Abstract
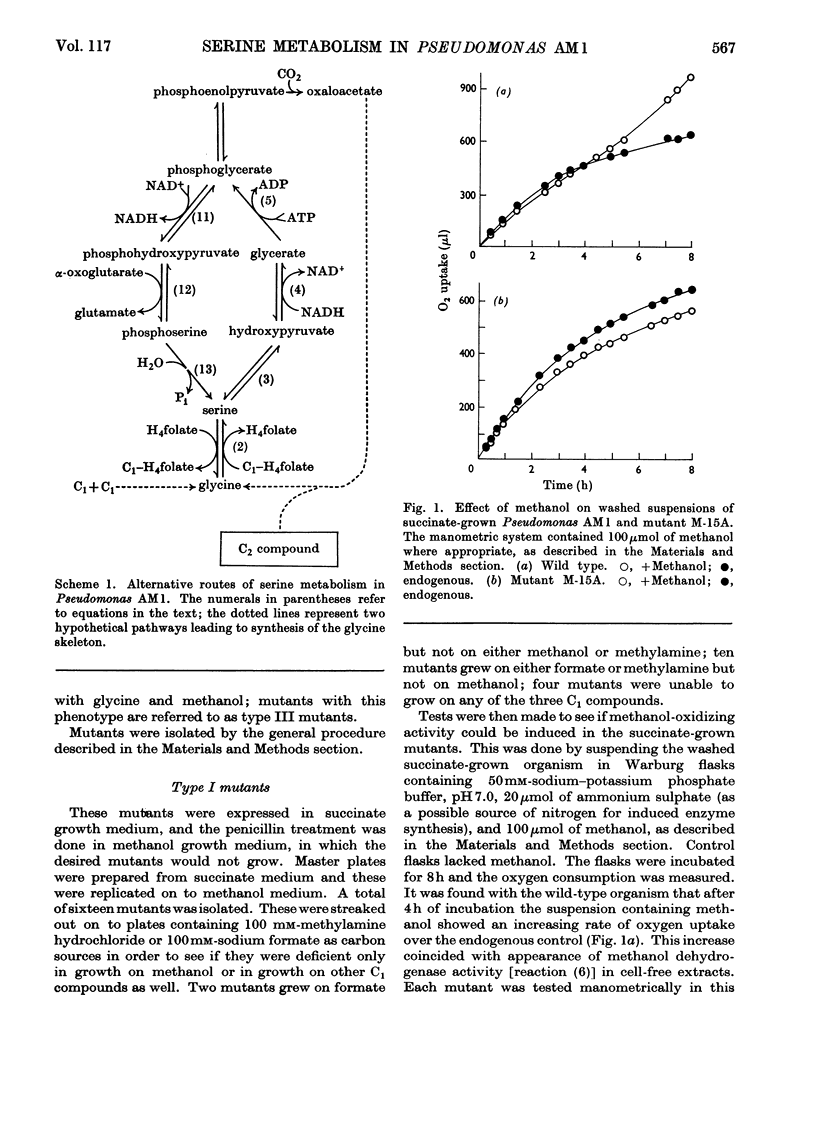
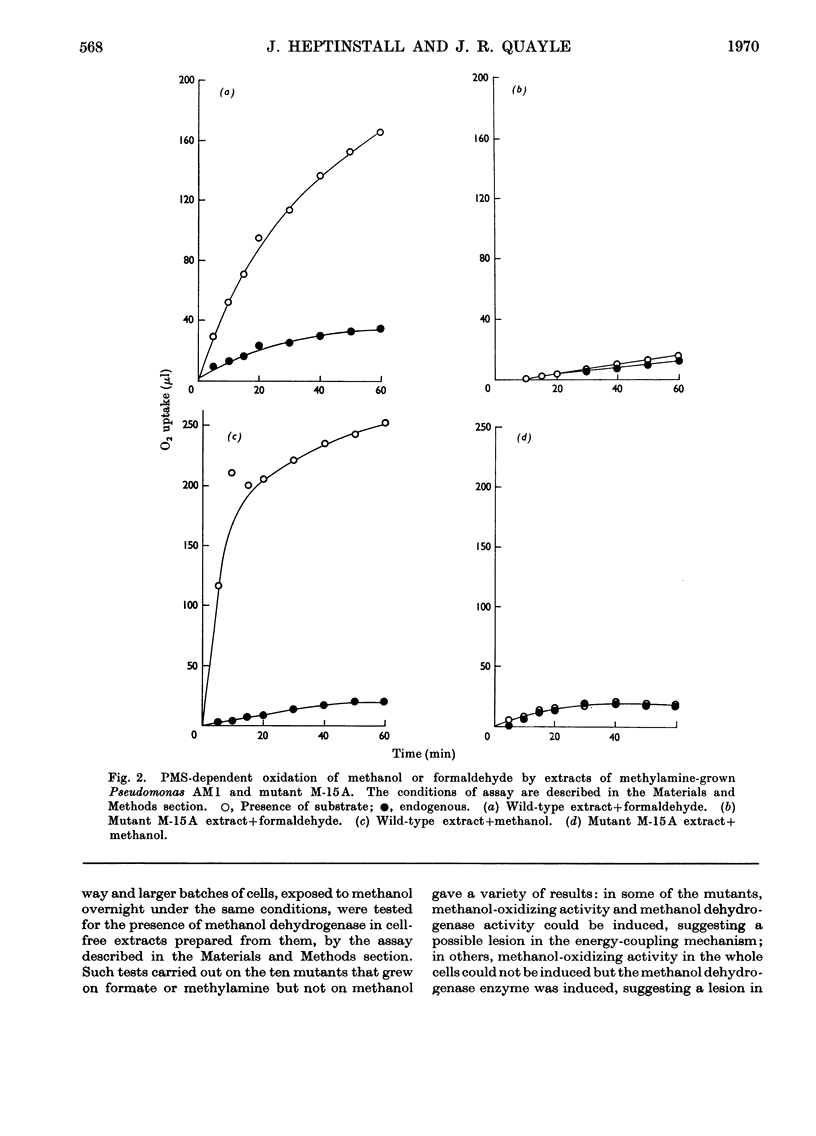
1. The following enzymes of the phosphorylated pathway of serine biosynthesis have been found in methanol- and succinate-grown Pseudomonas AM1: phosphoglycerate dehydrogenase, phosphoserine-α-oxoglutarate aminotransferase and phosphoserine phosphohydrolase. Their specific activities were similar in the organism grown on either substrate. 2. A procedure for preparation of auxotrophic mutants of Pseudomonas AM1 is described involving N-methyl-N′-nitro-N-nitrosoguanidine as mutagen and a penicillin enrichment step. 3. A mutant, M-15A, has been isolated that is unable to grow on methanol and that lacks phenazine methosulphate-linked methanol dehydrogenase. The mutant is able to grow on methylamine, showing that the amine is not oxidized by way of methanol. 4. Loss of methanol dehydrogenase activity in mutant M-15A led to loss of phenazine methosulphate-linked formaldehyde dehydrogenase activity showing that the same enzyme is probably responsible for both activities. 5. A mutant, 20B-L, has been isolated that cannot grow on any C1 compound tested but can grow on succinate. 6. Mutant 20B-L lacks hydroxypyruvate reductase, and revertants that regained the ability to grow on methanol, methylamine and formate contained hydroxypyruvate reductase activity at specific activities similar to that of the wild-type organism. This shows that hydroxypyruvate reductase is necessary for growth on methanol, methylamine and formate but not for growth on succinate. 7. The results suggest that during growth of Pseudomonas AM1 on C1 compounds, serine is converted into 3-phosphoglycerate by a non-phosphorylated pathway, whereas during growth on succinate, phosphoglycerate is converted into serine by a phosphorylated pathway.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. 