Abstract
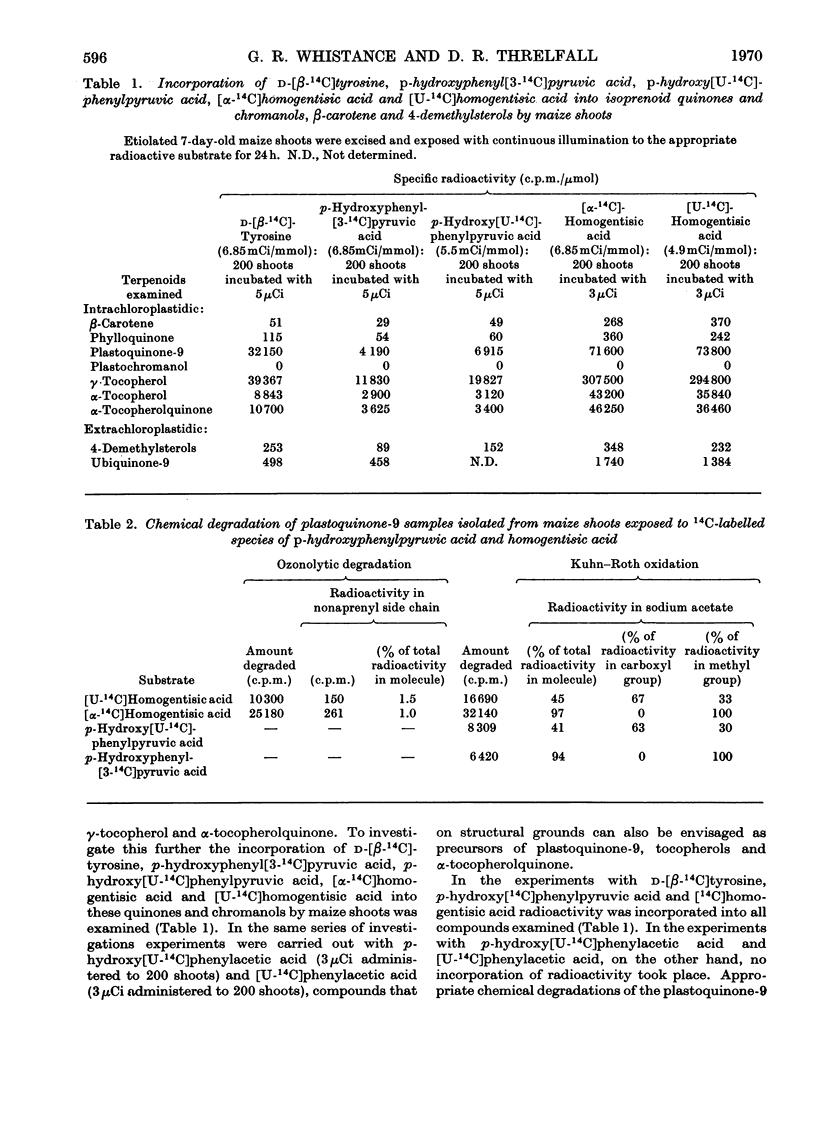
