Significance
Itaconate is an abundant antimicrobial metabolite whose production is increased during macrophage activation. However, the exact mode of bacterial inhibition remains unclear. Here, we report that itaconate inhibits aldolase and inosine monophosphate (IMP) dehydrogenase from Mycobacterium tuberculosis. Studies have shown that bacterial pathogens encode for three enzymes that are involved in itaconate degradation, namely itaconate:succinyl-CoA transferase, itaconyl-CoA hydratase, and (S)-citramalyl-CoA lyase. Here, we have unambiguously identified IcT (Rv2503c and Rv3272) and IcH (Rv2499c and Rv3389c) enzymes from M. tuberculosis. We also show that the attenuated phenotype of ΔRv3389c strain of M. tuberculosis is due to disruption of the itaconate dissimilation pathway. Thus, these enzymes represent targets for developing antitubercular agents that sensitize M. tuberculosis against the host immune response.
Keywords: Mycobacterium tuberculosis, itaconate, dissimilation, pathogenesis
Abstract
Itaconate, an abundant metabolite produced by macrophages upon interferon-γ stimulation, possesses both antibacterial and immunomodulatory properties. Despite its crucial role in immunity and antimicrobial control, its mechanism of action and dissimilation are poorly understood. Here, we demonstrate that infection of mice with Mycobacterium tuberculosis increases itaconate levels in lung tissues. We also show that exposure to itaconate inhibits M. tuberculosis growth in vitro, in macrophages, and mice. We report that exposure to sodium itaconate (ITA) interferes with the central carbon metabolism of M. tuberculosis. In addition to the inhibition of isocitrate lyase (ICL), we demonstrate that itaconate inhibits aldolase and inosine monophosphate (IMP) dehydrogenase in a concentration-dependent manner. Previous studies have shown that Rv2498c from M. tuberculosis is the bona fide (S)-citramalyl-CoA lyase, but the remaining components of the pathway remain elusive. Here, we report that Rv2503c and Rv3272 possess itaconate:succinyl-CoA transferase activity, and Rv2499c and Rv3389c possess itaconyl-CoA hydratase activity. Relative to the parental and complemented strains, the ΔRv3389c strain of M. tuberculosis was attenuated for growth in itaconate-containing medium, in macrophages, mice, and guinea pigs. The attenuated phenotype of ΔRv3389c strain of M. tuberculosis is associated with a defect in the itaconate dissimilation and propionyl-CoA detoxification pathway. This study thus reveals that multiple metabolic enzymes are targeted by itaconate in M. tuberculosis. Furthermore, we have assigned the two remaining enzymes responsible for the degradation of itaconic acid into pyruvate and acetyl-CoA. Finally, we also demonstrate the importance of enzymes involved in the itaconate dissimilation pathway for M. tuberculosis pathogenesis.
Tuberculosis (TB) is an air-borne infectious disease caused by Mycobacterium tuberculosis. Approximately 25% of the individuals worldwide are latently infected with TB, and 5 to 10% of these individuals develop active TB during their lifetime (1). M. tuberculosis exhibits significant metabolic flexibility that enables it to persist inside host tissues (2, 3). Inside the host, M. tuberculosis preferentially utilizes host-derived fatty acids and cholesterol as nutrient sources through various pathways such as the glyoxylate shunt pathway, methylcitrate cycle, and methylmalonyl pathway (4–6). Macrophages are the main line of defense against infectious agents and secrete various antimicrobials such as reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI) to combat bacterial infections (7). Itaconate is another antimicrobial metabolite produced by macrophages in response to bacterial infection and upon stimulation with either lipopolysaccharide (LPS) or interferon-γ (IFN-γ) (8, 9). In mammals, the immune-responsive gene (Irg1) catalyzes the decarboxylation of cis-aconitate to produce itaconate (10). Previously, it has been shown that Irg1 levels increase upon infection with M. tuberculosis, and Irg1−/− mice are more susceptible to infection with M. tuberculosis compared to wild type mice (10–12). This increased susceptibility of the Irg1−/− mice was associated with neutrophil infiltration and lung damage (12).
Itaconate, an unsaturated dicarboxylic acid, has been shown to alkylate cysteine residues of several proteins such as kelch like ECH-associated protein 1 (KEAP1), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and gasdermin D (GSDMD) (13–15). Several studies have shown that itaconate regulates the levels of proinflammatory cytokines, mainly IL-1β, through various pathways, including Nrf2 activation, ATF3 activation, GAPDH inhibition, and inflammasome formation inhibition (13–16). It has also been reported that administration of 4-octyl itaconate (4-OI) in mice alleviates LPS-induced acute lung injury by reducing the accumulation of neutrophils, oxidative stress, and cytokines release (17). Itaconate has been shown to restrict the growth of several microorganisms, such as M. tuberculosis, Salmonella enterica, Pseudomonas indigofera, Yersinia pestis, and Staphylococcus aureus in nutrient-limiting conditions and nontuberculous mycobacteria in a pH-dependent manner (10, 18–21). Recently, it has also been shown that itaconate inhibits the growth of Vibrio sp. by disrupting the central carbon metabolism (22). The enzymatic activity associated with isocitrate lyase (ICL) enzymes from M. tuberculosis, P. indigofera, C. elegans, and Ascaris suum is inhibited by itaconate (20, 23–25). In M. tuberculosis, ICL performs the dual roles of ICL in the glyoxylate shunt pathway and methylisocitrate lyase (MCL) in the methylcitrate cycle and is required for the utilization of fatty acids as a carbon source. The MCL activity associated with ICL is responsible for propionyl-CoA detoxification and is required for the in vivo survival of M. tuberculosis (4, 26, 27). It has also been demonstrated that itaconyl-CoA forms a stable biradical with the 5′-deoxyadenosyl moiety of coenzyme B12, inactivates methylmalonyl-CoA mutase (MCM), and inhibits M. tuberculosis growth on propionate (28). Having an itaconate dissimilation pathway is a strategy adopted by several bacterial pathogens to degrade itaconate (29, 30). In the first step of the itaconate dissimilation pathway, itaconate is converted to itaconyl-CoA by an itaconate:succinyl-CoA transferase (IcT). Subsequently, itaconyl-CoA is converted to (S)-citramalyl CoA by itaconyl-CoA hydratase (IcH). In the last step of this pathway, (S)-citramalyl CoA is cleaved by (S)-citramalyl-CoA lyase (CcL) into acetyl-CoA and pyruvate (29, 31). Recently, it has been shown that Rv2498c is a bifunctional enzyme involved in both itaconate dissimilation and the L-leucine catabolism pathway (32). It has been demonstrated that Rv2498c possesses CcL activity and is essential for M. tuberculosis survival in mice (32). We also previously reported that M. tuberculosis strain harboring deletions in both Rv2498c and Rv3075c (CitE2) was attenuated for growth in THP-1 macrophages and guinea pigs compared to the parental strain (33).
In this study, we demonstrate that itaconate exposure inhibits M. tuberculosis growth in liquid cultures, THP-1 macrophages, and mice. Using an untargeted metabolomics approach, we show that exposure to itaconate perturbs central carbon metabolism in M. tuberculosis. We also report that in addition to ICL, itaconate binds and inhibits aldolase and inosine monophosphate (IMP) dehydrogenase in a concentration-dependent manner. This study shows that the M. tuberculosis genome possesses functional homologs for IcT and IcH. We further show that the IcH enzyme, Rv3389c, is necessary for the intracellular growth of M. tuberculosis in macrophages, mice, and guinea pigs. The attenuated phenotype of the mutant strain is most likely associated with disruption of the itaconate dissimilation and propionyl-CoA detoxification pathway. Taken together, the results shown in the present study expand our understanding of the itaconate mechanism of action and dissimilation in bacterial pathogens.
Results
M. tuberculosis Induces Itaconate Production in Mice.
Previously, it has been reported that itaconate production is enhanced upon LPS or IFN-γ-stimulation or S. aureus infection in macrophages and mice (10, 21). Irg1 expression has been reported to increase in bone marrow–derived macrophages (BMDMs) after M. tuberculosis infection (11). We first investigated whether infection of mice with M. tuberculosis results in increased Irg1 expression with a concomitant increase in itaconate synthesis in the lung tissues. As shown in Fig. 1A, compared to uninfected mice, the transcript levels for Irg1 were increased by ~100- to 130-fold at 2- and 4-wk postinfection. Also, the lungs of mice infected with M. tuberculosis produced more itaconate (~4.5 µM) relative to uninfected mice at 4-wk postinfection (Fig. 1B). These findings reconfirm earlier findings that M. tuberculosis infection in mice results in increased Irg1 expression and itaconate synthesis (8, 11).
Fig. 1.
Itaconate restricts the growth of Mycobacterium tuberculosis in vitro and in vivo. (A and B) The relative levels of Irg1 (A) and itaconate (B) in the lung tissues of mice infected with M. tuberculosis at 2- and 4-wk postinfection were calculated as described in Materials and Methods. The data shown in panels (A) and (B) are obtained from three or four mice, respectively. (C) The growth of M. tuberculosis in MB7H9 medium was determined after exposure to various concentrations of sodium itaconate (ITA) or dimethyl itaconate (DI). (D) THP-1 macrophages were infected with M. tuberculosis strain at 1:10 MOI and treated with different concentrations of DI or ITA for 4 d. The data shown in panels C and D are the mean ± SD of log10 CFU obtained from two independent experiments performed in either duplicates or triplicates. (E and F) 6 to 8 wk old female BALB/c mice were infected with M. tuberculosis via aerosol route. The animals were administered intraperitoneally with 50 mg/kg DI for either 7 or 21 d. The data shown in these panels are mean ± SD of log10 CFU of lungs (E) and splenic (F) bacillary loads obtained from five animals per group. (G–K) The intracellular levels of IL-1β (G), TNF-α (H), IFN–γ (I), IL-10 (J), and IL-6 (K) were determined in naïve, untreated, and DI treated groups at day 21 posttreatment by ELISA. The data shown in these panels are mean ± SD obtained from five animals. The data were statistically analyzed using one-way ANOVA (A, G, H, I, J, and K) or a two-tailed “paired” t test (B, E, and F). *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Itaconate Restricts the Growth of M. tuberculosis In Vitro, in Macrophages, and Mice.
