI] [PubMed] [Google Scholar]
- WISE E. M., Jr, BALL E. G. MALIC ENZYME AND LIPOGENESIS. Proc Natl Acad Sci U S A. 1964 Nov;52:1255–1263. doi: 10.1073/pnas.52.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG J. W., SHRAGO E., LARDY H. A. METABOLIC CONTROL OF ENZYMES INVOLVED IN LIPOGENESIS AND GLUCONEOGENESIS. Biochemistry. 1964 Nov;3:1687–1692. doi: 10.1021/bi00899a015. [DOI] [PubMed] [Google Scholar]


