Abstract
Hydrocarbon fuels are widely recognized as significant contributors to climate change and the rising levels of CO2 in the atmosphere. As a result, it is crucial to reduce the net carbon intensity of energy derived from these fuels. This study explores the feasibility of using dimethyl ether (DME), produced through the hydrogenation of CO2, as a low-carbon method for generating electricity from hydrocarbon fuels. The proposed approach involves capturing the emitted CO2 during combustion and utilizing it to produce the necessary DME in a closed cycle. It is shown that for a mature reservoir in the Middle East, this method can mitigate approximately 75% of the CO2 emissions released from burning the produced oil. By incorporating zero-carbon electricity throughout the process, the total abatement of CO2 can reach 85%. Furthermore, the study highlights the importance of improving the DME utilization factor (bbl-oil/tDME). By optimizing this factor, high abatement rates can be achieved. However, it is important to note that implementing this method comes with a high exergetic cost. During a certain period in the field’s lifetime, the invested energy exceeds the energy produced. The stages with the highest exergy consumption are CO2 capture and hydrogen production.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-87981-x.
Subject terms: Environmental impact, Carbon capture and storage, Fossil fuels
Introduction
Hydrocarbon fuels currently dominate the global energy supply and remain the primary choice for transportation. In the absence of major technological advancements, hydrocarbon fuels are expected to continue meeting a significant portion of the growing energy demand in the near future. However, the combustion of hydrocarbons to release their chemical energy leads to the release of substantial quantities of carbon dioxide (CO2) into the atmosphere, which is the primary driver of climate change and global warming. Additionally, the extraction of hydrocarbons from subsurface reservoirs necessitates energy, and the energy requirements escalate as the producing fields age. Consequently, the resulting CO2 emissions from these operations contribute to over 20% of the total emissions in prominent oil-producing countries such as Russia, Norway, and Canada1,2.
Considering the urgent need to limit global temperature rise to below 2 °C above pre-industrial levels, the reduction of CO2 emissions has become a matter of utmost importance. To achieve this, it is crucial to develop innovative production schemes that can effectively decrease the CO2 intensity of energy (defined as the mass of CO2 emitted to atmosphere per unit energy, or gr-CO2/MJ) derived from hydrocarbons. These efforts are essential until alternative low-carbon energy sources can be scaled up and widely adopted. To accomplish this objective, it is necessary to integrate low-carbon energy sources into existing hydrocarbon production systems. Additionally, the utilization of materials or processes with minimal environmental impact can contribute to reducing CO2 emissions from hydrocarbon-based energy production3.
Enhanced oil recovery (EOR) methods, typically employed during the later stages of hydrocarbon reservoir life, offer the potential to produce oil with lower carbon intensity (measured in g-CO2/bbl oil) by optimizing the utilization factor of injectants, defined as the mass or volume of injected fluids required to produce a unit volume of oil4,5. These methods involve the injection of various substances such as chemicals (e.g., surfactants and polymers), gases (CO2, CH4, steam), and solvents6.
EOR techniques utilizing anthropogenic CO2 captured from concentrated sources like power plants or directly from the air appear particularly attractive. This approach has the potential to reduce both the net CO2 intensity of oil production and the costs associated with subsurface CO2 storage7. However, the efficiency of CO2 EOR, from an oil extraction perspective, relies on the development of miscibility between the injected CO2 and the oil within the porous reservoir media. Achieving miscibility typically requires high pressures, making CO2 injection into shallower reservoirs less economically viable. Moreover, the efficiency of CO2 in extracting oil diminishes with increasing reservoir heterogeneity, characterized by variations in permeability, leading to premature breakthrough of the injected CO2 in production wells8. To overcome these limitations, the injection of solvents in combination with gas or water has been explored9. However, it is crucial to ensure that the selected solvent does not contribute to the overall CO2 footprint of hydrocarbon fuels.
A recent advancement in EOR techniques involves the utilization of dimethyl ether (DME) as an EOR agent. DME exhibits high solubility in water and demonstrates first contact miscibility with hydrocarbons10,11. The chemical properties of DME are summarized in Table A.1. in the appendix. DME is dissolved in water and injected into the oil-bearing reservoir and therefore the process is referred to as DME-enhanced waterflooding (DEW). Within the reservoir pores, DME exhibits a strong affinity for oil, leading to its partitioning into the trapped oil phase. This process effectively reduces the viscosity of the oil and increases the volume of the oleic phase12,13. These combined effects ultimately result in an enhanced oil recovery and a reduction in the remaining oil saturation10,14. The economic viability of this process is heavily dependent on the volume of injected DME that can be recycled15,16. Despite the potential benefits, the large-scale application of DME-EOR has not yet been realized, primarily due to the high cost associated with DME. Nevertheless, studies have advanced the understanding of the phase behavior of Dimethyl Ether (DME) in contact with oil and water17, its impact on relative permeability18, and the development of efficient simulation methods12,13,19. Moreover, DME has been proposed as an efficient additive to steam to enhance production of heavy oils under gravity drainage20–22. Conventional methods for manufacturing DME typically rely on natural gas or coal as feedstock to produce syngas, which is then used to produce DME. However, the generation of syngas is both energy-intensive and costly. To simplify the process and reduce costs, this step should be eliminated23. An innovative alternative is the hydrogenation of CO2, which allows to produce methanol using either a homogeneous or heterogeneous catalyst24. The necessary hydrogen can be obtained through water electrolysis using renewable energy sources, while the required CO2 can be captured from industrial plant emissions (such as cement plants or power plants) or directly from the air. Methanol production follows the Eq. (1):
| 1 |
The methanol produced is subsequently dehydrated to obtain DME through a process known as indirect DME synthesis. This method of methanol production has already been implemented in several countries, including Japan, where commercial plants have been established23,24. Additionally, DME can also be produced through direct catalytic CO2 hydrogenation25,26.