It has been reported that itaconate inhibits the growth of several bacteria, including M. tuberculosis, S. enterica, S. aureus, nontuberculous mycobacteria, and Vibrio sp. (10, 18, 21, 22). Since itaconate is highly polar and poorly permeable to the cell wall, we also performed killing experiments using dimethyl itaconate (DI), a cell wall permeable derivative of itaconate, as reported in earlier studies (15, 34). We observed that exposure to either sodium itaconate (ITA) or DI inhibited M. tuberculosis growth in a dose-dependent manner (Fig. 1C). As shown in Fig. 1D, exposure to either 1 mM or 10 mM of ITA for 4 d inhibited M. tuberculosis intracellular growth by ~10-fold. In comparison, exposure to either 125 µM or 500 µM of DI inhibited the intracellular growth of M. tuberculosis by 10- and 52-fold, respectively. As expected, isoniazid exposure significantly inhibited M. tuberculosis growth in macrophages (Fig. 1D). We also determined the effect of DI administration on M. tuberculosis growth in aerosol-infected mice. We show that intraperitoneal administration of DI at 50 mg/kg reduced the lung bacillary load by ~4-fold at days 7 and 21 posttreatment, respectively (Fig. 1E). In spleens, DI administration reduced M. tuberculosis growth by 17-fold and 2-fold at days 7 and 21 posttreatment, respectively (Fig. 1F). These findings are in agreement with a recent study demonstrating that DI administration inhibited the growth of M. tuberculosis, M. bovis BCG, and M. avium in macrophages and mice tissues (35). In the same study, it has been shown that the administration of DI suppresses the release of IL-6 and IL-10 and activation of STAT3 signaling and promotes autophagy and phagolysosomal maturation in BMDMs (35). In earlier studies, administration of itaconate and DI has been demonstrated to control inflammation by reducing the levels of proinflammatory cytokines in lung tissues and macrophages (13–15). In agreement with earlier reports, we noticed that infection of mice with M. tuberculosis for 4 wk resulted in increased levels of TNF-α, IFN–γ, and IL–6 in comparison to naïve mice (36, 37) (Fig. 1 H, I, and K). As expected, DI administration to M. tuberculosis–infected mice resulted in a ~2.0-fold reduction in TNF-α levels (Fig. 1H). However, we did not observe any differences in the IL-1β levels between naïve, infected, and treated animals (Fig. 1G). As shown in Fig. 1 J and K, the administration of DI in infected mice resulted in increased IL-10 and IL-6 levels compared to naïve mice. Taken together, these findings imply that itaconate inhibited the growth of M. tuberculosis in liquid cultures, macrophages, and mice.
Itaconate has Wide-Ranged Metabolic Effects in M. tuberculosis.
Previously, itaconate has been shown to impart an antimicrobial effect by modulating the activity of enzymes such as ICL and aldolase (Ald) involved in central carbon metabolism (21–24). To ascertain the effect of itaconate exposure on the metabolite profile of M. tuberculosis, we next performed untargeted metabolomics as described in Materials and Methods. At designated time points, metabolites were extracted and subjected to LC-MS analysis. Principal component analysis revealed that the metabolic profiles obtained from ITA-treated cultures were distinct from untreated samples (Fig. 2 A–C). The metabolites with a P value < 0.05 and log2 fold change of ≤1.0 or ≥1.0 were considered as significantly differentially altered after exposure to ITA. Compared to the untreated cultures, 56, 59, and 73 metabolites were significantly changed in M. tuberculosis after exposure to 10 mM, 20 mM, or 50 mM of ITA for 3 d (Datasets S1, S3 and S5). Among the metabolites with altered levels, we observed that the levels of 34 metabolites (22 and 12 metabolites were increased and decreased, respectively) were altered in ITA-treated M. tuberculosis cultures in comparison to untreated cultures (SI Appendix, Table S1). Relative to the untreated cultures, we observed that levels of 29, 75, and 99 metabolites were altered in M. tuberculosis after exposure to either 10 mM, 20 mM, or 50 mM ITA, respectively, for 6 d (Datasets S2, S4 and S6). Among these, in comparison to untreated cultures, the levels of 23 metabolites were altered (11 and 12 metabolites increased and decreased, respectively) in all three ITA treatment conditions (SI Appendix, Table S2).
Fig. 2.
(A–K) The effect of itaconate exposure on the metabolite profiles of M. tuberculosis. (A–C) Principal component analysis of the metabolite profiles obtained from either untreated or from early log phase cultures of M. tuberculosis exposed to either 10 mM (A), 20 mM (B), or 50 mM (C) ITA for either 3 or 6 d. (D–K) This panel depicts the relative abundance of itaconate (D), citramalate (E), acetyl-CoA (F), glucose-6-phosphate (G6P, G), fructose-6-phosphate (F6P, H), dihydroxyacetone phosphate (DHAP, I), inosine monophosphate (IMP, J), and xanthosine monophosphate (XMP, K) in untreated and ITA-treated cultures. RPI represents relative peak intensity. The data were statistically analyzed using one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. (L–U) Itaconate binds and inhibits the enzymatic activity of aldolase (Ald) and IMP dehydrogenase (GuaB2). (L and M) The binding of Ald (L) and GuaB2 (M) in the presence of various concentrations of ITA was measured by tryptophan fluorescence quenching assay as described in Materials and Methods. (N and O) The enzymatic assays of Ald (N) and GuaB2 (O) were performed in the presence of different concentrations of ITA. The data shown in these panels are mean ± SD of percentage inhibition of the enzymatic activity of Ald or GuaB2 obtained from two independent experiments performed in duplicates or triplicates. (P and Q) These panels show mean ± SD of Ald (P) or GuaB2 (Q) activity in the presence of 10 mM ITA or cis-aconitic acid (CAA) or methyl succinic acid (MSA). The data shown in these panels are obtained from two independent experiments performed in duplicates. (R and S) These panels show the molecular docking of Ald with either sodium itaconate (R) or fructose-1,6 biphosphate (S). (T and U) The molecular docking of GuaB2 with sodium itaconate (T) or IMP (U) is shown in these panels.
The detailed analysis of the metabolomics data revealed that metabolic pathways belonging to central carbon processes such as glycolysis, gluconeogenesis, TCA, pentose phosphate pathway, and purine metabolism were perturbed in ITA-treated cultures (SI Appendix, Fig. S1A). We observed increased intracellular levels of itaconate, citramalate, and acetyl-CoA in ITA-treated samples relative to untreated cultures (Fig. 2 D–F). However, we did not observe any significant differences in the levels of pyruvate between untreated and ITA-treated cultures (SI Appendix, Fig. S1B). These observations confirm that an itaconate dissimilation pathway is functional in M. tuberculosis. We also observed that in comparison to the untreated cultures, ITA-treated cultures had higher levels of glucose-6-phosphate (G6P) and fructose-6-phosphate (F6P) (Fig. 2 G and H). The pentose phosphate pathway utilizes G6P to produce ribose-5-phosphate, which is reversibly converted into glyceraldehyde-3-phosphate and F6P (38). Hence, we also looked at the abundance of these metabolites in ITA-treated cultures compared to untreated cultures. As expected, after exposure to ITA, M. tuberculosis showed increased levels of 6-phosphogluconic acid and ribose-5-phosphate compared to untreated cultures (SI Appendix, Fig. S1 C and D). We also observed that ITA-treated samples had significantly reduced levels of dihydroxyacetone phosphate (DHAP) and glycerol-3-phosphate relative to untreated cultures (Fig. 2I and SI Appendix, Fig. S1E). Previously, it has been shown that itaconate inhibits the conversion of isocitrate into succinate and glyoxylate by covalently modifying the catalytic cysteine residue of M. tuberculosis ICL1 and ICL2 enzymes (24). In agreement with the reported inhibition of ICL enzymes, we observed isocitrate and α-ketoglutarate accumulation in ITA-treated samples compared to untreated cultures (SI Appendix, Fig. S1 F and G) (24). We did not observe any significant differences in the levels of succinate and malate between the untreated and ITA-treated cultures. Importantly, increased levels of intermediates of glycolysis and pentose phosphate pathway cannot be easily explained by assuming ICL is the sole target of itaconate in M. tuberculosis. Previous studies have shown that inhibition of glycolysis might have several consequences on related metabolic pathways, such as the pentose phosphate pathway, amino acid biosynthesis, and nucleotide metabolism (39, 40). Previously, it has been reported that phosphoribosylpyrophosphate synthetase (PrsA) catalyzes the first committed step of purine biosynthesis by converting ribose-5-phosphate to 5-phosphoribosyl pyrophosphate (PRPP) (41) (SI Appendix, Fig. S1A). Next, we compared the levels of intermediates involved in purine metabolism in ITA-treated and untreated cultures. We observed increased levels of intermediates belonging to purine metabolism, such as 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), and inosine in ITA-treated cultures relative to untreated cultures (SI Appendix, Fig. S1 H and I). We also observed increased and reduced levels of IMP and xanthosine monophosphate (XMP), respectively, in ITA-treated cultures relative to untreated samples (Fig. 2 J and K). These observations suggest that exposure to itaconate disrupts central carbon metabolism in M. tuberculosis widely, with effects that cannot be solely attributed to ICL inhibition.
Itaconate Inhibits Aldolase and IMP Dehydrogenase from M. tuberculosis.
