Abstract
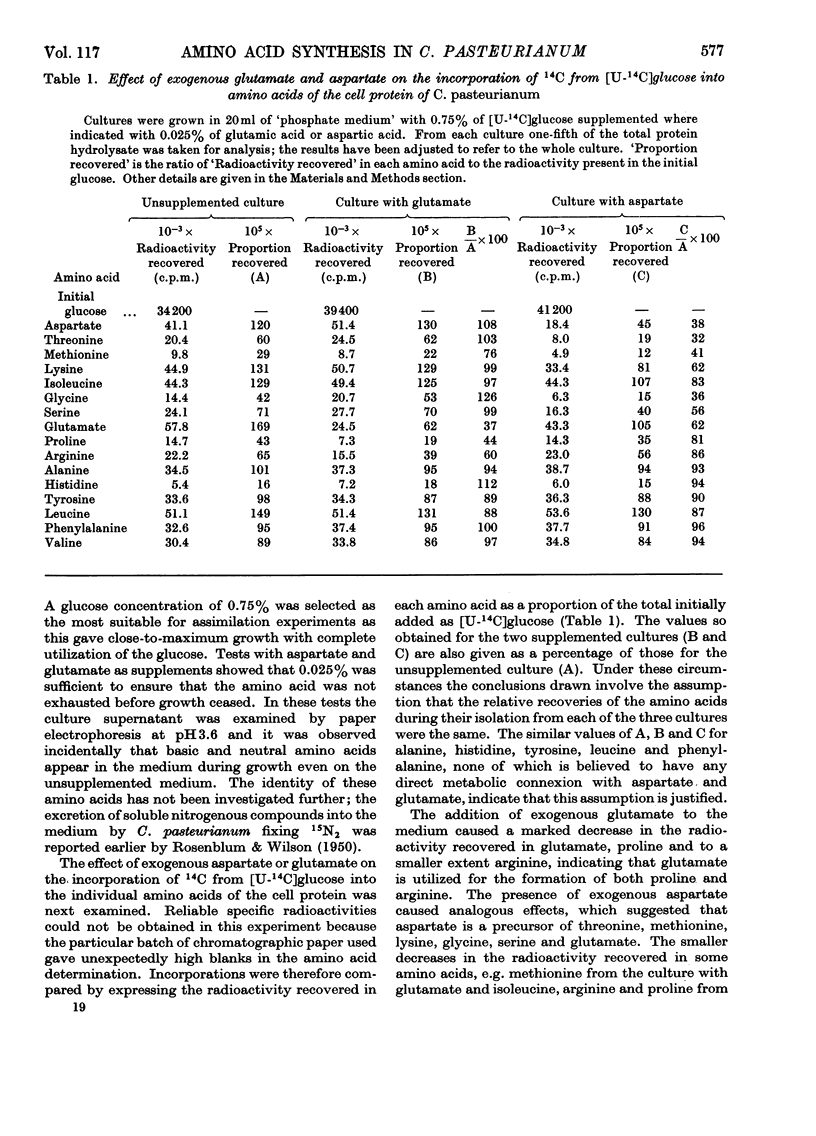
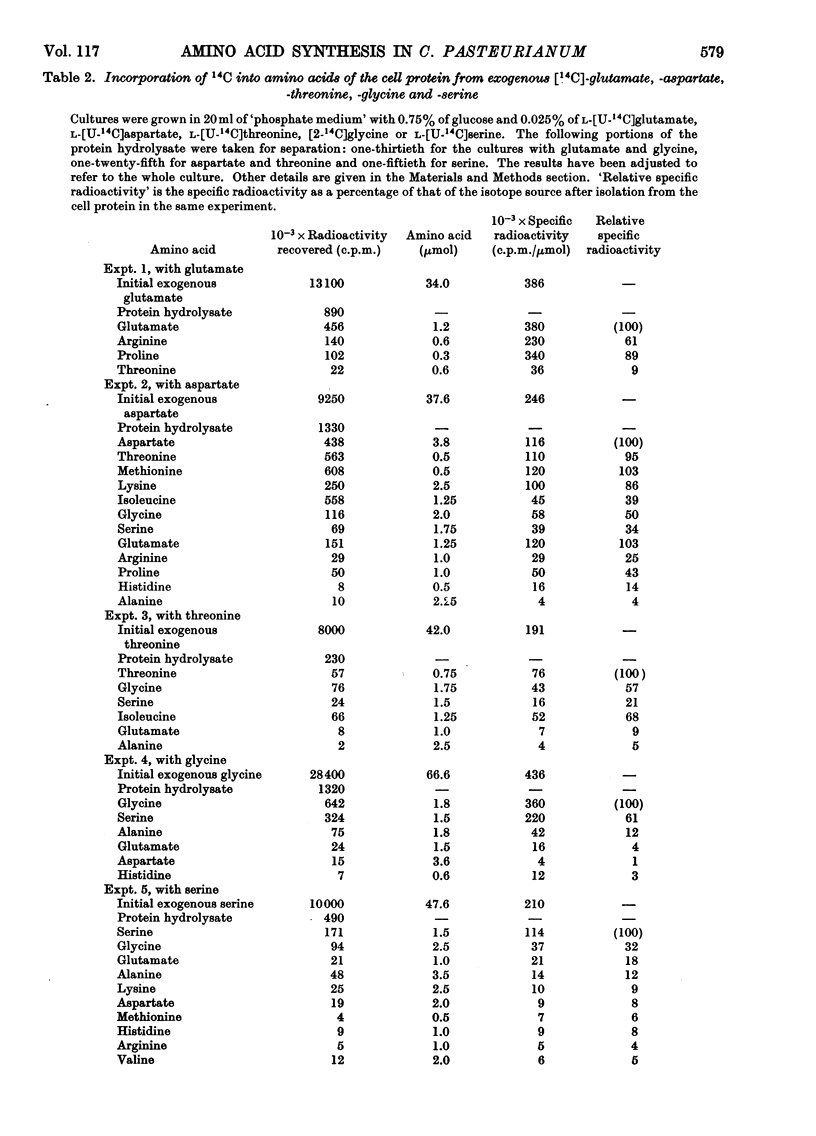
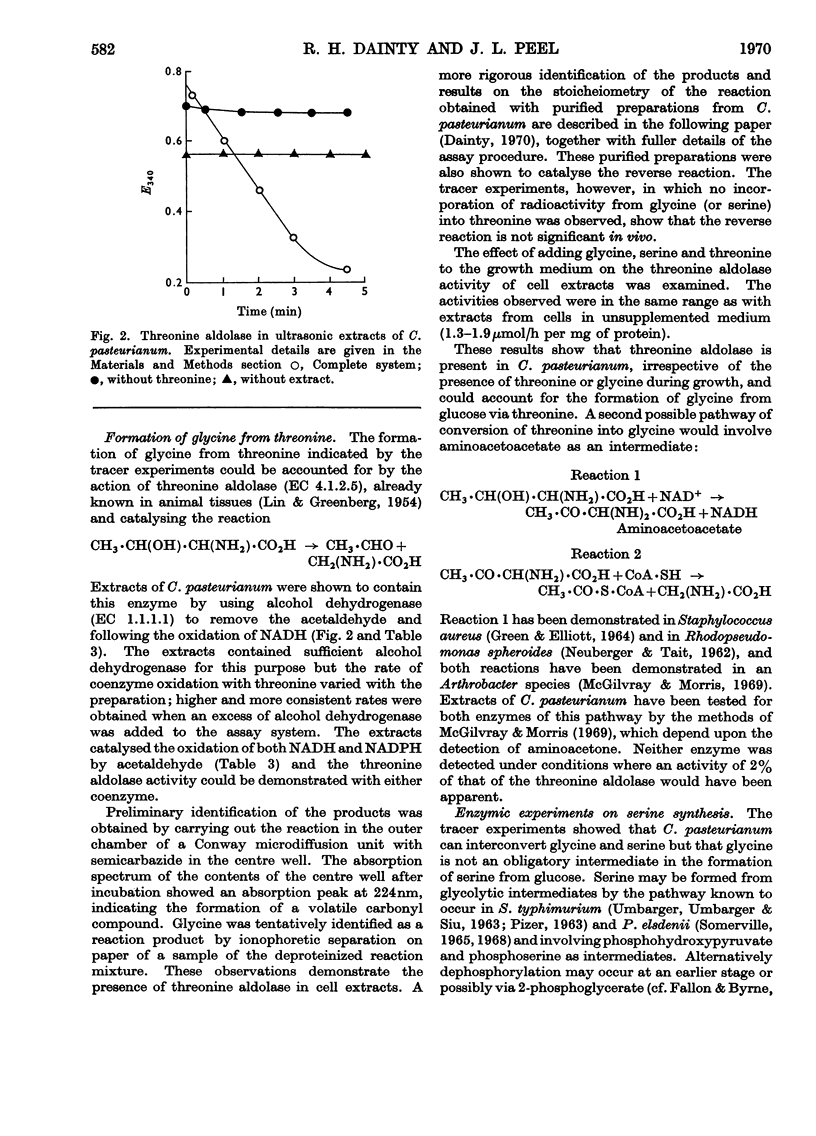
1. Clostridium pasteurianum was grown on a synthetic medium with the following carbon sources: (a) 14C-labelled glucose, alone or with unlabelled aspartate or glutamate, or (b) unlabelled glucose plus 14C-labelled aspartate, glutamate, threonine, serine or glycine. The incorporation of 14C into the amino acids of the cell protein was examined. 2. In both series of experiments carbon from exogenous glutamate was incorporated into proline and arginine; carbon from aspartate was incorporated into glutamate, proline, arginine, lysine, methionine, threonine, isoleucine, glycine and serine. Incorporations from the other exogenous amino acids indicated the metabolic sequence: aspartate → threonine → glycine ⇌ serine. 3. The following activities were demonstrated in cell-free extracts of the organism: (a) the formation of aspartate by carboxylation of phosphoenolpyruvate or pyruvate, followed by transamination; (b) the individual reactions of the tricarboxylic acid route to 2-oxoglutarate from oxaloacetate; glutamate dehydrogenase was not detected; (c) the conversion of aspartate into threonine via homoserine; (d) the conversion of threonine into glycine by a constitutive threonine aldolase; (e) serine transaminase, phosphoserine transaminase, glycerate dehydrogenase and phosphoglycerate dehydrogenase. This last activity was abnormally high. 4. The combined evidence indicates that in C. pasteurianum the biosynthetic role of aspartate and glutamate is generally similar to that in aerobic and facultatively aerobic organisms, but that glycine is synthesized from glucose via aspartate and threonine.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S., WRIGHT N. G. Homoserine dehydrogenase. J Biol Chem. 1955 Mar;213(1):51–60. [PubMed] [Google Scholar]