| 2 |
The method works by using hybrid catalysts consisting of a methanol synthesis catalyst and a dehydration catalyst25.
The purpose of this study is to assess the feasibility of using CO2 hydrogenation-derived DME injection as a carbon-neutral method for oil recovery. This paper differs from previous studies related to DME-enhanced waterflooding in two ways. Firstly, the focus of this paper is to investigate the use of renewable DME in the process with the aim to abate CO2 released from produced oil. Secondly, this paper presents an integrated view of the surface and subsurface components of the process and aims to identify the key parameters that contribute to the sustainability of the process. To achieve this, a comprehensive life cycle assessment of the DEW injection process is conducted, considering the principles of the second law of thermodynamics. Additionally, the concept of exergy-return on exergy-investment (ERoEI) is employed to determine the exergetic efficiency and CO2 intensity of the oil produced through the DEW process.
Methodology
To conduct a life cycle exergy analysis, it is essential to identify the primary work and material streams involved3,27. Work streams refer to the components that consume exergy and are therefore the primary sources of CO2 emissions in the process. Typically, the analysis focuses on the main contributors to simplify the assessment. On the other hand, material streams are the components that generate exergy. This exergy can be in the form of electricity (in power plants), chemical exergy (in fuels), or heat. In the case of the DME-EOR process, two indicators are utilized to evaluate the exergy efficiency. The exergy-return on exergy-investment (ERoEI), and the exergy recovery factor, 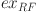 , defined as
, defined as
| 3 |
| 4 |
In hydrocarbon recovery techniques, the chemical exergy of the produced hydrocarbons (oil and gas) serves as the exergy of the fuel. The volumes of these hydrocarbons are determined through numerical or analytical models. Subsequently, the chemical exergy of the produced hydrocarbons is converted into electricity or heat considering the conversion efficiency of hydrocarbon-fueled power plants or boilers. Calculating the invested exergy involves determining the amounts of exergy and material consumed during the production of hydrocarbons. The volumes of injected fluids (such as water, solvents, and gas) and the materials (e.g., chemicals or solvents) are obtained from simulations. The invested exergy can be in the form of electricity or material. For instance, the injection of fluids requires electric-driven equipment like pumps or compressors. The CO2 emissions associated with this equipment depend on the power source and the conversion efficiency of the power generator. When chemicals such as polymers or surfactants are used to enhance oil production, the conversion of exergy to CO2 emissions is more complex. These chemicals often possess a significant embodied chemical exergy. However, since they are not oxidized and are typically retained in the reservoir, their primary contribution to the CO2 footprint of the oil recovery process arises from their manufacturing and transportation stages.
DME-based enhanced oil recovery
The proposed DME-based Enhanced Oil Recovery (EOR) system, aimed at producing low-carbon oil, is illustrated in Fig. 1. The system begins by capturing CO2 emissions from oil-fueled power plants but can also be applied to CO2 from other sources. To facilitate the hydrogenation reaction (Eq. 2), the required hydrogen is obtained through water electrolysis, powered by renewable or low-carbon electricity sources, such as solar electricity in this case. A portion of the produced DME is utilized to generate more oil, albeit with reduced CO2 intensity. The excess or recycled DME can be reinjected into nearby fields or used for various purposes, including electricity generation, methanol production, or the production of light olefins like propylene or ethylene. Alternatively, it can be used as a replacement fuel for diesel. In this analysis, any additional DME is converted to electricity using fuel cells. The produced oil is transported to an oil-fueled power plant, where it is utilized to generate electricity.
Fig. 1.
Schematic of the considered system for the DME-enhanced waterflooding or DME EOR process. The exergy consumed is calculated for each process. All the red squares are CO2 emitters.
Material stream
The material stream associated with the DME-EOR process consists of the produced oil and gas, as well as DME. In this study we assume that the main exergy source is produced oil. The chemical exergy of the crude oil is dependent on its composition. In the case of a light oil with a composition as reported in Farajzadeh et al., 2020, the estimated chemical exergy is 45.63 MJ/kg-oil. The chemical exergy values for natural gas (CH4) and DME are considered to be 52 MJ/kg-CH4 and 30.57 MJ/kg-DME, respectively28.
Work stream
Based on Fig. 1, the DME-EOR process involves several key work streams. These include hydrogen production, CO2 capture and transportation, DME synthesis, injection of DME/water mixture, lifting of produced fluids, transportation of produced oil to the power plant, and the work required for electricity generation.