Based on our metabolomics results, we hypothesized that increased and decreased levels of F6P and DHAP, respectively, in ITA-treated cultures might be associated with inhibition of aldolase (Ald) enzymatic activity. Similarly, increased and decreased levels of IMP and XMP, respectively, in ITA-treated samples might be associated with the inhibition of IMP dehydrogenase (GuaB2) enzymatic activity. To test our hypothesis, the gene encoding for Rv0363c (Ald) and Rv3411c (GuaB2) was PCR amplified, cloned in pET28b, and purified as (His)6-tagged proteins. Next, we performed tryptophan quenching experiments and enzyme inhibition assays to determine ITA binding with purified (His)6-Ald and (His)6-GuaB2. We observed that ITA binding reduced the intrinsic fluorescence of purified Ald and GuaB2 in a dose-dependent manner (Fig. 2 L and M). In agreement with metabolomics and binding assays, we also demonstrate that ITA inhibits Ald and GuaB2 enzymatic activity in a concentration-dependent manner (Fig. 2 N and O). Inhibition of Ald and GuaB2 by itaconate was specific, as we did not observe inhibition in the presence of other itaconate similar structures such as cis-aconitic acid (CAA) or methyl succinic acid (MSA) (Fig. 2 P and Q). Further, we also performed molecular docking studies to determine itaconate binding with Ald and GuaB2. We observed that the binding energy for itaconate and fructose-1,6-bisphosphate with Ald was −4.7 kcal/mol and −6.4 kcal/mol, respectively (Fig. 2 R and S). In our molecular docking studies, we observed binding energy associated with itaconate and IMP with GuaB2 of −4.5 kcal/mol and −8.7 kcal/mol, respectively (Fig. 2 T and U). We observed that itaconate interacted with key residues of Ald and GuaB2 via hydrogen bonds, coordinate bonds, and π-cation and π-anion bonds. As shown in Fig. 2R, itaconate interacted with Asp95, His96, His212, His252, and Gly253 of Ald. In comparison, fructose-1,6-bisphosphate interacted with His96, His212, His252, Gly253, Ser255, Asn274, Asp276, and Thr277 of Ald (Fig. 2S. In the case of GuaB2, we observed that itaconate interacted with Met85, Asp283 and Asp374, Ala285, and Ser339 (Fig. 2T). In comparison, in addition to these residues, IMP interacted with Arg108, His286, and Val345 of GuaB2 (Fig. 2U). Taken together, these findings imply that itaconate binds and inhibits the enzymatic Ald and IMP dehydrogenase from M. tuberculosis.
Identification of Enzymes Required for Itaconate Dissimilation in M. tuberculosis.
Previously, it has been shown that the genomes of P. aeruginosa and Y. pestis encode for IcT, IcH, and CcL homologs (29). Recently, it has been reported that Rv2498c encodes for the CcL enzyme from M. tuberculosis (32). However, homologs for IcT and IcH proteins belonging to itaconate dissimilation pathways are still unknown in M. tuberculosis (Fig. 3A). For identification of IcT and IcH in M. tuberculosis, we performed homology search using the IcT and IcH sequences of various microorganisms. Homology search and multiple sequence alignment revealed that Rv2503c and Rv3272 share 20 to 25% sequence identity with IcT homologs from other bacterial pathogens (SI Appendix, Fig. S2A). Phylogenetic analysis revealed that the Rv2503c homolog is more similar to IcT homologs from Y. pestis, S. enterica, and Legionella longbeachae (SI Appendix, Fig. S2B). As shown in SI Appendix, Fig. S3A, Rv2499c and Rv3389c shared between 20 to 45% sequence identity with the IcH homolog of other bacterial pathogens. Phylogenetic analysis revealed that Rv2499c is more similar to the IcH homolog from L. longbeachae Y. pestis and S. enterica (SI Appendix, Fig. S3B). In comparison Rv3389c was closer to homologs from P. aeruginosa, Bordetella parapertussis, Campylobacter coli, and Burkholderia xenovorans (SI Appendix, Fig. S3B). The phylogenetic analysis of IcH reveals the divergence and presence of two distinct subgroups of sequences. It has been demonstrated that Rv3389c belongs to the hydratase two family, possesses asymmetric double hot dog fold, and effectively catalyzes the hydration of C8-C16 enoyl-CoA substrates. Rv3389c also catalyzes the hydration of 3-hydroxyacyl-CoA substrates of fatty acid synthase-II (FAS-II), which are involved in mycolic acid biosynthesis (42, 43). To biochemically characterize IcT and IcH homologs from M. tuberculosis, genes encoding for Rv2503c, Rv3272, Rv2499c, and Rv3389c were PCR amplified, cloned into either pET28b or pGEX4T-1 or pMALc2x and purified as (His)6- or GST- and MBP-tagged proteins. IcT (PA0882) and IcH (PA0878) homologs from P. aeruginosa were also cloned in pET28b and purified as (His)6-tagged proteins to serve as positive controls in our enzymatic reactions. The enzymatic assays for IcT and IcH enzymes were performed and subjected to LC-MS analysis as described in Materials and Methods (Fig. 3B). We were able to identify peaks corresponding to itaconyl-CoA in enzymatic reactions performed using either (His)6-PA0882 or (His)6-Rv2503c or GST-Rv3272 (Fig. 3C). Further, we performed IcH enzymatic assays using either purified (His)6-PA0878 or MBP-Rv2499c or (His)6-Rv3389c and subjected to LC-MS analysis. As shown in Fig. 3D, all three proteins displayed IcH activity as peaks corresponding to citramalyl-CoA were identified in their enzymatic reactions. Recently, it has been demonstrated that Rv2498c possesses CcL activity and converts (S)–citramalyl-CoA into acetyl-CoA and pyruvate (32). We had previously reported that Rv3075c shares 25 to 30% sequence identity with Rv2498c (33). Therefore, we hypothesized that Rv3075c might also exhibit CcL activity in addition to Rv2498c and performed enzymatic assays using purified proteins. CcL (PA0883) homolog from P. aeruginosa served as a positive control for this assay. In enzymatic reactions involving purified (His)6-PA0883 or (His)6-Rv2498c, peaks corresponding to acetyl-CoA and pyruvate were identified (Fig. 3E). However, we did not observe CcL activity in enzymatic reactions performed using (His)6-Rv3075c.
Fig. 3.
Functional characterization of enzymes from M. tuberculosis involved in itaconate dissimilation pathway. (A) Schematic representation of itaconate dissimilation pathway in bacteria. IcT, IcH, and CcL enzymes represent itaconate:succinyl-CoA transferase, itaconyl-CoA hydratase, and citramalyl-CoA lyase, respectively. (B) This panel shows that representative LC-chromatogram of the succinyl-CoA, itaconyl-CoA, citramalyl-CoA, and acetyl-CoA extracted at their corresponding m/z. (C–E) This panel shows the relative abundance of peaks corresponding to itaconyl-CoA (C), citramalyl-CoA (D), pyruvate and acetyl-CoA (E) in enzymatic assays performed using purified proteins. The data shown in these panels are the mean ± SD of data obtained from five independent experiments. (F–H) This panel shows the percentage activity of purified Rv2503c (F), Rv2499c (G), or Rv3389c (H) mutant proteins relative to the wild type protein. The data shown are mean ± SD of percentage activity for various purified proteins obtained from two independent experiments performed in triplicates. The data were statistically analyzed using one-way ANOVA. ***P < 0.001, ****P < 0.0001.
We searched for homologs of Rv2503c and Rv2499c against the PDB database using BLAST. We performed multiple sequence alignment using Clustal Omega to identify and predict the conserved residues involved in substrate binding (44). BLAST highlighted various PDBs with sequence similarity to Rv2503c and Rv2499c. We used AlphaFold2 to predict the structures for Rv2503c and Rv2499c and compared these to the sequence homologs obtained from BLAST analysis. These included 3OXO (rmsd = 0.929 Å), 4KGB (rmsd = 0.714 Å), and 3DLX (rmsd = 0.621 Å) for Rv2503c and 4E3E (rmsd = 0.643 Å), 2BI0 (rmsd = 1.279 Å), 4FFU (rmsd = 1.692 Å), and 3EXZ (rmsd = 2.504 Å) for Rv2499c (SI Appendix, Table S3). We selected Q29551 (PDB ID: 3OXO, the crystal structure of succinyl-CoA: ketoacyl-CoA transferase from pig heart covalently bound to CoA) for analysis of the binding pocket for Rv2503c given that the structure of human 3-oxoacid transferase was solved in complex with acetoacetyl-CoA (45). Since Glu305 in human 3-oxoacid transferase is highly conserved and forms a covalent bond with the CoA group, we selected its structural equivalent, Glu50 in Rv2503c for mutagenesis studies (SI Appendix, Fig. S4 A and C). We also selected Met108 for mutagenesis studies based on sequence conservation and its involvement in interaction with the aromatic group of acetyl-CoA (45, 46) (SI Appendix, Fig. S4 A and C). To further validate IcT activity associated with Rv2503c, Rv2503c variants (Rv2503cE50A and Rv2503cM108A) were PCR amplified, cloned in pET28b and purified using affinity chromatography. In comparison to the wild-type protein, the itaconate:succinyl-CoA transferase activity of (His)6-Rv2503cE50A and (His)6-Rv2503cM108A was reduced by 60 to 70% (Fig. 3F).
We selected 2BI0 (another conserved hypothetical protein from M. tuberculosis Rv0216) for further analysis of the binding pocket of Rv2499c (47). Based on the sequence conservation and previous studies suggesting residues involved in substrate binding, in the case of Rv2499c, we selected Asn114 and Phe117 positions for mutagenesis studies (SI Appendix, Fig. S4 B and D) (47). In the case of Rv3389c, we mutated Gln199 and Cys212 positions to alanine as previously described (43, 48). The sequences with desired mutations were cloned and purified as either MBP-tagged or (His)6-tagged proteins. As shown in Fig. 3G, MBP-Rv2499cF117A and MBP-Rv2499cN114A displayed 80 to 90% reduced activity compared to the wild-type enzyme. The mutation of Gln199 and Cys212 residues to Ala in Rv3389c decreased IcH activity by ~40 to 50% (Fig. 3H). Taken together, these experiments unambiguously assign the enzymes responsible for the first and second steps of itaconate dissimilation in M. tuberculosis. Rv2503c and Rv3272 catalyze the transacylation of itaconate and succinyl-CoA into succinate and itaconyl-CoA, and (ii) Rv2499c and Rv3389c catalyze the hydration of itaconyl-CoA forming (S)-citramalyl-CoA.