- BLACK S., WRIGHT N. G. beta-Aspartokinase and beta-aspartyl phosphate. J Biol Chem. 1955 Mar;213(1):27–38. [PubMed] [Google Scholar]
- BRYANT F., OVERELL B. T. Quantitative chromatographic analysis of organic acids in plant tissue extracts. Biochim Biophys Acta. 1953 Mar;10(3):471–476. doi: 10.1016/0006-3002(53)90279-0. [DOI] [PubMed] [Google Scholar]
- CARNAHAN J. E., CASTLE J. E. Some requirements of biological nitrogen fixation. J Bacteriol. 1958 Feb;75(2):121–124. doi: 10.1128/jb.75.2.121-124.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARNAHAN J. E., MORTENSON L. E., MOWER H. F., CASTLE J. E. Nitrogen fixation in cell-free extracts of Clostridium pasteurianum. Biochim Biophys Acta. 1960 Nov 18;44:520–535. doi: 10.1016/0006-3002(60)91606-1. [DOI] [PubMed] [Google Scholar]
- DIXON G. H., KAUFFMAN D. L., NEURATH H. Amino acid sequence in the region of diisopropylphosphoryl binding in diisopropylphosphoryl-trypsin. J Biol Chem. 1958 Dec;233(6):1373–1381. [PubMed] [Google Scholar]
- Dainty R. H. Purification and properties of threonine aldolase from Clostridium pasteurianum. Biochem J. 1970 Apr;117(3):585–592. doi: 10.1042/bj1170585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent C. E. The amino-aciduria in Fanconi syndrome. A study making extensive use of techniques based on paper partition chromatography. Biochem J. 1947;41(2):240–253. doi: 10.1042/bj0410240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRY B. A. THE RELATIONSHIP BETWEEN THE COMPOSITION OF THE ENVIRONMENT AND THE CONTROL OF BIOSYNTHESIS IN BACTERIA. Proc Nutr Soc. 1964;23:170–188. doi: 10.1079/pns19640030. [DOI] [PubMed] [Google Scholar]