Hydrogen production: The exergy of hydrogen production through water electrolysis has been reported to range from 180 to 220 MJe/kg-H2, with a CO2 intensity of 0.7–2.0 kg-CO2/kg-H229,30. In this study, the exergy of DME synthesis includes the exergy of hydrogen production.
DME synthesis: The exergy of DME manufacturing via CO2 hydrogenation is estimated to be 74 MJe/kg-DME31,32. This value does not account for the exergy of CO2 capture and transportation. It is assumed that DME is produced near the field site, thus neglecting the transportation exergy of DME.
CO2 capture and transport: Power plants with high CO2 concentrations typically use monoethanolamine (MEA) as a solvent to absorb CO2 from flue gas. This process requires 3.5-6 MJe/kg-CO2, with a capturing efficiency of 80–95%33,34. It is assumed that the capturing energy is supplied by the power plant fueled by the oil produced from the DME-EOR process. The overall process efficiency of the oil-fueled power plants is assumed to be 40%35.
Renewable electricity: When specified, low-carbon electricity is assumed to be sourced from solar power plants with a specific CO2 emission of 12.5 gr-CO2/MJe36.
Other processes: It is assumed that an additional 10% exergy investment is required for processes such as fluid separation, well stimulation, gas processing, and pigging of pipelines, which are not considered in this study37,38.
Efficiency of DME fuel cells: Fuel cells directly convert the chemical exergy of fuels like H2 and DME into electricity. The conversion efficiency of DME fuel cells is like that of hydrogen fuel cells, ranging from 40 to 85%39, with an average practical conversion efficiency of 70%.
In summary, for the DME-EOR system depicted in Fig. 1, the terms in Eqs. (3) and (4) are defined as follows:
| 5 |
| 6 |
| 7 |
where  is the chemical exergy of the oil,
is the chemical exergy of the oil,  is the chemical exergy of DME,
is the chemical exergy of DME,  is the exergy of DME manufacturing,
is the exergy of DME manufacturing, 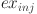 is the exergy of injection pumps,
is the exergy of injection pumps,  is the exergy of water treatment,
is the exergy of water treatment, 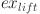 is the exergy of lift pumps,
is the exergy of lift pumps, 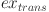 is the exergy of oil transportation to the refinery,
is the exergy of oil transportation to the refinery,  is the exergy of CO2 capture from the oil-fueled power plant, and
is the exergy of CO2 capture from the oil-fueled power plant, and 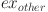 is the exergy of the other processes (assumed to be 10% of the total exergy invested).
is the exergy of the other processes (assumed to be 10% of the total exergy invested). 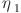 and
and 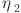 are the conversion efficiency of oil and DME to electricity, i.e., the conversion efficiency of the power plant and the DME fuel cell, respectively. Specific chemical exergy values of oil, methane, and DME are assumed to 45.63, 52.0, and 30.75 MJ/kg, respectively.
are the conversion efficiency of oil and DME to electricity, i.e., the conversion efficiency of the power plant and the DME fuel cell, respectively. Specific chemical exergy values of oil, methane, and DME are assumed to 45.63, 52.0, and 30.75 MJ/kg, respectively.
The work stream and the main assumptions of the calculations are summarized in Table 1, respectively.
Table 1.
| Work stream | |
|---|---|
| DME synthesis | Exergy: 74 MJe/kg-DME |
| CO2 capture and transport |
MEA-solvent: 4.0 MJ/kg-CO2 with capture efficiency of 90% |
|
Conversion efficiency of gas power plant |
40% |
|
Conversion efficiency of DME fuel cell |
70% |
| Water treatment | Exergy: 18 (5 kWh/m3) |
| Injection pumps | Exergy rate: 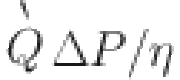
|
| Lift pumps |

|
Average overall pump efficiency, 
|
0.36 |
| Transportation of oil | 188 Je/kg/km |
| Distance to power plant | 500 km |
| Specific CO2 emission for gas | 0.055 kg-CO2/MJ |
| Specific CO2 emission for oil | 0.073 kg-CO2/MJ |
| Specific CO2 emission for solar electricity | 0.0125 kg-CO2/MJe |
| Water density | 1000 kg/m3 |
| Oil density | 885 kg/m3 |
| DME density | 668 kg/m3 (@ 25 °C) |
| Injection pressure | Reservoir pressure + 50 bar |
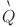 (m3/s): flow rate,
(m3/s): flow rate,  (Pa): pressure,
(Pa): pressure,  (-): pump efficiency,
(-): pump efficiency,  (kg/m3): density, g (m/s2): acceleration due to gravity, h (m): reservoir depth.
(kg/m3): density, g (m/s2): acceleration due to gravity, h (m): reservoir depth.
Injection and production data
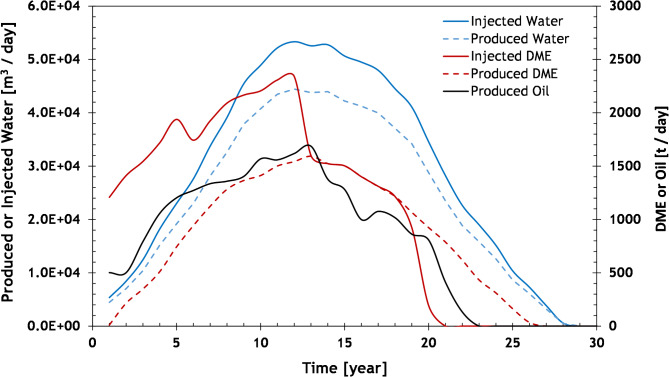
The work and material stream calculations are derived from the injection and production histories of the DME EOR process in a field located in the Middle East. These histories are obtained through numerical simulations with parameters calibrated to experimental data. Further details can be found in refs10,15,16. Figure 2 illustrates the total volumes of injected and produced fluids.