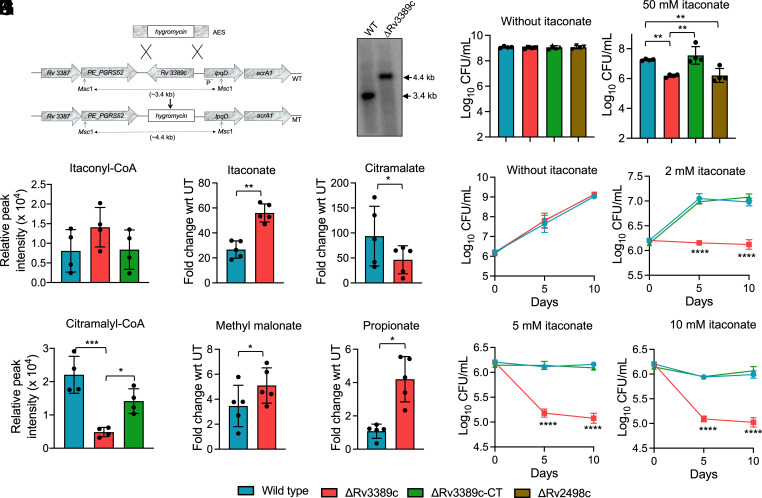
Itaconate Degradation Pathway Is Impaired in the Absence of Rv3389c.
To determine the contribution of Rv3389c in the itaconate dissimilation pathway and disease pathogenesis, we constructed ΔRv3389c and Rv3389c complemented strains of M. tuberculosis as described in Materials and Methods. As shown in Fig. 4A, the Rv3389c-specific probe hybridized with 3.4 kb and 4.4 kb in Msc I digested genomic DNA isolated from parental and mutant strains, respectively, in our Southern blot analysis. We observed that the growth patterns of parental, ΔRv3389c and Rv3389c complemented strains were comparable in the absence of ITA (Fig. 4B). As shown in Fig. 4B, ΔRv3389c displayed a significant growth defect (~11.5-fold) upon exposure to ITA relative to the parental strain and Rv3389c complemented strain (Fig. 4B). In agreement with published results, we observed that in comparison to the parental strain, ΔRv2498c was more susceptible to ITA mediated killing (Fig. 4B) (32). Next, we performed activity assays using cell-free protein extracts (CFPE) prepared from parental or ΔRv3389c or Rv3389c complemented strains. There were no statistically significant differences in the levels of itaconyl-CoA in reactions using CFPE from parental and ΔRv3389c strain (Fig. 4C). However, the levels of citramalyl-CoA were significantly reduced in reactions performed using CFPE from ΔRv3389c strain relative to the parental strain (Fig. 4D). As shown in Fig. 4 C and D, the amounts of itaconyl-CoA and citramalyl-CoA in reactions performed using CFPE prepared from the parental and Rv3389c complemented strain were comparable to each other. Further, we performed untargeted metabolomics of samples prepared from parental and ΔRv3389c strains cultured in the absence or presence of ITA. Relative to untreated cultures, exposure to ITA resulted in concomitant itaconate accumulation and depletion of citramalate in mid-log phase cultures of ΔRv3389c strain compared to the parental strain (Fig. 4 E and F).
Fig. 4.
Itaconate dissimilation pathway is disrupted in ΔRv3389c strain of M. tuberculosis. (A) A schematic representation of the Rv3389c genetic locus in the genome of parental and ΔRv3389c strains is shown in this panel. The replacement of Rv3389c with the hygromycin resistance gene was confirmed by Southern blot. (B) The growth patterns of parental, ΔRv3389c, Rv3389c complemented, and ΔRv2498c strains were compared in the absence or presence of ITA for 8 d. The data shown in this panel are mean ± SD of log10 CFU/ml obtained from two independent experiments performed in duplicates. (C and D) The enzymatic reactions were performed using CFPE prepared from mid-log phase cultures of either parental or ΔRv3389c or Rv3389c complemented strain. The data shown in these panels are mean ± SD of the relative peak intensity of itaconyl-CoA (C) or citramalyl-CoA (D) obtained from four independent experiments. (E–H) This panel depicts the relative fold change of itaconate (E), citramalate (F), methylmalonate (G), and propionate (H) in 50 mM ITA exposed mid-log phase cultures of parental and ΔRv3389c strain for 3 d. The data shown in this panel are the mean ± SD of five independent experiments. (I–L) The growth patterns of parental, ΔRv3389c, and Rv3389c complemented strains were compared in a medium containing 10 mM propionate in the absence (I) or presence of 2 mM (J), 5 mM (K), and 10 mM sodium itaconate (L). The data shown in these panels are mean ± SD of log10 CFU obtained from two independent experiments performed in duplicates. The data were statistically analyzed using one-way ANOVA (B, C, D, J, K, and L) or two-tailed “paired” t test (E, F, G, and H). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
M. tuberculosis genome encodes for functional methylcitrate and methylmalonyl pathways that are involved in propionyl-CoA synthesis and catabolism and are required for M. tuberculosis growth in the presence of propionate (5, 49–51). Studies have confirmed that propionyl-CoA is involved in odd-chain fatty acid biosynthesis in M. tuberculosis (52). It has also been reported that itaconate exposure inhibits vitamin B12–facilitated M. tuberculosis growth in liquid cultures having propionate as the sole carbon source (28). This growth inhibition is probably due to the inhibition of enzymatic activity associated with MCL and MCM enzymes by itaconate and itaconyl-CoA, respectively (28, 49). In agreement with increased itaconate levels, we also observed slight yet significantly increased levels of propionate and methylmalonate in ΔRv3389c strain relative to the parental strain (Fig. 4 G and H). Additionally, it has been demonstrated that accumulation of propionate inhibits M. tuberculosis growth in vitro (53). Since the itaconate dissimilation pathway is nonfunctional in the ΔRv3389c strain of M. tuberculosis and accumulation of propionate upon exposure to itaconate, we hypothesized that intracellular accumulation of itaconate and itaconyl-CoA in the mutant strain might inhibit the formation of propionyl-CoA in the propionate-containing medium. Next, we compared the growth of parental, ΔRv3389c, and Rv3389c complemented strain in a medium containing propionate as the sole carbon source in the absence or presence of ITA. We observed that the growth patterns of various strains were comparable in a propionate-containing medium (Fig. 4I). We observed that upon exposure to ITA, ΔRv3389c displayed a growth defect in a medium containing propionate as the sole carbon source in comparison to the parental and Rv3389c complemented strains in a concentration-dependent manner (Fig. 4 J–L). These observations validate that in addition to 3-hydroxyacyl-CoA thioester hydratase activity, Rv3389c also possesses itaconyl-CoA hydratase activity, and itaconate dissimilation pathway is impaired in ΔRv3389c strain (42, 43). Taken together, these findings suggest that deletion of Rv3389c might result in additional propionate toxicity in M. tuberculosis in the presence of itaconate and odd-chain fatty acids.
Rv3389c Is Important for the Pathogenesis of M. tuberculosis in Mice and Guinea Pigs.
We next evaluated the impact of Rv3389c deletion in M. tuberculosis physiology and pathogenesis. The growth kinetics of the parental, ΔRv3389c and Rv3389c complemented strains were similar in 7H9 medium (SI Appendix, Fig. S5A). We also compared the survival of parental, ΔRv3389c and Rv3389c complemented strains upon exposure to different host-relevant stress conditions. The bacterial growth in the case of ΔRv3389c strain was reduced by ~12.5- and 7-fold, respectively, in comparison to the parental strain after being exposed to oxidative stress (i.e., H2O2) for 24 h and 72 h, respectively (Fig. 5A). This growth defect associated with the mutant strain upon exposure to oxidative stress was completely restored upon complementation with the parental copy. The survival of parental, ΔRv3389c and Rv3389c complemented strains were comparable after exposure to other stresses such as nitrosative or nutritional or acidic stress (SI Appendix, Fig. S5 B–D). In agreement with our published results, we show that the survival of parental and ΔRv2498c was comparable upon exposure to oxidative stress (SI Appendix, Fig. S5E) (33). Next, we compared the survival of various strains in THP-1 macrophages at days 2, 4, and 6 d postinfection. We found that the ability of the mutant strain to infect macrophages was comparable to that observed for the parental strain. However, ΔRv3389c strain displayed a significant growth defect (~10-fold) in comparison to the parental strain at days 2 and 4 postinfection (Fig. 5B). This growth defect associated with the mutant strain increased to ~26-fold after infection for 6 d (Fig. 5B). As shown in Fig. 5B, both parental and complemented strain displayed comparable growth kinetics in macrophages at 2, 4, and 6 d postinfection. This result demonstrates that deletion of Rv3389c attenuated M. tuberculosis growth inside THP-1 macrophages. Several studies have performed experiments using the A549 human lung epithelial cell line to mimic itaconate deficient condition ex vivo (10, 54, 55). Next, we compared the survival of parental, ΔRv3389c and Rv3389c complemented strains in A549 epithelial cell line in the absence or presence of itaconate. As shown in Fig. 5C, the growth patterns of these strains were comparable in A549 epithelial cell line at days 2, 4, and 6 postinfection. However, upon exposure to 10 mM ITA, the intracellular growth of ΔRv3389c was reduced by ~4.8, ~5.0, and ~9.7-fold relative to the parental strain at days 2, 4, and 6 postinfection (Fig. 5D). We also observed that this growth defect associated with the mutant was restored in Rv3389c complemented strain (Fig. 5D). We also compared the intracellular growth of parental and ΔRv2498c strains in THP-1 macrophages, and in A549 lung epithelial cell line in the absence or presence of itaconate. As shown in SI Appendix, Fig. S5F, the deletion of Rv2498c impaired the growth of M. tuberculosis in THP-1 macrophages. In comparison to the parental strain, we observed ~11-, 13-, and 8.5-fold reduction in the intracellular growth of ΔRv2498c strain at days 2, 4, and 6 postinfection, respectively (SI Appendix, Fig. S5F). In agreement, we also observed that the intracellular growth of ΔRv2498c was significantly reduced in the A549 cell line compared to parental strain upon exposure to itaconate (SI Appendix, Fig. S5H). Also, the survival of parental and ΔRv2498c was comparable in A549 epithelial cell line at days 2, 4, and 6 postinfection (SI Appendix, Fig. S5G).
