Abstract
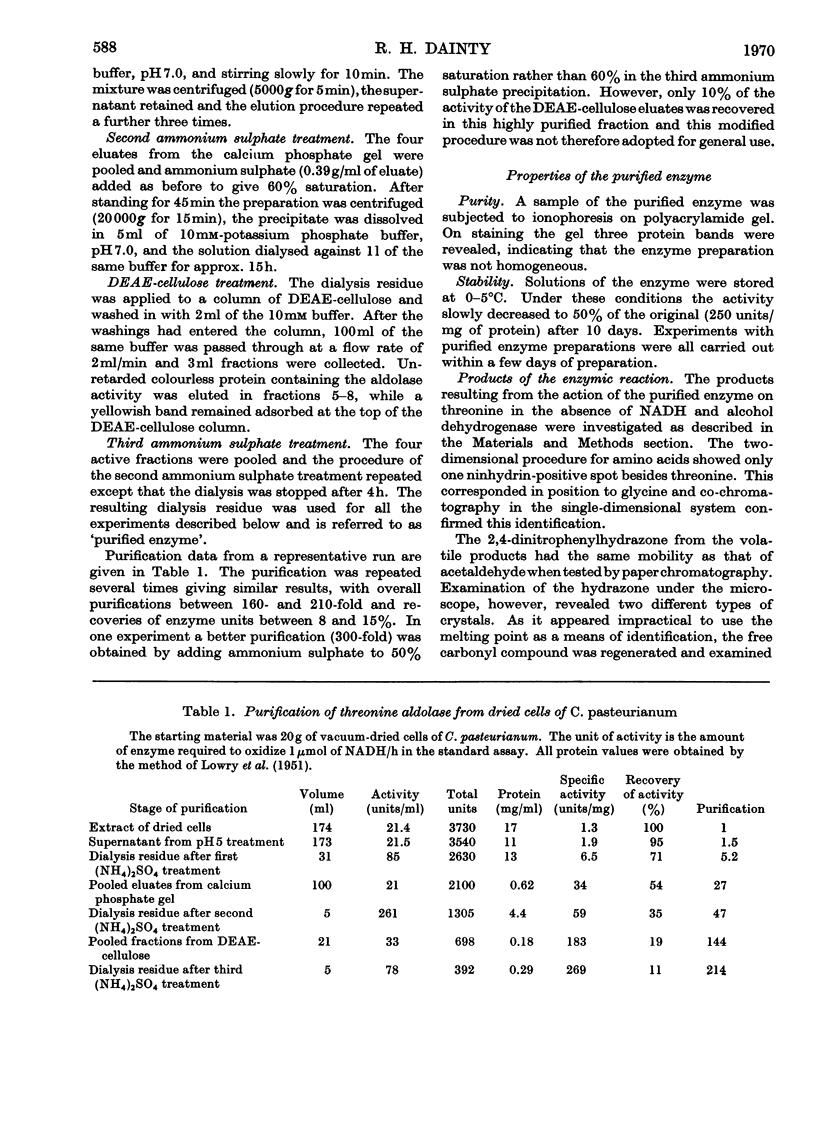
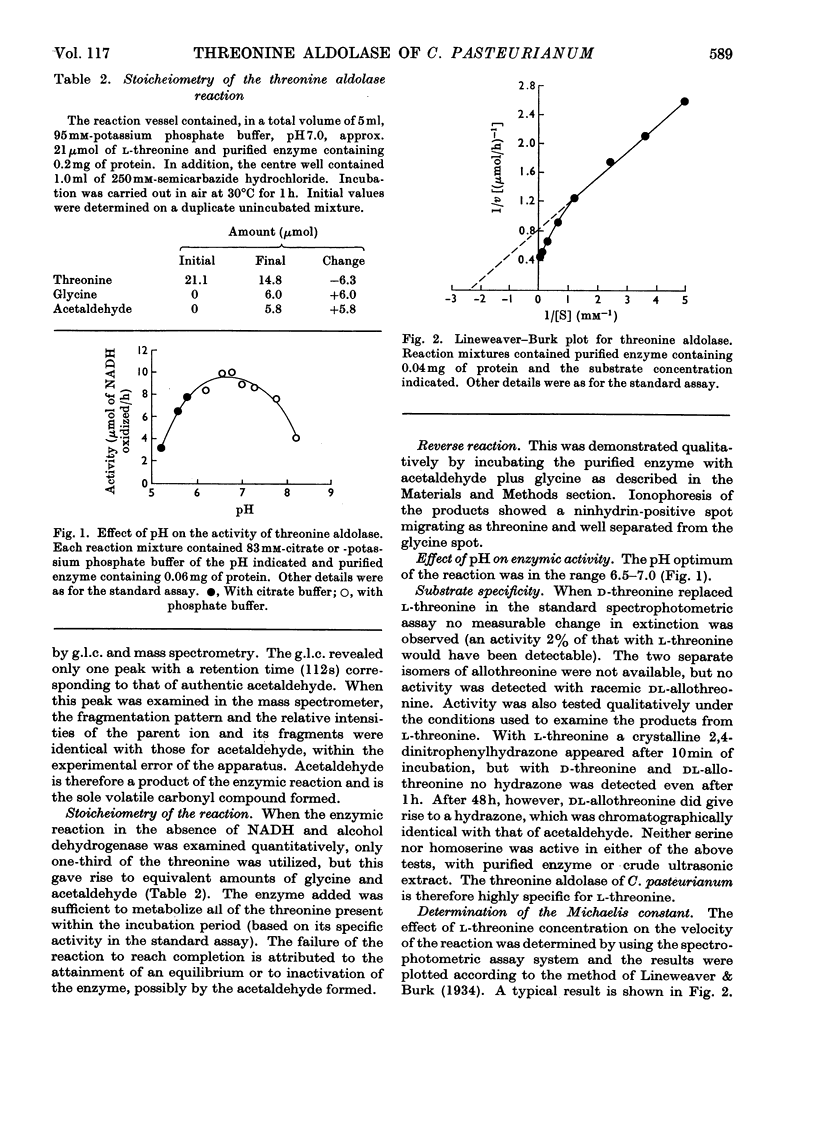
1. Threonine aldolase was purified about 200-fold in 10% yield from Clostridium pasteurianum and its properties were examined. The final preparation gave three bands after ionophoresis on polyacrylamide gel. 2. The purified enzyme was shown to produce glycine and acetaldehyde in stoicheiometric amounts from threonine. The reverse reaction was demonstrated qualitatively. 3. The enzyme has a broad pH optimum at 6.5–7.0. 4. The enzyme is highly specific for l-threonine. 5. The enzyme is completely inhibited by 1mm concentrations of hydroxylamine and semicarbazide. Activity is decreased to 20% of the original by treatment with cysteine plus mercaptoethanol; most of the loss is regained on incubation with pyridoxal phosphate. It is concluded that pyridoxal phosphate is a prosthetic group. 6. The relationship between velocity and substrate concentration is atypical but indicates a Km value of 0.42mm. 7. The enzyme was demonstrated in several other strictly anaerobic bacteria.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALMOND H. R., Jr, NIEMANN C. The consequences of systematic error in enzyme kinetics. Biochim Biophys Acta. 1960 Oct 21;44:143–150. doi: 10.1016/0006-3002(60)91532-8. [DOI] [PubMed] [Google Scholar]
- BURBRIDGE T. N., HINE C. H., SCHICK A. F. A simple spectrophotometric method for the determination of acetaldehyde in blood. J Lab Clin Med. 1950 Jun;35(6):983–987. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dainty R. H., Peel J. L. Biosynthesis of amino acids in Clostridium pasteurianum. Biochem J. 1970 Apr;117(3):573–584. doi: 10.1042/bj1170573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupourque D., Newton W. A., Snell E. E. Purification and properties of D-serine dehydrase from Escherichia coli. J Biol Chem. 1966 Mar 10;241(5):1233–1238. [PubMed] [Google Scholar]
- HUELIN F. E. Volatile products of apples. III. Identification of aldehydes and ketones. Aust J Sci Res B. 1952 Aug;5(3):328–334. doi: 10.1071/bi9520328. [DOI] [PubMed] [Google Scholar]
- Ingles D. W., Knowles J. R. The alpha-chymotryptic ydrolysis of glycine esters. Biochem J. 1966 May;99(2):275–282. doi: 10.1042/bj0990275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARASEK M. A., GREENBERG D. M. Studies on the properties of threonine aldolases. J Biol Chem. 1957 Jul;227(1):191–205. [PubMed] [Google Scholar]