Fig. 2.
The simulated history of the injection and production fluids of the DME EOR for a reservoir in the Middle East.
Results and discussion
Producible DME volume
In the DME EOR process, it is common to inject significant volumes of solvents into subsurface formations to displace and recover oil. Figure 2 presents the oil production data, which is utilized to assess whether the produced oil from the field, along with the resulting CO2 emissions from the power plant, can generate the necessary volume of DME required for the field operations. To determine the amount of CO2 generated by burning oil, a stoichiometric balance is employed. Oil is assumed to be CH2 with the combustion reaction:
| 8 |
According to Eq. (8), burning 1 kg of CH2 releases 3.14 kg of CO2. With a CO2 capturing efficiency of 90%, 10% of the produced CO2 in the power plant will be emitted to the atmosphere. According to Eq. (2), 1.91 kg of CO2 is needed to produce 1 kg of DME, therefore, the minimum amount of the manufactured DME per barrel of oil can be calculated as
| 9 |
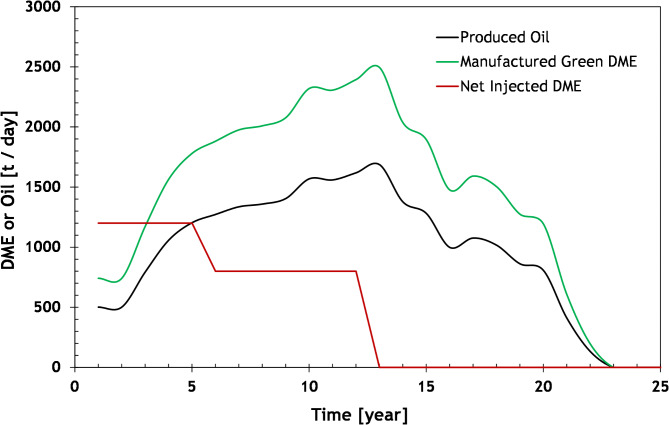
Based on the analysis, it is observed that approximately 0.21 tons of DME (equivalent to 1.48 kg-DME/kg-oil) can be produced from every barrel of oil burned in the power plants. Figure 3 illustrates the total producible DME using the hydrogenation method, considering the oil production data presented in Fig. 2. Except for the first two years, the DME production is sufficient to meet the field’s requirements. The surplus DME can be utilized in other fields or for various purposes, such as electricity generation, methanol production, or transportation.
Fig. 3.
Oil Production, manufactured DME, and net DME Injection (injected–back produced) rates as a function of time using the field data shown in Fig. 2. In the calculation it has been assumed that 0.21 tDME/bbl-oil can be produced (see Eq. 9).
Power requirement to manufacture DME
The estimated maximum installed capacity needed to supply the power required for producing approximately 3000 tons per day of DME (as shown in Fig. 3) is around 2.5 GW, based on an energy requirement of 74 MJe/kg-DME. In recent years, solar and wind power have accounted for over 60% of the installed capacity from renewable energy sources, which amounts to around 3300 GW globally42. This growth has been primarily driven by significant investments in solar power. In the Middle East and North Africa region, where there is abundant sunlight, there has been a notable increase in solar PV investments. As of 2022, approximately 16 GW of power is produced using solar PV in the Middle East, and this number is expected to rise rapidly to over 35 GW by 202642.
However, a challenge lies in obtaining the required capacity from a single solar farm. Currently, the largest solar farm in the UAE has an installed capacity of around 1.0 GW, which is projected to increase to 5 GW by 2030. While the capacity in the region is expected to grow, meeting the energy requirements of the DME EOR project would likely necessitate sourcing from multiple solar farms. Furthermore, it is important to note that the estimated installed capacity of 2.5 GW assumes that all the captured CO2 is used for DME production, which exceeds the requirements of the field. Depending on availability, a lower installed capacity may be sufficient to meet the field’s needs. For instance, to produce the net DME requirement of 1200 tons per day (as shown in Fig. 3), an installed capacity of 1 GW would be necessary. Alternatively, dividing the DME EOR project into several smaller projects could be considered to reduce the size of the initial investments.