Fig. 5.
Deletion of Rv3389c impairs the survival of M. tuberculosis in macrophages, mice and guinea pigs (A) The survival of parental, ΔRv3389c and Rv3389c complemented strain was compared after exposure to oxidative stress for either 6 h or 24 h or 72 h. The data shown in this panel are mean ± SD of log10 CFU obtained from two independent experiments performed in duplicates. (B–D) The growth kinetics of parental, ΔRv3389c and Rv3389c complemented strain was compared in THP-1 macrophage (B) or A549 lung epithelial cell line in the absence (C) or presence of 10 mM itaconate (D) after 2, 4, and 6 d postinfection. The data shown in these panels are mean ± SD of log10 CFU obtained from two or three independent experiments performed in triplicates. (E–H) The data shown in these panels are mean ± SD of log10 CFU of lungs (E and G) and splenic (F and H) bacillary loads obtained from 5 BALB/c mice infected with various strains at 4 and 8 wk postinfection. (I) The growth of parental, ΔRv3389c and Rv3389c complemented strain was compared in BMDMs isolated from wild type or Irg1−/− C57BL/6 mice after 4 d postinfection. The data shown in this panel are mean ± SD of log10 CFU obtained from two independent experiments performed in triplicates or quadruplicates. (J) The growth patterns of parental, ΔRv3389c and Rv3389c complemented, Rv3389cQ199A complemented, and Rv3389cC212A complemented strain in THP-1 macrophages after day 6-post infection. The data shown in this panel are mean ± SD of log10 CFU obtained from two independent experiments performed in triplicates. (K and L) The data shown in these panels are mean ± SD of log10 CFU obtained from lungs (K), and spleens (L) bacillary loads in BALB/c mice (n= 5) infected with either parental or ΔRv3389c or Rv3389c complemented, Rv3389cQ199A complemented, and Rv3389cC212A complemented strains at 4 wk postinfection. (M and N) The data shown in these panels are mean ± SD of log10 CFU of lungs (M) and splenic (N) bacillary loads obtained from 5 or 6 guinea pigs infected with either parental or ΔR3389c strain at 4 and 8 wk postinfection. The data obtained were statistically analyzed using one-way ANOVA (panels A, B, D, G, H, I, J, K, and L) or two-tailed “paired” t test (panels E, F, M, and N). **P < 0.01, ***P < 0.001, and ****P < 0.0001.
In the present study, we used mice and guinea pig animal models to investigate the contribution of Rv3389c in M. tuberculosis pathogenesis. We observed that ΔRv3389c strain was significantly impaired in establishing disease in mice compared to the parental strain at 4- and 8-wk postinfection. The lung and splenic bacillary loads were reduced by 625- and 17-fold, respectively, in ΔRv3389c strain-infected mice compared to parental strain-infected animals at 4 wk postinfection (Fig. 5 E and F). In comparison to the parental strain, mice infected with ΔRv3389c strain had reduced lung and splenic bacillary loads by 82-fold and 450-fold, respectively, at 8 wk postinfection (Fig. 5 E and F). In agreement with Wang et al., we also observed that in comparison to the parental strain, ΔRv2498c strain was attenuated for growth in the lungs and spleens of mice at both 4- and 8-wk postinfection via aerosol route (32). As shown in SI Appendix, Fig. S5I, compared to the parental strain–infected mice, the lung bacillary loads were reduced by 133- and 266-fold, respectively, in ΔRv2498c infected mice at days 28 and 56 postinfection. Similarly, splenic bacillary loads were significantly reduced by ~20- and 177-fold in ΔRv2498c infected mice compared to parental strain–infected mice at days 28 and 56 postinfection, respectively (SI Appendix, Fig. S5J). To further confirm our findings that Rv3389c is essential for M. tuberculosis pathogenesis, we repeated mice experiments using parental, ΔRv3389c and Rv3389c complemented strains. In agreement with our earlier experiments, the bacterial loads in the lungs and spleens of mice infected with ΔRv3389c strain were reduced in comparison to parental strain–infected mice at both 28 and 56 d postinfection (Fig. 5 G and H). We also observed that the in vivo growth defect associated with ΔRv3389c strain was restored in mice infected with the Rv3389c complemented strain (Fig. 5 G and 5 H). Next, we performed experiments to compare the growth of parental, ΔRv3389c and Rv3389c complemented strains in BMDMs isolated from wild type and Irg1−/− mice. In agreement with the earlier study, we observed the intracellular growth of the parental strain was increased by ~5.0-fold in BMDMs isolated from Irg1−/− mice compared to BMDMs isolated from wild type mice (Fig. 5I, **P < 0.01) (12). We noticed that relative to the parental and Rv3389c complemented strain, the intracellular growth of ΔRv3389c was significantly reduced in BMDMs isolated from wild type mice (Fig. 5I). In comparison, the intracellular growth of parental, ΔRv3389c and Rv3389c complemented strain was comparable in BMDMs isolated from Irg1−/− mice (Fig. 5I).
We also performed experiments to determine whether the complementation with catalytic-impaired variants of Rv3389c could restore the growth defect associated with ΔRv3389c strain in macrophages and mice. We observed that complementation of ΔRv3389c strain with Rv3389cQ199A or Rv3389cC212A was unable to restore the growth defect associated with the survival of ΔRv3389c strain in THP-1 macrophages and in lungs and spleens of mice at 4 wk postinfection (Fig. 5 J–L). In agreement with the mice data, bacterial loads in the lungs of guinea pigs infected with ΔRv3389c strain were reduced by 65- and 342-fold, respectively, compared to the parental strain–infected guinea pigs at 4- and 8-wk postinfection (Fig. 5M). We also observed that splenic bacillary loads in guinea pigs infected with ΔRv3389c strain were reduced by 180- and 325-fold in comparison to parental strain–infected guinea pigs at 4- and 8-wk postinfection, respectively (Fig. 5N). Gross pathology and histological evaluation revealed significant differences in the extent of tissue damage of the lungs of parental or ΔRv3389c strain infected mice at 4- and 8 wk postinfection (SI Appendix, Fig. S6 A and B). In agreement with the changes observed in bacterial burden, the tissue damage was significantly decreased in hematoxylin and eosin-stained lung tissues of guinea pigs infected with the ΔRv3389c strain in comparison to the parental strain–infected guinea pigs at days 28 and 56 postinfection (SI Appendix, Fig. S6 A and B). These results imply that itaconyl-CoA hydratase associated with Rv3389c is essential for M. tuberculosis survival in macrophages, mice, and guinea pigs. We surmise that the disruption of the itaconate dissimilation pathway and propionyl-CoA metabolism is associated with the attenuated phenotype of ΔRv3389c strain in host tissues, in addition to potential host-directed effects.
Discussion
Itaconate is an abundant host metabolite that possesses both immune-modulating and antibacterial properties (56). It has been demonstrated that itaconate confers protection against bacterial infection either by enhancing host innate immune pathways or by reducing proinflammatory cytokines synthesis (13, 15, 57, 58). Irg1−/− deficient mice infected with M. tuberculosis have been previously reported to exhibit higher vulnerability, neutrophil infiltration, and inflammation (12). In agreement with earlier reports, we demonstrate that infection of mice with M. tuberculosis increases Irg1 transcript levels and itaconate synthesis (8, 11). We show that exposure to itaconate inhibited M. tuberculosis growth in liquid culture, macrophages, and mice. In agreement with our data, it has been recently demonstrated that intraperitoneal administration of DI suppressed M. tuberculosis growth in mice by activating innate immune pathways (35). It has also been previously shown that DI administration decreases IL-6 and IL-10 synthesis, STAT-3 activation and increases autophagy and phagosome maturation in BMDMs (35). DI regulates inflammation by lowering the levels of proinflammatory cytokines such as IL-1β, TNF–α, IFN-γ and IL-6 (15, 34). We also observed significantly reduced levels of TNF-α in lung homogenates of DI-treated mice as compared to untreated mice. However, no significant differences were observed in IL-1β and INF-γ levels in lung homogenates from untreated and DI-treated mice. DI is a cell-permeable derivative of itaconate and has been used in various studies to delineate its function (13, 15, 35). It has been shown that compared to itaconate, exposure of macrophages to DI induces a strong electrophilic response (59). Further, in contrast to itaconate treatment, exposure of macrophages to DI inhibits the induction of pro-IL-1β and IκBζ and secretion of IL-10, IFN-β, and IL-6 in an Nrf2 independent manner (59).
