Abstract
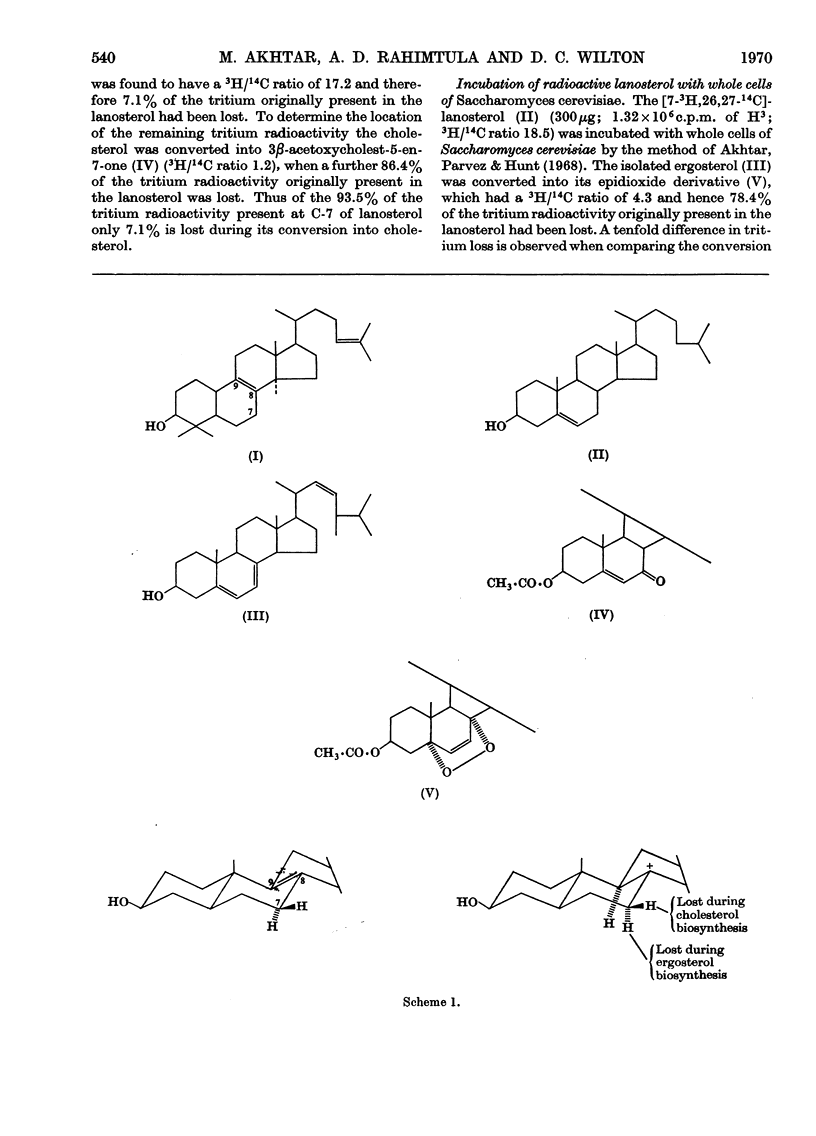
The synthesis of [7α-3H]lanosterol is described. It is shown that in the conversion of [7α-3H,26,27-14C2]lanosterol into cholesterol by a rat liver system, it is the 7β-hydrogen atom that is predominantly removed. On the other hand, the conversion of doubly labelled lanosterol into ergosterol by whole yeast cells results in the loss of the 7α-hydrogen atom. These results therefore suggest that the C-7 hydrogen atoms with opposite stereochemistry are labilized by the rat liver and the yeast Δ8–Δ7 steroid isomerases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., Hunt P. F., Parvez M. A. The transfer of hydrogen from C-24 to C-25 in ergosterol biosynthesis. Biochem J. 1967 Jun;103(3):616–622. doi: 10.1042/bj1030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M., Parvez M. A., Hunt P. F. Studies on the biosynthesis of the ergosterol side chain. Biochem J. 1969 Jul;113(4):727–732. doi: 10.1042/bj1130727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M., Parvez M. A., Hunt P. F. The introduction of the C-22-C-23 ethylenic linkage in ergosterol biosynthesis. Biochem J. 1968 Feb;106(3):623–626. doi: 10.1042/bj1060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M., Rahimtula A. D., Watkinson I. A., Wilton D. C., Munday K. A. The status of C-6, C-7, C-15 and C-16 hydrogen atoms in cholesterol biosynthesis. Eur J Biochem. 1969 May 1;9(1):107–111. doi: 10.1111/j.1432-1033.1969.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Akhtar M., Watkinson I. A., Rahimtula A. D., Wilton D. C., Munday K. A. The role of a cholesta-8,14-dien-3-beta-ol system in cholesterol biosynthesis. Biochem J. 1969 Mar;111(5):757–761. doi: 10.1042/bj1110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonica L., Fiecchi A., Kienle M. G., Scala A., Galli G., Paoletti E. G., Paoletti R. The biological conversion of 5alpha-cholest-8-en-3beta-ol to 5alpha-cholest-7-en-3beta-ol in the biosynthesis of cholesterol. Steroids. 1968 Mar;11(3):287–298. doi: 10.1016/s0039-128x(68)80141-2. [DOI] [PubMed] [Google Scholar]
- Caspi E., Greig J. B., Ramm P. J., Varma K. R. Stereochemistry of tritium at C-1 and C-7 in cholesterol derived from (3R,2R)-2T-mevalonic acid. Tetrahedron Lett. 1968 Jul;(35):3829–3832. doi: 10.1016/s0040-4039(01)99112-9. [DOI] [PubMed] [Google Scholar]
- Cornforth J. W., Cornforth R. H., Popják G., Yengoyan L. Studies on the biosynthesis of cholesterol. XX. Steric course of decarboxylation of 5-pyrophosphomevalonate and of the carbon to carbon bond formation in the biosynthesis of farnesyl pyrophosphate. J Biol Chem. 1966 Sep 10;241(17):3970–3987. [PubMed] [Google Scholar]
- Katsuki H., Bloch K. Studies on the biosynthesis of ergosterol in yeast. Formation of methylated intermediates. J Biol Chem. 1967 Jan 25;242(2):222–227. [PubMed] [Google Scholar]
- Lee W. H., Kammereck R., Lutsky B. N., McCloskey J. A., Schroepfer G. J. Studies on the mechanism of the enzymatic conversion of delta 8-cholesten-3 beta-ol to delta 7-cholesten-3 beta-ol. J Biol Chem. 1969 Apr 25;244(8):2033–2040. [PubMed] [Google Scholar]
- Rees H. H., Goad L. J., Goodwin T. W. Studies in phytosterol biosynthesis. Mechanism of biosynthesis of cycloartenol. Biochem J. 1968 Apr;107(3):417–426. doi: 10.1042/bj1070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton D. C., Munday K. A., Skinner S. J., Akhtar M. The biological conversion of 7-dehydrocholesterol into cholesterol and comments on the reduction of double bonds. Biochem J. 1968 Feb;106(4):803–810. doi: 10.1042/bj1060803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton D. C., Rahimtula A. D., Akhtar M. The reversibility of the delta8-cholestenol-delta7-cholestenol isomerase reaction in cholesterol biosynthesis. Biochem J. 1969 Aug;114(1):71–73. doi: 10.1042/bj1140071. [DOI] [PMC free article] [PubMed] [Google Scholar]