Exergy balance
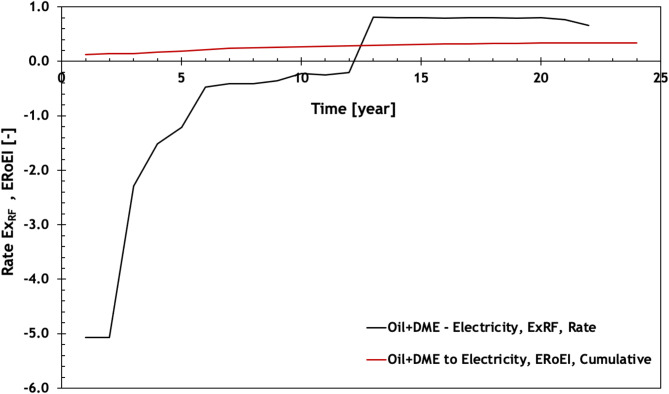
This section presents the findings of the exergy analysis conducted on the DME-EOR process with the system depicted in Fig. 1 and input parameter in Table 1. Figure 4 depicts the history of the exergy-recovery-factor (ExRF) for the scenario where the produced oil and excess DME are converted into electricity. The injection of DME leads to additional oil production until year 13; however, this necessitates the manufacturing of large volumes of DME. Due to the significant exergy involved in DME synthesis, the amount of invested exergy is considerably greater than the recovered exergy. Consequently, the overall ExRF for the process is negative.
Fig. 4.
Rate exergy recovery factor (defined in Eq. 3) and cumulative ERoEI as functions of time for the case where the produced oil and the excess DME are converted to electricity. The resulting CO2 assumed to be captured.
Nevertheless, after year 13, the ExRF starts to increase and becomes positive as the volume of back-produced DME is sufficient to meet the injection requirements. By the end of year 20, there is no DME injection or oil production, resulting in no exergy being used for DME synthesis. The injected DME is fully recovered even after oil production ceases. The cumulative Energy Return on Energy Invested (ERoEI) for the entire process reaches 0.34 at the project’s conclusion, indicating an overall exergetic efficiency of 34%, as depicted in Fig. 4.
Figure 5 illustrates the distribution of the invested exergy among different components of the system depicted in Fig. 1. As shown in Fig. 5, during the initial phase of the project, the exergy required for DME synthesis accounts for approximately 80% of the total invested exergy until year 13 when the net injected DME reaches zero. During this period, CO2 capture consumes less than 20% of the total invested exergy. However, once the back-produced DME from the reservoir is sufficient to sustain oil recovery, the exergy investment in CO2 capture and transport becomes the largest. This trend continues until year 20 when oil production ceases, resulting in no CO2 emissions and, consequently, no exergy required for CO2 capture.
Fig. 5.
Fractions of the invested exergy in different Components of the considered system in Fig. 1.
After this point, the lift and injection pumps become the primary exergy consumers, accounting for approximately 50% and 30% of the total exergy investment, respectively. It is important to note that after oil production stops, water is injected to recover the retained DME in the reservoir. The contribution of oil transportation to the overall exergy invested is minimal and does not have a significant impact. Figure 5 also reveals a general increase in the exergy investment associated with oil and water. This is attributed to the increasing oil production over time. Additionally, the exergy investment related to lift and injection pumps and water treatment increases due to the larger volume of injected water required for back-producing the injected DME. However, the magnitude of this increase is not comparable to the exergy invested in DME synthesis and CO2 capture from the power plant. The corresponding Sankey diagram of the process is plotted in Fig. 6 for year 5 of the injection.
Fig. 6.
Sankey diagram of the DME EOR process at time = 5 years.
Figure 6 further illustrates the CO2 intensity of the DME-EOR process over time, particularly in terms of specific emission reduction performance, measured in kg-CO2/bbl-oil. This includes CO2 emissions from DME synthesis, injection, and production operations, as well as the oil-fueled power plant. The reservoir oil has a calculated CO2 intensity of approximately 442 kg-CO2/bbl-oil. With a 90% CO2 capturing efficiency, the dashed red line in Fig. 6 represents the emission of 44.2 kg of CO2 for every barrel of oil consumed in the power plant. Most the total CO2 emissions is attributed to the manufacturing of the required DME, particularly in the early stages of the project. To reduce this emission, efforts can be made to lower the carbon footprint of the exergy needed for CO2 capture and H2 production. In this study, it is assumed that solar energy with a specific CO2 emission of 12.5 gr-CO2/MJe is used to supply the exergy for DME synthesis. The field equipment operates on electricity generated from gas turbines.
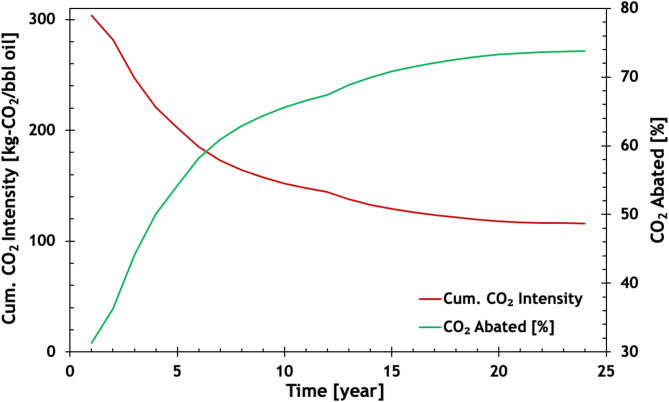
In summary, Fig. 7 presents the cumulative CO2 intensity of the process and the fraction of abated CO2 Over time, the cumulative CO2 intensity decreases, and by the end of the project, with the assumptions made in this paper, it is reduced to less than 50 kg-CO2/bbl-oil. This means that approximately 74% of the CO2 resulting from burning the produced oil is prevented from entering the atmosphere. If zero-carbon electricity is used throughout the entire process, the total abated CO2 can reach 85%.