Several studies have shown that exposure to itaconate inhibits the growth of P. indigofera, S. enterica, Y. pestis, V. cholerae, S. aureus, M. tuberculosis, and nontuberculous mycobacteria (10, 18–22). The activities of various enzymes, such as ICL, succinate dehydrogenase, or Ald, are inhibited by itaconate (20, 21, 24, 34). Metabolomic studies revealed that exposure to itaconate affects central carbon metabolism and the relative levels of 25 metabolites in V. cholerae (22). In the present study, ITA-treated M. tuberculosis cultures had higher levels of G6P, F6P, 6-phosphogluconate, and ribose-5-phosphate compared to untreated cultures. Also, the levels of DHAP were significantly reduced in M. tuberculosis after exposure to ITA. Compared to untreated cultures, intermediates of purine metabolism such as AICAR, IMP, and inosine accumulated in ITA-treated cultures. However, the levels of XMP were significantly reduced in ITA-treated cultures relative to untreated cultures. These findings suggest disruption of the central carbon metabolism and an increased influx of carbon into the pentose phosphate pathway in ITA-treated M. tuberculosis cultures. We further demonstrate that ITA directly binds and inhibits the Ald and GuaB2 enzymes from M. tuberculosis. Previously, it has been reported that M. tuberculosis strains deficient in Ald and GuaB2 are attenuated for growth in both acute and chronic stages of infection (60, 61). In summary, we propose that itaconate inhibits M. tuberculosis growth by reducing the activity of Ald and GuaB2 enzymes involved in glycolysis and purine biosynthesis, respectively, in addition to ICL, as previously reported (25).
Several studies have shown that bacterial pathogens encode enzymes involved in the itaconate dissimilation pathway, and itaconate is converted to pyruvate and acetyl-CoA (29, 31, 62). The canonical itaconate dissimilation pathway comprises three enzymes: IcT, IcH, and CcL. In agreement with earlier studies, metabolomic studies revealed an accumulation of itaconate, citramalate, and acetyl-CoA in ITA-treated M. tuberculosis cultures in vitro (32). These observations suggest that the itaconate dissimilation pathway is functional in M. tuberculosis. Rv2498c has been demonstrated to possess CcL activity and is essential for the virulence of M. tuberculosis in mice (32). However, the homologs for IcT and IcH enzymes have not yet been identified in M. tuberculosis. In the present study, using biochemical assays, we revealed that both Rv2503c and Rv3272 possess IcT activity. Rv2503c is a putative succinyl-CoA:3-ketoacyl-CoA transferase and belongs to the oxoacid Class I transferase family. Rv3272 has been classified as a family III CoA transferase and binds to acyl-CoA of varying carbon chain lengths, including palmitoyl-CoA (63). We further demonstrated that Rv2499c and Rv3389c enzymes possess IcH activity. Rv2499c is a putative (S)—specific enoyl-CoA hydratase, and Rv3389c belongs to the family of (R)—specific hydratase and dehydratases family (42, 43). In addition to IcH activity, Rv3389c also catalyzes the hydration of [C(8)–C(16)] enoyl-CoA substrates and is involved in mycolic acid biosynthesis (43). Taken together, these findings imply that M. tuberculosis encodes for a functional itaconate dissimilation pathway. Intriguingly, the first two steps are catalyzed by two different enzymes and, therefore, represent examples of metabolic functional convergence and redundancy.
It has been shown that in Y. pestis and S. enterica, the expression of enzymes involved in itaconate dissimilation is increased during infection, and this increase is important for their survival in macrophages (29, 64, 65). We generated a mutant strain lacking Rv3389c to delineate further its role in itaconate dissimilation, physiology, and pathogenesis of M. tuberculosis. In agreement with the findings from enzymatic assays, we observed increased and decreased levels of itaconyl-CoA and citramalyl-CoA, respectively, in ΔRv3389c strain relative to the parental and complemented strains. We also observed increased and reduced levels of itaconate and citramalate, respectively, in ΔRv3389c strain compared to the parental strain upon exposure to ITA. As observed in the case of ΔRv2498c strain, ΔRv3389c was also more susceptible to itaconate-mediated killing in liquid cultures relative to the parental and Rv3389c complemented strains. This observation suggests that the itaconate dissimilation pathway is only partially functional in the ΔRv3389c strain, consistent with the observation that Rv2499c can also catalyze the same reaction. We also noticed that in comparison to the parental strain, ΔRv3389c strain was attenuated for growth in macrophages, mice, and guinea pigs. In agreement, it has also been reported that Rv2498c is required to establish infection in mice (32). However, we also observed that deletion of Rv3389c does not impair the survival of M. tuberculosis in itaconate-deficient A549 lung epithelial cell lines and BMDMs isolated from Irg1−/− mice. However, exposure to itaconate significantly reduced the survival of ΔRv3389c strain in A549 epithelial cells compared to parental and Rv3389c complemented strains. Relative to the parental strain, we also observed a slight accumulation of methylmalonate and propionate in ΔRv3389c strain upon exposure to ITA compared to untreated cultures. M. tuberculosis utilizes fatty acids as nutrients in host tissues, and oxidation of odd-chain fatty acids and branched-chain amino acids results in propionyl-CoA formation (50, 66–68). Propionate is a key precursor for synthesizing lipids such as phthiocerol dimycocerosates, sulfolipid-1, and odd-chain fatty acids and is toxic if accumulated (52, 69). M. tuberculosis encodes for enzymes involved in methylcitrate and methylmalonyl pathways, which are involved in propionate detoxification (4, 5). It has been shown that itaconic acid inhibits the MCL enzyme in the methylcitrate cycle, which converts 2-methylisocitrate into pyruvate and succinate (4, 26, 49). It has also been shown that the MCM enzyme of the methylmalonyl pathway converts methylmalonyl-CoA to succinyl-CoA, and its enzymatic activity is inhibited by itaconyl-CoA (5, 28). M. tuberculosis strains with a nonfunctional methylcitrate cycle or methylmalonyl pathway accumulate propionate and are impaired for growth in the propionate-containing medium (4, 5). The attenuation of M. tuberculosis harboring deletions in ICL enzymes in mice tissues has been linked to propionate toxicity or nonfunctionality of the methylcitrate cycle or glyoxylate shunt (26, 49). In agreement with these observations, we also demonstrate that after exposure to ITA, the ΔRv3389c strain displayed a growth defect in the propionate-containing medium relative to the parental and Rv3389c complemented strain. The reduced growth of ΔRv3389c strain in the propionate-containing medium in comparison to the parental strain is most simply explained by the inhibition of ICL and MCM enzymes by the increased intracellular levels of itaconate and itaconyl-CoA, respectively.
Taken together, we conclude that infection of mice with M. tuberculosis leads to increased Irg1 expression and itaconate synthesis in lung tissues. The accumulation of itaconate in infected lung tissues is associated with enhanced antimicrobial pathways of host tissues, such as autophagy, phagosome lysosome fusion, and lysosomal biogenesis. This study shows that M. tuberculosis encodes for functional IcT and IcH homologs and, therefore, has a complete and canonical itaconate dissimilation pathway, ultimately converting itaconate into acetyl-CoA and pyruvate. We demonstrate that itaconate toxicity is not only limited to ICL inhibition, as itaconate suppresses glycolysis and enhances the pentose phosphate pathway and purine biosynthesis in M. tuberculosis. We also show that exposure to itaconate modulates central carbon metabolism in M. tuberculosis, with prominent inhibition of aldolase and IMP dehydrogenase. The deletion of Rv3389c (itaconyl-CoA hydratase) in M. tuberculosis results in a defective itaconate dissimilation pathway, subsequently leading to the accumulation of itaconyl-CoA and reduced levels of citramalyl-CoA. We propose that increased levels of intracellular itaconate and itaconyl-CoA in ΔRv3389c strain inhibit the enzymatic activity of enzymes belonging to propionate detoxification pathways such as MCL/ICL and MCM, leading to propionate accumulation and attenuated phenotype of ΔRv3389c strain in mice and guinea pigs (Fig. 6). In addition to propionate detoxification, the attenuated phenotype of ΔRv3389c strain in host tissues might also be associated with enhanced susceptibility toward oxidative stress. Since enzymes involved in itaconate dissimilation pathways are important for M. tuberculosis pathogenesis, these represent attractive targets for designing improved therapeutics.
Fig. 6.
Proposed model for itaconate dissimilation pathway and attenuation of ΔRv3389c strain in vivo. Our findings demonstrate that itaconate production is increased in the lung tissues of mice upon M. tuberculosis infection. We also show that itaconate inhibits M. tuberculosis growth by inhibiting the enzymatic Ald or IMP dehydrogenase (GuaB2) in addition to isocitrate lyase (ICL) enzyme. The exposure of M. tuberculosis to itaconate increases the carbon flux from glycolytic to pentose phosphate pathway and purine biosynthesis in M. tuberculosis. We also unambiguously assign the enzymes from M. tuberculosis responsible for IcT and IcH activity. These enzymes collectively degrade itaconate into acetyl CoA and pyruvate. We also demonstrate that the Rv3389c (IcH) mutant strain has a defective itaconate degradation pathway, which results in itaconyl CoA accumulation in the strain. This accumulation of itaconyl CoA might inhibit MCM enzymatic activity that is involved in the propionyl CoA detoxification pathway. Further, we show that disruption of the itaconate degradation pathway leads to reduced intracellular survival of ΔRv3389c in vivo. This figure is prepared using Biorender.
Materials and Methods
The detailed description of all protocols used in the study is provided in SI Appendix, Material and Methods.
Bacterial Strains and Culture Conditions.
The plasmids and strains used in the present study are listed in SI Appendix, Table S4.
ITA and DI Killing Experiments in Liquid Cultures.
For ITA or DI killing experiments, M. tuberculosis cultures (OD600nm ~ 0.05) were exposed to various concentrations of ITA or DI, and bacterial enumeration was performed as per standard protocols.
THP-1 Cell Viability Experiments.
The cell viability of THP-1 macrophages upon exposure to ITA and DI was determined using the cell proliferation reagent WST-1, as per the manufacturer’s recommendations.
ITA and DI Killing Experiments in Macrophages.
For killing experiments, macrophages were infected with M. tuberculosis at a multiplicity of infection of 1:10. Subsequently, macrophages were washed with 1x PBS, overlaid with RPMI medium containing different concentrations of ITA or DI and bacterial enumeration was performed as per standard protocols.
DI Treatment in Mice.