- Gottschalk G., Barker H. A. Presence and stereospecificity of citrate synthase in anaerobic bacteria. Biochemistry. 1967 Apr;6(4):1027–1034. doi: 10.1021/bi00856a011. [DOI] [PubMed] [Google Scholar]
- Gottschalk G. The stereospecificity of the citrate synthase in sulfate-reducing and photosynthetic bacteria. Eur J Biochem. 1968 Aug;5(3):346–351. doi: 10.1111/j.1432-1033.1968.tb00376.x. [DOI] [PubMed] [Google Scholar]
- Green M. L., Elliott W. H. The enzymic formation of aminoacetone from threonine and its further metabolism. Biochem J. 1964 Sep;92(3):537–549. doi: 10.1042/bj0920537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- KAWERAU E., WIELAND T. Conservation of amino-acid chromatograms. Nature. 1951 Jul 14;168(4263):77–78. doi: 10.1038/168077b0. [DOI] [PubMed] [Google Scholar]
- KEMBLE A. R., MACPHERSON H. T. Determination of monoamino monocarboxylic acids by quantitative paper chromatography. Biochem J. 1954 Apr;56(4):548–555. doi: 10.1042/bj0560548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIGHT M. The photometabolism of propionate by Rhodospirillum rubrum. Biochem J. 1962 Jul;84:170–185. doi: 10.1042/bj0840170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN S. C., GREENBERG D. M. Enzymatic breakdown of threonine by threonine aldolase. J Gen Physiol. 1954 Nov 20;38(2):181–196. doi: 10.1085/jgp.38.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGilvray D., Morris J. G. Utilization of L-threonine by a species of Arthrobacter. A novel catabolic role for "aminoacetone synthase". Biochem J. 1969 May;112(5):657–671. doi: 10.1042/bj1120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUBERGER A., TAIT G. H. Production of aminoacetone by Rhodopseudomonas spheroides. Biochem J. 1962 Aug;84:317–328. doi: 10.1042/bj0840317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I. THE PATHWAY AND CONTROL OF SERINE BIOSYNTHESIS IN ESCHERICHIA COLI. J Biol Chem. 1963 Dec;238:3934–3944. [PubMed] [Google Scholar]