Fig. 7.
CO2 intensity of the low-carbon DME-EOR process (Fig. 1) as a function of time.
Figure 8 presents a comparison of the CO2 intensity between the electricity generated in the system of Fig. 1 and the oil produced through water injection, with CO2 capture considered in both cases. Following refs.40,41. the CO2 emissions of the water-injection project is
Fig. 8.
Cumulative CO2 intensity of the low-carbon DME-EOR process and the percentage of the abated CO2. The CO2 intensity of burning oil is 442 kg-CO2/bbl oil.
| 10 |
Here, fw represents the overall water cut of the reservoir, and the factor a (kg-CO2/m3-water) represents the CO2 emission associated with handling the injected water. For water cuts of 95% and 98%, CO2 intensities of 25 and 64 kg-CO2/bbl oil are calculated, respectively. Assuming a 90% CO2 capture efficiency and a 40% power plant efficiency, the CO2 intensity of the electricity is calculated to be 30 and 47 g-CO2/MJe, respectively. It is worth noting that the emitted CO2 for the low-carbon DME EOR is lower (approximately 33% for fw = 0.95 and 65% for fw = 0.98) compared to the water flooding case. This difference is more pronounced in the initial stages of the project, as less energy is required for DME manufacturing due to lower oil production. The exergetic efficiency of the DME-EOR process starts off relatively low but reaches about 80% by the end of the project.
Figure 9 illustrates the relationship between the DME utilization factor (bbl-oil / tDME) and the total emitted CO2 per barrel of oil in the DME-EOR process. DME utilization factor is defined as the volume of oil produced per mass of DME injected. Utilization factor in (CO2 and chemical) enhanced oil recovery is a good indicator of the efficiency of injectants in extracting oil. The utilization factor usually.
Fig. 9.
CO2 intensity of the electricity generated from the system considered in Fig. 1. The black dashed line is the CO2 intensity of the oil production from waterflood project with 95% water cut. Both cases consider Carbon Capture and Storage (CCS) option. The red is the exergetic efficiency of the low-carbon DME EOR process.
The DME utilization factor is quantitatively defined as the volume of oil produced per unit mass of DME injected into the reservoir. The utilization factor is commonly used to evaluate efficiency of injectants in (CO2 and chemical) EOR projects and is influenced by the physicochemical properties of the reservoir, degree of reservoir heterogeneity, thermodynamic conditions (pressure, temperature, salinity), the type of injectant, the characteristics of the crude oil, the interaction between the injectant and the reservoir rock, and the operational parameters of the injection process.
The CO2 emissions considered in Fig. 10include the 10% of CO2 released from burning oil (with 90% of the released CO2 captured) as well as the CO2 emissions from DME synthesis and injection. As shown in this figure, the DME utilization factor significantly impacts the life-cycle analysis of the DME-EOR process, like the CO2 EOR process (Farajzadeh et al., 2020). Improving the DME utilization factor can be achieved by enhancing the mobility control between the injected DME solution and the in-situ oil. One approach to achieve this is by adding polymers into the DME solution9. Under favorable conditions, such as a DME utilization factor of 20 bbl/tDME, the total CO2 intensity of oil decreases to less than 30 kg-CO2/bbl oil. This indicates that more than 93% of the resulting CO2 from burning oil can be abated. This is a significant reduction compared to other EOR methods. In particular, with typical utilization factors of 2–4 bbl-oil/tCO2, CO2 EOR reduces CO2 intensity of oil by 75–80%7.
Fig. 10.

Total CO2 intensity of a barrel of oil produced as a function of DME utilization factor. The numbers include CO2 emissions from burning oil and CO2 released from synthesis of DME.
Conclusions
Indirect CO2 Enhanced Oil Recovery (EOR) is a promising approach that offers multiple benefits for oil production and carbon mitigation. It enables the utilization of CO2 that would otherwise be emitted into the atmosphere, thereby reducing greenhouse gas emissions. This paper evaluates the use of dimethyl-ether (DME), produced from anthropogenic CO2 and renewable or green hydrogen, as an EOR agent in a closed system. Our analysis demonstrates several advantages of the proposed indirect CO2 EOR method, including CO2 capture and utilization, increased energy extraction, utilization of existing infrastructure, and reduced CO2 intensity. In the case studied, DME synthesis accounts for the largest fraction of the total exergy investment followed by CO2 capture, specially at the initial stages of the project. Furthermore, our findings indicate that the proposed indirect EOR method emits less CO2 compared to the water flooding process, with a difference of approximately 33% for a water cut of fw=0.95 and 65% for fw=0.98. This difference is more pronounced in the initial stages of the project due to lower energy requirements for DME manufacturing resulting from lower oil production. The CO2 intensity of the system is strongly influenced by the DME utilization factor, which can be improved by incorporating a mobility-control agent into the DME-water solution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge Shell Gloabl Solutions International B.V. for granting permission to publish this work.