Mice were infected with M. tuberculosis H37Rv via aerosol route, and DI treatment was initiated one week postinfection for either 7 d or 21 d. The bacterial enumeration and cytokine quantification in lung homogenates was performed as per standard protocols.
RNA Extraction from Mice Lung Tissues.
The total RNA was extracted using an RNAeasy column as per standard protocols. The transcript levels of Irg1 were quantified using gene-specific SYBR primer and normalized to the levels of the housekeeping gene, GAPDH.
Itaconate Quantification by GC-MS.
The quantification of itaconate in lung tissues from uninfected and infected mice was performed as previously described (70). The extracted samples and standards were subjected to GC-MS analysis using GCMS-TQ8050 NX, as described previously (10).
Metabolomic Sample Preparation and Analysis.
For metabolomics experiments, mid-log phase cultures of M. tuberculosis (OD600nm ~ 0.3 to 0.4) were exposed to different concentrations of ITA for either 3 or 6 d. At designated time points, metabolites were extracted, separated on UPLC ultimate 3,000 and subjected to hydrophilic interaction liquid chromatography (HILIC) phase and reverse phase chromatography.
Intrinsic Fluorescence Quenching Studies.
The binding of itaconate with purified proteins was determined using an intrinsic fluorescence quenching assay as previously described (71).
Aldolase and IMP Dehydrogenase Activity Assays.
Ald and GuaB2 activity assays were performed in assay buffer in the absence or presence of various concentrations of ITA or CAA or MSA as described earlier (61, 72).
Docking Studies.
For molecular docking studies, the three-dimensional crystal structures of mycobacterial proteins were obtained from the Protein Data Bank [PDB ID: 3EKL (Ald); PDB ID: 4ZQM (GuaB2)] (73, 74). The protein structure was appropriately modified before it was submitted to AutoDock Vina for docking studies. Chimera software was employed to illustrate the protein with the ligand binding site.
IcT and IcH Activity Assays.
The activity assays for purified IcT and IcH were performed using LC-MS as previously described (29, 75). The products of the enzymatic reactions were analyzed by LC-MS in HILIC negative mode. The CoA esters and organic acids were extracted at their specific m/z and quantified by the relative peak intensity (area under the curve).
Generation of Mutant and Complemented Strains of M. tuberculosis.
The mutant strain of M. tuberculosis with a deletion in Rv3389c was constructed using temperature-sensitive mycobacteriophage as previously described (76). For the construction of the complemented strain, Rv3389c wild type, Rv3389cQ199A, and Rv3389cC212A were PCR amplified and cloned into pJEB402.
Activity Assays with CFPE.
For activity assays, mid-log phase cultures of various strains (OD600nm ~ 0.8) were harvested, washed with 1xPBS, resuspended in lysis buffer, and lysed by bead beating with intermittent cooling. The reactions were performed using 5 µg of CFPE, and products of the enzymatic reactions were analyzed by LC-MS in HILIC negative mode.
In Vitro Stress and Macrophage Experiments.
To determine the contribution of Rv3389c in the stress adaptation of M. tuberculosis, early log phase cultures of various strains (OD600nm ~ 0.2 to 0.3) were exposed to various stress conditions as previously described (77). To determine the role of Rv3389c in the intracellular survival of M. tuberculosis, THP-1 macrophages or A549 lung epithelial cells or BMDMs isolated from wild type and Irg1−/− mice were infected with various strains at a multiplicity of infection of 1:10, and bacterial enumeration was performed as previously described (11, 33).
Animal Experiments.
For aerosol infection studies, mice (6 to 8 wk old, female BALB/c) and guinea pigs (6 to 8 wk old, female Hartley strain) were infected with the single cell suspension of parental or ∆Rv3389c or Rv3389c complemented strains using Glass Col Inhalation exposure system. The disease progression in infected animals was determined at day 28 and day 56 postinfection by CFU enumeration and histopathology analysis as previously described (78).
Statistical Analysis.
The graphs and statistical analysis were performed using GraphPad Prism software version 9.5.1. Student’s paired t test or one-way ANOVA was used for statistical analysis, and P < 0.05 was considered statistically significant. The number of replicates and statistical tests used to analyze the data is mentioned in the respective figure legends.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Dataset S05 (XLSX)
Dataset S06 (XLSX)
Acknowledgments
We thank Dr. Subhadeep Chatterjee for providing P. aeruginosa PAO1 strain. We acknowledge Dr. Tushar Kanti Maiti for his guidance in performing Tryptophan quenching experiments. The authors sincerely thank Dr. Sheetal Gandotra for scientific discussions. We are thankful to Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar, and small animal facility, THSTI, for providing female guinea pigs and female BALB/c mice, respectively. We sincerely thank Dr. Maxim Artyomov for providing femurs from wild type and Irg1−/− knock-out C57BL/6 mice for BMDM experiments. We acknowledge the staff members of biosafety level III for the technical help. MP acknowledges the Council of Scientific & Industrial Research for her fellowship. A.K. and S.T. acknowledge the University Grants Commission and the Ministry of Human Resource Development, respectively, for their fellowship. R.S. is a senior fellow of Wellcome Trust-DBT India Alliance and recipient of the Ramalingaswami fellowship and National Bioscience Award. We acknowledge Ms. Simran and Mr. Sayan Das for help with animal experiments. We thank Dr. Saurabh Chugh and Dr. Shikha Singh for providing input on the manuscript. We sincerely thank lab attendants Mr. Rajesh, Mr. Ashish, and Mr. Sher Singh for their technical help. The authors acknowledge the funding received from the Department of Science and Technology, Science and Engineering Research Board (CRG/2021/004042). The funders had no role in study design, results analysis, and manuscript preparation.
Author contributions
M.P., and R.S. designed research; M.P., S.K.G., A.K., S.Kapoor, S.T., S.Kidwai, and Y.K. performed research; L.P.S.d.C., K.G.T., D.S., Y.K., and R.S. contributed new reagents/analytic tools; M.P., S.K.G., A.K., S.Kapoor, S.T., L.P.S.d.C., K.G.T., D.M., D.S., Y.K., and R.S. analyzed data; and M.P., L.P.S.d.C., and R.S. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information.
Supporting Information
References
- 1.Bagcchi S., WHO’s global tuberculosis report 2022. Lancet Microbe 4, e20 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Chang D. P. S., Guan X. L., Metabolic versatility of mycobacterium tuberculosis during infection and dormancy. Metabolites 11, 88 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hubert C. B., de Carvalho L. P. S., Convergence and divergence in the metabolic network of Mycobacterium tuberculosis. Curr. Opin. Syst. Biol. 28, 100384 (2021). [Google Scholar]
- 4.Munoz-Elias E. J., Upton A. M., Cherian J., McKinney J. D., Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol. Microbiol. 60, 1109–1122 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Savvi S., et al. , Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: Implications for propionate metabolism during growth on fatty acids. J. Bacteriol. 190, 3886–3895 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrt S., Schnappinger D., Rhee K. Y., Metabolic principles of persistence and pathogenicity in Mycobacterium tuberculosis. Nat. Rev. Microbiol. 16, 496–507 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guirado E., Schlesinger L. S., Kaplan G., Macrophages in tuberculosis: Friend or foe. Semin Immunopathol. 35, 563–583 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin J. H., et al. , 1)H NMR-based metabolomic profiling in mice infected with Mycobacterium tuberculosis. J. Proteome Res. 10, 2238–2247 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Strelko C. L., et al. , Itaconic acid is a mammalian metabolite induced during macrophage activation. J. Am. Chem. Soc. 133, 16386–16389 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michelucci A., et al. , Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. U.S.A. 110, 7820–7825 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bomfim C. C. B., et al. , Mycobacterium tuberculosis induces irg1 in murine macrophages by a pathway involving both TLR-2 and STING/IFNAR signaling and requiring bacterial phagocytosis. Front Cell Infect Microbiol. 12, 862582 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair S., et al. , Irg1 expression in myeloid cells prevents immunopathology during M. tuberculosis infection. J. Exp. Med. 215, 1035–1045 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills E. L., et al. , Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao S. T., et al. , 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat. Commun. 10, 5091 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bambouskova M., et al. , Electrophilic properties of itaconate and derivatives regulate the IkappaBzeta-ATF3 inflammatory axis. Nature 556, 501–504 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooftman A., et al. , The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab 32, 468–478.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., et al. , 4-octyl itaconate alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting oxidative stress and inflammation. Drug Des. Devel Ther 14, 5547–5558 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breen P., Zimbric M., Caverly L. J., Itaconic acid inhibits nontuberculous mycobacterial growth in pH dependent manner while 4-octyl-itaconic acid enhances THP-1 clearance of nontuberculous mycobacteria in vitro. PLoS One 19, e0303516 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillier S., Charnetzky W. T., Glyoxylate bypass enzymes in Yersinia species and multiple forms of isocitrate lyase in Yersinia pestis. J. Bacteriol. 145, 452–458 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFadden B. A., Purohit S., Itaconate, an isocitrate lyase-directed inhibitor in Pseudomonas indigofera. J. Bacteriol. 131, 136–144 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson K. L., et al. , Staphylococcus aureus induces an itaconate-dominated immunometabolic response that drives biofilm formation. Nat. Commun. 12, 1399 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen T. V., et al. , Itaconic acid inhibits growth of a pathogenic marine Vibrio strain: A metabolomics approach. Sci. Rep. 9, 5937 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel T. R., McFadden B. A., Caenorhabditis elegans and Ascaris suum: Inhibition of isocitrate lyase by itaconate. Exp. Parasitol. 44, 262–268 (1978). [DOI] [PubMed] [Google Scholar]