- REDFIELD R. R. Two-dimensional paper chromatographic systems with high resolving power for amino acids. Biochim Biophys Acta. 1953 Feb;10(2):344–345. doi: 10.1016/0006-3002(53)90260-1. [DOI] [PubMed] [Google Scholar]
- ROSENBLUM E. D., WILSON P. W. Molecular hydrogen and nitrogen fixation by Clostridium. J Bacteriol. 1950 Jan;59(1):83–91. doi: 10.1128/jb.59.1.83-91.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHMAN F., HIGA A. A new two-dimensional system for the separation of amino acids on paper. Anal Biochem. 1962 Mar;3:173–177. doi: 10.1016/0003-2697(62)90052-0. [DOI] [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville H. J. Enzymic studies on the biosynthesis of amino acids from lactate by peptostreptococcus elsdenii. Biochem J. 1968 Jun;108(1):107–119. doi: 10.1042/bj1080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville H. J., Peel J. L. Tracer studies on the biosynthesis of amino acids from lactate by Peptostreptococcus elsdenii. Biochem J. 1967 Oct;105(1):299–310. doi: 10.1042/bj1050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., UMBARGER M. A., SIU P. M. BIOSYNTHESIS OF SERINE IN ESCHERICHIA COLI AND SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Jun;85:1431–1439. doi: 10.1128/jb.85.6.1431-1439.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON P. W., BURRIS R. H. Biological nitrogen fixation; a reappraisal. Annu Rev Microbiol. 1953;7:415–432. doi: 10.1146/annurev.mi.07.100153.002215. [DOI] [PubMed] [Google Scholar]