- KOGUT M., PODOSKI E. P. Oxidative pathways in a fluorescent Pseudomonas. Biochem J. 1953 Dec;55(5):800–811. doi: 10.1042/bj0550800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., Wolfe R. S., Elsden S. R. The synthesis of amino acids by Methanobacterium omelianskii. Biochem J. 1966 Apr;99(1):76–86. doi: 10.1042/bj0990076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- London J., Knight M. Concentrations of nicotinamide nucleotide coenzymes in micro-organisms. J Gen Microbiol. 1966 Aug;44(2):241–254. doi: 10.1099/00221287-44-2-241. [DOI] [PubMed] [Google Scholar]
- MALKIN L. I., GREENBERG D. M. PURIFICATION AND PROPERTIES OF THREONINE OR ALLOTHREONINE ALDOLASE FROM RAT LIVER. Biochim Biophys Acta. 1964 Apr 6;85:117–131. doi: 10.1016/0926-6569(64)90172-5. [DOI] [PubMed] [Google Scholar]
- McGilvray D., Morris J. G. Utilization of L-threonine by a species of Arthrobacter. A novel catabolic role for "aminoacetone synthase". Biochem J. 1969 May;112(5):657–671. doi: 10.1042/bj1120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G. Utilization of L-threnonine by a pseudomonad: a catabolic role for L-threonine aldolase. Biochem J. 1969 Nov;115(3):603–605. doi: 10.1042/bj1150603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMMONDS S., MILLER D. A. Metabolism of glycine and serine in Escherichia coli. J Bacteriol. 1957 Dec;74(6):775–783. doi: 10.1128/jb.74.6.775-783.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirch L., Gross T. Serine transhydroxymethylase. Identification as the threonine and allothreonine aldolases. J Biol Chem. 1968 Nov 10;243(21):5651–5655. [PubMed] [Google Scholar]
- Somerville H. J. Enzymic studies on the biosynthesis of amino acids from lactate by peptostreptococcus elsdenii. Biochem J. 1968 Jun;108(1):107–119. doi: 10.1042/bj1080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF J. P., 3rd, WALLACE R. A., PETERSON R. L., NIEMANN C. THE ALPHA-CHYMOTRYPSIN-CATALYZED HYDROLYSIS OF A SERIES OF ACYLATED GLYCINE METHYL ESTERS. II. BEHAVIOR AT LOW AND HIGH SUBSTRATE CONCENTRATIONS. Biochemistry. 1964 Jul;3:940–944. doi: 10.1021/bi00895a016. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C., REISSIG J. L. L-Serine dehydrase of Neurospora. J Biol Chem. 1953 Jun;202(2):567–577. [PubMed] [Google Scholar]