- 24.Kwai B. X. C., et al. , Itaconate is a covalent inhibitor of the Mycobacterium tuberculosis isocitrate lyase. RSC Med. Chem. 12, 57–61 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon S., Chun H. L., Ha H. J., Lee S. Y., Park H. H., Heterogeneous multimeric structure of isocitrate lyase in complex with succinate and itaconate provides novel insights into its inhibitory mechanism. PLoS One 16, e0251067 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz-Elias E. J., McKinney J. D., Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 11, 638–644 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKinney J. D., et al. , Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406, 735–738 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Ruetz M., et al. , Itaconyl-CoA forms a stable biradical in methylmalonyl-CoA mutase and derails its activity and repair. Science 366, 589–593 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasikaran J., Ziemski M., Zadora P. K., Fleig A., Berg I. A., Bacterial itaconate degradation promotes pathogenicity. Nat. Chem. Biol. 10, 371–377 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Cooper R. A., Kornberg H. L., The utilization of itaconate by Pseudomonas sp. Biochem J 91, 82–91 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adler J., Wang S. F., Lardy H. A., The metabolism of itaconic acid by liver mitochondria. J. Biol. Chem. 229, 865–879 (1957). [PubMed] [Google Scholar]
- 32.Wang H., et al. , An essential bifunctional enzyme in Mycobacterium tuberculosis for itaconate dissimilation and leucine catabolism. Proc. Natl. Acad. Sci. U.S.A. 116, 15907–15913 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arora G., Chaudhary D., Kidwai S., Sharma D., Singh R., CitE enzymes are essential for mycobacterium tuberculosis to establish infection in macrophages and guinea pigs. Front. Cell Infect. Microbiol. 8, 385 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lampropoulou V., et al. , Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab 24, 158–166 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y. J., et al. , Dimethyl itaconate is effective in host-directed antimicrobial responses against mycobacterial infections through multifaceted innate immune pathways. Cell Biosci. 13, 49 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appelberg R., Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. avium infections. Immunobiology 191, 520–525 (1994). [DOI] [PubMed] [Google Scholar]
- 37.Bhengu K. N., et al. , Cytokine responses during Mycobacterium tuberculosis H37Rv and ascaris lumbricoides costimulation using human THP-1 and jurkat cells, and a pilot human tuberculosis and helminth coinfection study. Microorganisms 11, 1846 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rytter H., et al. , The pentose phosphate pathway constitutes a major metabolic hub in pathogenic Francisella. PLoS Pathog 17, e1009326 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandel N. S., Amino acid metabolism. Cold Spring Harb. Perspect. Biol. 13, a040584 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGuinness O. P. “The pentose phosphate pathway & other pathways of hexose metabolism” in Harper's Illustrated Biochemistry, Kennelly P. J., Eds. (McGraw Hill, New York, 2023). [Google Scholar]
- 41.Warner D. F., Evans J. C., Mizrahi V., Nucleotide metabolism and DNA replication. Microbiol. Spectr. 2, 1–20 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Gurvitz A., Hiltunen J. K., Kastaniotis A. J., Heterologous expression of mycobacterial proteins in Saccharomyces cerevisiae reveals two physiologically functional 3-hydroxyacyl-thioester dehydratases, HtdX and HtdY, in addition to HadABC and HtdZ. J. Bacteriol. 191, 2683–2690 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacco E., et al. , Rv3389C from Mycobacterium tuberculosis, a member of the (R)-specific hydratase/dehydratase family. Biochim. Biophys. Acta 1774, 303–311 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Sievers F., Higgins D. G., Clustal omega. Curr. Protoc. Bioinform. 48, 3.13.1–3.13.16 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Fraser M. E., Hayakawa K., Brown W. D., Catalytic role of the conformational change in succinyl-CoA:3-oxoacid CoA transferase on binding CoA. Biochemistry 49, 10319–10328 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Zhang M., Xu H. Y., Wang Y. C., Shi Z. B., Zhang N. N., Structure of succinyl-CoA:3-ketoacid CoA transferase from Drosophila melanogaster. Acta Crystallogr. Sect F Struct. Biol. Cryst. Commun. 69, 1089–1093 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castell A., Johansson P., Unge T., Jones T. A., Bäckbro K., Rv0216, a conserved hypothetical protein from Mycobacterium tuberculosis that is essential for bacterial survival during infection, has a double hotdog fold. Protein Sci. 14, 1850–1862 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leesong M., Henderson B. S., Gillig J. R., Schwab J. M., Smith J. L., Structure of a dehydratase-isomerase from the bacterial pathway for biosynthesis of unsaturated fatty acids: Two catalytic activities in one active site. Structure 4, 253–264 (1996). [DOI] [PubMed] [Google Scholar]
- 49.Eoh H., Rhee K. Y., Methylcitrate cycle defines the bactericidal essentiality of isocitrate lyase for survival of Mycobacterium tuberculosis on fatty acids. Proc. Natl. Acad. Sci. U.S.A. 111, 4976–4981 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffin J. E., et al. , Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem. Biol. 19, 218–227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serafini A., et al. , Mycobacterium tuberculosis requires glyoxylate shunt and reverse methylcitrate cycle for lactate and pyruvate metabolism. Mol. Microbiol. 112, 1284–1307 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee W., VanderVen B. C., Fahey R. J., Russell D. G., Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J. Biol. Chem. 288, 6788–6800 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Upton A. M., McKinney J. D., Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis. Microbiology (Reading) 153, 3973–3982 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Cordes T., et al. , Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. J. Biol. Chem. 291, 14274–14284 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sohail A., et al. , Itaconate and derivatives reduce interferon responses and inflammation in influenza A virus infection. PLoS Pathog 18, e1010219 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cordes T., Michelucci A., Hiller K., Itaconic acid: The surprising role of an industrial compound as a mammalian antimicrobial metabolite. Annu. Rev. Nutr. 35, 451–473 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z., et al. , Itaconate is a lysosomal inducer that promotes antibacterial innate immunity. Mol. Cell 82, 2844–2857.e10 (2022). [DOI] [PubMed] [Google Scholar]
- 58.Bambouskova M., et al. , Itaconate confers tolerance to late NLRP3 inflammasome activation. Cell Rep. 34, 108756 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swain A., et al. , Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat. Metab 2, 594–602 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puckett S., et al. , Inactivation of fructose-1,6-bisphosphate aldolase prevents optimal co-catabolism of glycolytic and gluconeogenic carbon substrates in Mycobacterium tuberculosis. PLoS Pathog 10, e1004144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh V., et al. , The inosine monophosphate dehydrogenase, gua B2, is a vulnerable new bactericidal drug target for tuberculosis. ACS Infect Dis. 3, 5–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin W. R., Frigan F., Bergman E. H., Noninductive metabolism of itaconic acid by Pseudomonas and Salmonella species. J. Bacteriol. 82, 905–908 (1961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karade S. S., et al. , Rv3272 encodes a novel Family III CoA transferase that alters the cell wall lipid profile and protects mycobacteria from acidic and oxidative stress. Biochim. Biophys. Acta Proteins Proteom 1867, 317–330 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Pujol C., Grabenstein J. P., Perry R. D., Bliska J. B., Replication of Yersinia pestis in interferon gamma-activated macrophages requires ripA, a gene encoded in the pigmentation locus. Proc. Natl. Acad. Sci. U.S.A. 102, 12909–12914 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi L., et al. , Proteomic analysis of Salmonella enterica serovar typhimurium isolated from RAW 264.7 macrophages: Identification of a novel protein that contributes to the replication of serovar typhimurium inside macrophages. J. Biol. Chem. 281, 29131–29140 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Wilburn K. M., Fieweger R. A., VanderVen B. C., Cholesterol and fatty acids grease the wheels of Mycobacterium tuberculosis pathogenesis. Pathog Dis. 76, fty021 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beste D. J., McFadden J., System-level strategies for studying the metabolism of Mycobacterium tuberculosis. Mol. Biosyst. 6, 2363–2372 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pandey A. K., Sassetti C. M., Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. U.S.A. 105, 4376–4380 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brock M., Buckel W., On the mechanism of action of the antifungal agent propionate. Eur. J. Biochem. 271, 3227–3241 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Fernández-García M., et al. , Comprehensive examination of the mouse lung metabolome following mycobacterium tuberculosis infection using a multiplatform mass spectrometry approach. J. Proteome Res. 19, 2053–2070 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khurana H., et al. , Identification of diphenyl furan derivatives via high throughput and computational studies as ArgA inhibitors of Mycobacterium tuberculosis. Int. J. Biol. Macromol. 193, 1845–1858 (2021). [DOI] [PubMed] [Google Scholar]
- 72.Ramsaywak P. C., Labbe G., Siemann S., Dmitrienko G. I., Guillemette J. G., Molecular cloning, expression, purification, and characterization of fructose 1,6-bisphosphate aldolase from Mycobacterium tuberculosis–a novel Class II A tetramer. Protein Expr Purif 37, 220–228 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Pegan S. D., Rukseree K., Franzblau S. G., Mesecar A. D., Structural basis for catalysis of a tetrameric class IIa fructose 1,6-bisphosphate aldolase from Mycobacterium tuberculosis. J. Mol. Biol. 386, 1038–1053 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Makowska-Grzyska M., et al. , Mycobacterium tuberculosis IMPDH in complexes with substrates, products and antitubercular compounds. PLoS One 10, e0138976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen M., Huang X., Zhong C., Li J., Lu X., Identification of an itaconic acid degrading pathway in itaconic acid producing Aspergillus terreus. Appl. Microbiol. Biotechnol. 100, 7541–7548 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Bardarov S., et al. , Specialized transduction: An efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology (Reading) 148, 3007–3017 (2002). [DOI] [PubMed] [Google Scholar]
- 77.Singh R., et al. , Polyphosphate deficiency in Mycobacterium tuberculosis is associated with enhanced drug susceptibility and impaired growth in guinea pigs. J. Bacteriol. 195, 2839–2851 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh M., et al. , Establishing virulence associated polyphosphate kinase 2 as a drug target for Mycobacterium tuberculosis. Sci. Rep. 6, 26900 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Dataset S05 (XLSX)
Dataset S06 (XLSX)
Data Availability Statement
All study data are included in the article and/or supporting information.