2. The methanol-oxidizing enzyme of Pseudomonas sp. M 27. Biochem J. 1964 Sep;92(3):614–621. doi: 10.1042/bj0920614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATT R. D., DICKENS F., WILLIAMSON D. H. The enzymic incorporation of the beta-carbon of serine into dihydroxyacetone. Biochim Biophys Acta. 1960 Dec 18;45:571–575. doi: 10.1016/0006-3002(60)91495-5. [DOI] [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J. 1968 Jan;106(1):245–255. doi: 10.1042/bj1060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- JAYASURIYA G. C. The isolation and characteristics of an oxalate-decomposing organism. J Gen Microbiol. 1955 Jun;12(3):419–428. doi: 10.1099/00221287-12-3-419. [DOI] [PubMed] [Google Scholar]
- JONES K. M., GUEST J. R., WOODS D. D. Folic acid and the synthesis of methionine by extracts of Escherichia coli. Biochem J. 1961 Jun;79:566–574. doi: 10.1042/bj0790566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARGE P. J., PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. II. Synthesis of cell constituents by methanol- and formate-grown Pseudomonas AM 1, and methanol-grown Hyphomicrobium vulgare. Biochem J. 1961 Dec;81:470–480. doi: 10.1042/bj0810470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ladner A., Zatman L. J. Formaldehyde oxidation by the methanol dehydrogenase of seudomonas PP. J Gen Microbiol. 1969 Mar;55(3):xvi–xvi. [PubMed] [Google Scholar]
- Large P. J., Peel D., Quayle J. R. Microbial growth on C(1) compounds. 3. Distribution of radioactivity in metabolites of methanol-grown Pseudomonas AM1 after incubation with [C]methanol and [C]bicarbonate. Biochem J. 1962 Mar;82(3):483–488. doi: 10.1042/bj0820483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large P. J., Peel D., Quayle J. R. Microbial growth on C(1) compounds. 4. Carboxylation of phosphoenolpyruvate in methanol-grown Pseudomonas AM1. Biochem J. 1962 Oct;85(1):243–250. doi: 10.1042/bj0850243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large P. J., Quayle J. R. Microbial growth on C(1) compounds. 