Author contributions
R.F. developed the idea, performed the analysis, prepared figures, and wrote the initial draft of the paper. N.K.performed part of the analysis and reviewed the paper. D.S. performed the initial exergy analysis. S.M. and J.B. helped with interpretation of the results and reviewed the final version of the paper.
Data availability
The datasets used and/or analysed during the current study available from the correspond-ing author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Masnadi, M. S. & Brandt, A. R. Climate impacts of oil extraction increase significantly with oilfield age. Nat. Clim. Change7, 551. 10.1038/nclimate3347 (2017). [Google Scholar]
- 2.Gür, T. M. Carbon dioxide emissions, capture, storage and utilization: review of materials, processes and technologies. Prog. Energy Combust. Sci.89, 100965 (2022). [Google Scholar]
- 3.Farajzadeh, R. Sustainable production of hydrocarbon fields guided by full-cycle exergy analysis. J. Pet. Sci. Eng.181, 569. 10.1016/j.petrol.2019.106204 (2019).
- 4.Jaramillo, P., Griffin, W. M. & McCoy, S. T. Life Cycle Inventory of CO2 in an Enhanced oil Recovery System (ACS, 2009). [DOI] [PubMed]
- 5.Chaturvedi, K. R. & Sharma, T. Comparative analysis of carbon footprint of various CO2-enhanced oil recovery methods: a short experimental study. Chem. Eng. Commun.2023, 1–8 (2023).
- 6.Lake, L., Johns, R. T., Rossen, W. R. & Pope, G. A. Fundamentals of Enhanced oil Recovery (Society of Petroleum Engineers, 2014).
- 7.Farajzadeh, R., Eftekhari, A. A., Dafnomilis, G., Lake, L. W. & Bruining, J. On the sustainability of CO2 storage through CO2 enhanced oil recovery. Appl. Energy261, 114467 (2020). [Google Scholar]
- 8.Masalmeh, S. K. et al. EOR options for heterogeneous carbonate reservoirs currently under waterflood. In Paper presented at the Abu Dhabi International Petroleum Exhibition and Conference, November 10–13, SPE-171900-MS. 10.2118/171900-MS (2014).
- 9.Chahardowli, M., Farajzadeh, R. & Bruining, H. Experimental investigation of the use of the dimethyl ether/polymer hybrid as a novel enhanced oil recovery method. J. Ind. Eng. Chem.38, 50–60 (2016). [Google Scholar]
- 10.te Riele, P. et al. Implementing a water-soluble solvent based enhanced oil recovery technology—aspects of field development planning. In SPE EOR Conference at Oil and Gas West Asia. Muscat, Oman, 21–23 March (2016).
- 11.Parsons, C. et al. Introducing a novel enhanced oil recovery technology. In IOR 2017-19th European Symposium on Improved Oil Recovery (EAGE Publications BV, 2017).
- 12.Ratnakar, R., Dindoruk, B. & Wilson, L. Experimental investigation of DME–water–crude oil phase behavior and PVT modeling for the application of DME-enhanced waterflooding. Fuel182(Supplement C), 188–197. 10.1016/j.fuel.2016.05.096 (2016).
- 13.Ratnakar, R., Dindoruk, B. & Wilson, L. Use of DME as an EOR agent: experimental and modeling study to capture interactions of DME, brine and crudes at reservoir conditions. In SPE Annual Technical Conference and Exhibition. Dubai, UAE, 26–28 September (2016).
- 14.Chernetsky, A. et al. A novel enhanced oil recovery technique: experimental results and modelling workflow of the DME enhanced waterflood technology. In Abu Dhabi International Petroleum Exhibition and Conference. Abu Dhabi, UAE, 9–12 November (2015).
- 15.Groot, J. et al. Representation of phase behavior and PVT workflow for DME enhanced water-flooding. In SPE EOR Conference at Oil and Gas West Asia, OnePetro (2016).
- 16.Groot, J., Eikmans, D., Fadili, A. & Romate, J. Field-scale modelling and sensitivity analysis of DME enhanced waterflooding. In SPE EOR Conference at Oil and Gas West Asia, OnePetro (2016).
- 17.Chai, M., Chen, Z., Nourozieh, H., Yang, M. & Chai, B. Introduce dimethyl ether (DME) as a solvent for steam-assisted gravity drainage (SAGD) co-injection: an effective and environmental application. Fuel341, 69. 10.1016/j.fuel.2023.127639 (2023).
- 18.Mahdizadeh, M., Eftekhari, A. A. & Nick, H. M. Numerical modeling of water-soluble solvents for enhancing oil recovery in heterogeneous chalk reservoirs. J. Petrol. Sci. Eng.175, 681–692. 10.1016/j.petrol.2018.12.083 (2019). [Google Scholar]
- 19.Cho, J., Kim, T. H. & Lee, K. S. Compositional modeling and simulation of dimethyl ether (dme)-enhanced waterflood to investigate oil mobility improvement. Petrol. Sci.15(2), 297–304. 10.1007/s12182-017-0212-z (2018). [Google Scholar]
- 20.Sheng, K., Okuno, R. & Wang, M. Dimethyl ether as an additive to steam for improved steam-assisted gravity drainage. SPE J.23(4), 1201–1222. 10.2118/184983-pa (2018). [Google Scholar]
- 21.Pratama, R. A. & Babadagli, T. A review of the mechanics of heavy-oil recovery by steam injection with chemical additives. J. Petrol. Sci. Eng.208, 859. 10.1016/j.petrol.2021.109717 (2022).
- 22.Parsons, C. et al. Introducing a novel enhanced oil recovery technology. In SPE Improved Oil Recovery Conference. Tulsa, Oklahoma, USA, 11–13 April (2016).
- 23.Goeppert, A., Czaun, M., Jones, J. P., Surya Prakash, G. K. & Olah, G. A. Recycling of carbon dioxide to methanol and derived products—closing the loop. Chem. Soc. Rev.43(23), 7995–8048. 10.1039/C4CS00122B (2014). [DOI] [PubMed] [Google Scholar]
- 24.Olah, G. A. Towards oil independence through renewable methanol chemistry. Angew. Chem. Int. Ed.52(1), 104–107. 10.1002/anie.201204995 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Olah, G. A., Goeppert, A. & Prakash, G. K. S. Beyond oil and gas. In The Methanol Wconomy (eds. George, A. O. et al.) (Wiley-VCH, 2009).
- 26.Matzen, M. & Demirel, Y. Methanol and dimethyl ether from renewable hydrogen and carbon dioxide: Alternative fuels production and life-cycle assessment. J. Clean. Prod.139(Supplement C), 1068–1077. 10.1016/j.jclepro.2016.08.163. (2016).
- 27.Farajzadeh, R., Lomans, B. P., Hajibeygi, H. & Bruining, J. Exergy return on exergy investment and CO2 intensity of the underground biomethanation process. ACS Sustain. Chem. Eng.. 10.1021/acssuschemeng.2c02931 (2022).
- 28.Semelsberger, T. A., Borup, R. L. & Greene, H. L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources156(2), 497–511. 10.1016/j.jpowsour.2005.05.082 (2006). [Google Scholar]
- 29.Babacan, O. et al. Assessing the feasibility of carbon dioxide mitigation options in terms of energy usage. Nat. Energy5, 720–728 (2020). [Google Scholar]
- 30.Simpson, A. P. & Lutz, A. E. Exergy analysis of hydrogen production via steam methane reforming. Int. J. Hydrogen Energy32, 4811–4820 (2007). [Google Scholar]
- 31.Martín, M. Optimal year-round production of DME from CO2 and water using renewable energy. J. CO2 Utiliz.13(Supplement C), 105–113. 10.1016/j.jcou.2016.01.003 (2016).
- 32.Martín, M. Artificial versus natural reuse of CO2 for DME production: are we any closer? Engineering3(2),166–170. 10.1016/J.ENG.2017.02.002 (2017).
- 33.Eftekhari, A. A., van der Kooi, H. & Bruining, H. Exergy analysis of underground coal gasification with simultaneous storage of carbon dioxide. In The 24th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy, ECOS 2011, vol. 45 729–745 (2012). 10.1016/j.energy.2012.07.019.
- 34.Eftekhari, A. A. & Farajzadeh, R. Environmental and technical advantages and bottlenecks of carbon dioxide capture and storage from a thermodynamic perspective. In 35th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems (2022).
- 35.Nierop, S. & Humperdinck, S. International comparison of fossil power efficiency and CO2 Intensity—Update 2018; MRI Research Associates: Japan, accessed October 15. https://guidehouse.com/-/media/www/site/downloads/energy/2018/intl-comparison-of-fossil-power-efficiency--co2-in.pdf (2023).
- 36.De Chalendar, J. A. & Benson, S. M. Why 100% renewable energy in not enough? Joule3, 1389 –1393 (2019).
- 37.Patzek, T. W. Thermodynamics of the corn-ethanol biofuel cycle. Crit. Rev. Plant Sci.23, 6. 10.1080/07352680490886905 (2004).
- 38.Farajzadeh, R., Zaal, C., van den Hoek, P. & Bruining, J. Life-cycle assessment of water injection into hydrocarbon reservoirs using exergy concept. J. Clean. Prod.235, 812–821. 10.1016/j.jclepro.2019.07.034 (2019). [Google Scholar]
- 39.Fuel Cells Fact Sheet. US Department of Energy (DOE) (2023, accessed 15 Oct 2023). https://www.energy.gov/sites/prod/files/2015/11/f27/fcto_fuel_cells_fact_sheet.pdf.
- 40.Farajzadeh, R., Kahrobari, S., Eftekhari, A. A., Mjnei, R. A. & Boersma, D. Bruining Chemical enhanced oil recovery and the dilemma of more and cleaner energy. Sci. Rep.11, 859. 10.1038/s41598-020-80369-z (2021). [DOI] [PMC free article] [PubMed]
- 41.Farajzadeh, R. et al. Improved oil recovery techniques and their role in energy efficiency and reducing CO2 footprint of oil production. J. Clean. Prod.369, 133308. 10.1016/j.jclepro.2022.133308 (2022). [Google Scholar]
- 42.International Energy Agency. Renewable electricity. https://www.iea.org/reports/renewables-2021/renewable-electricity?mode=market®ion=Middle+East+and+North+Africa&publication=2021&product=Total (2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the correspond-ing author on reasonable request.