5. Enzyme activities in extracts of Pseudomonas AM1. Biochem J. 1963 May;87(2):386–396. doi: 10.1042/bj0870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A. J., Kemp M. B., Quayle J. R. Synthesis of cell constituents by methane-grown Methylococcus capsulatus and Methanomonas methanooxidans. Biochem J. 1970 Feb;116(4):631–639. doi: 10.1042/bj1160631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston M. K., Ornston L. N. Two forms of D-glycerate kinase in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1227–1233. doi: 10.1128/jb.97.3.1227-1233.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. I. Isolation and characterization of Pseudomonas AM 1. Biochem J. 1961 Dec;81:465–469. doi: 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I. GLYCINE SYNTHESIS AND METABOLISM IN ESCHERICHIA COLI. J Bacteriol. 1965 Apr;89:1145–1150. doi: 10.1128/jb.89.4.1145-1150.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I., POTOCHNY M. L. NUTRITIONAL AND REGULATORY ASPECTS OF SERINE METABOLISM IN ESCHERICHIA COLI. J Bacteriol. 1964 Sep;88:611–619. doi: 10.1128/jb.88.3.611-619.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I. THE PATHWAY AND CONTROL OF SERINE BIOSYNTHESIS IN ESCHERICHIA COLI. J Biol Chem. 1963 Dec;238:3934–3944. [PubMed] [Google Scholar]
- UMBARGER H. E., UMBARGER M. A., SIU P. M. BIOSYNTHESIS OF SERINE IN ESCHERICHIA COLI AND SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Jun;85:1431–1439. doi: 10.1128/jb.85.6.1431-1439.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., UMBARGER M. A. The biosynthetic pathway of serine in salmonella typhimurium. Biochim Biophys Acta. 1962 Jul 30;62:193–195. doi: 10.1016/0006-3002(62)90515-2. [DOI] [PubMed] [Google Scholar]
- WYNGAARDEN J. B., ASHTON D. M. The regulation of activity of phosphoribosyl-pyrophosphate amidotransferase by purine ribonacleotides: a potential feedback control of purine biosyntoesis. J Biol Chem. 1959 Jun;234(6):1492–1496. [PubMed] [Google Scholar]