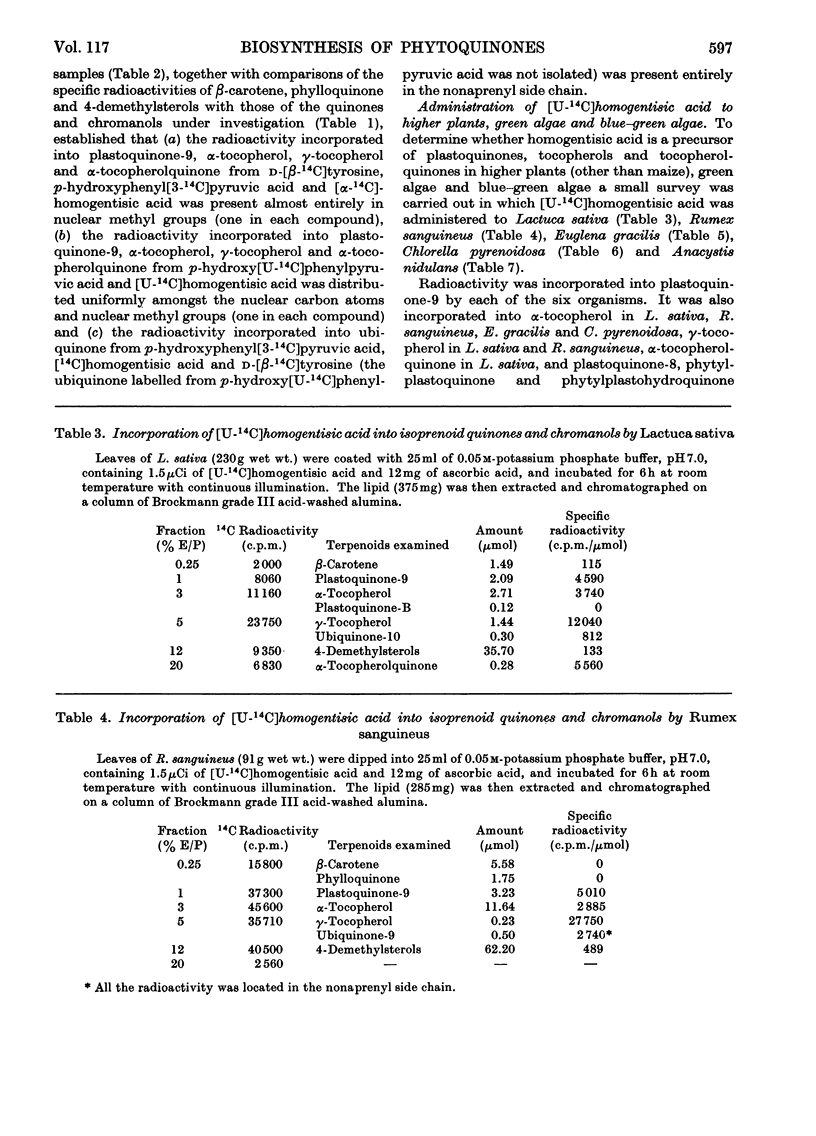
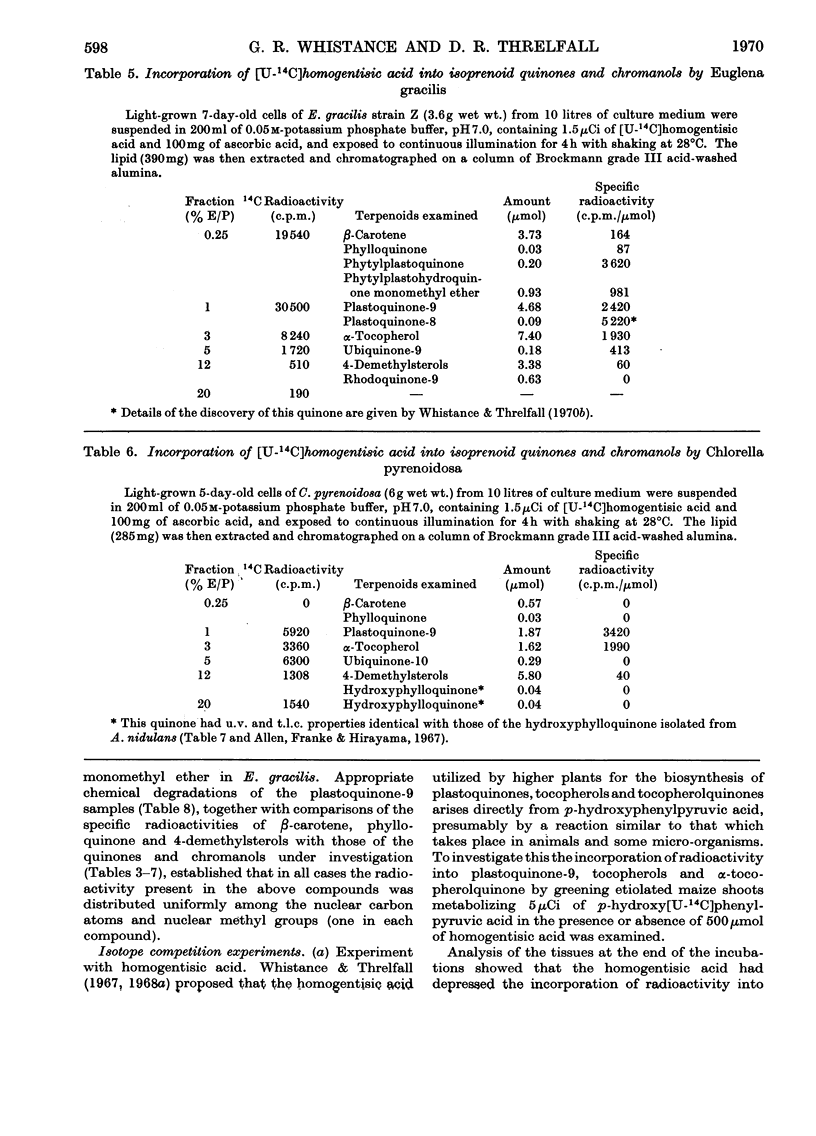
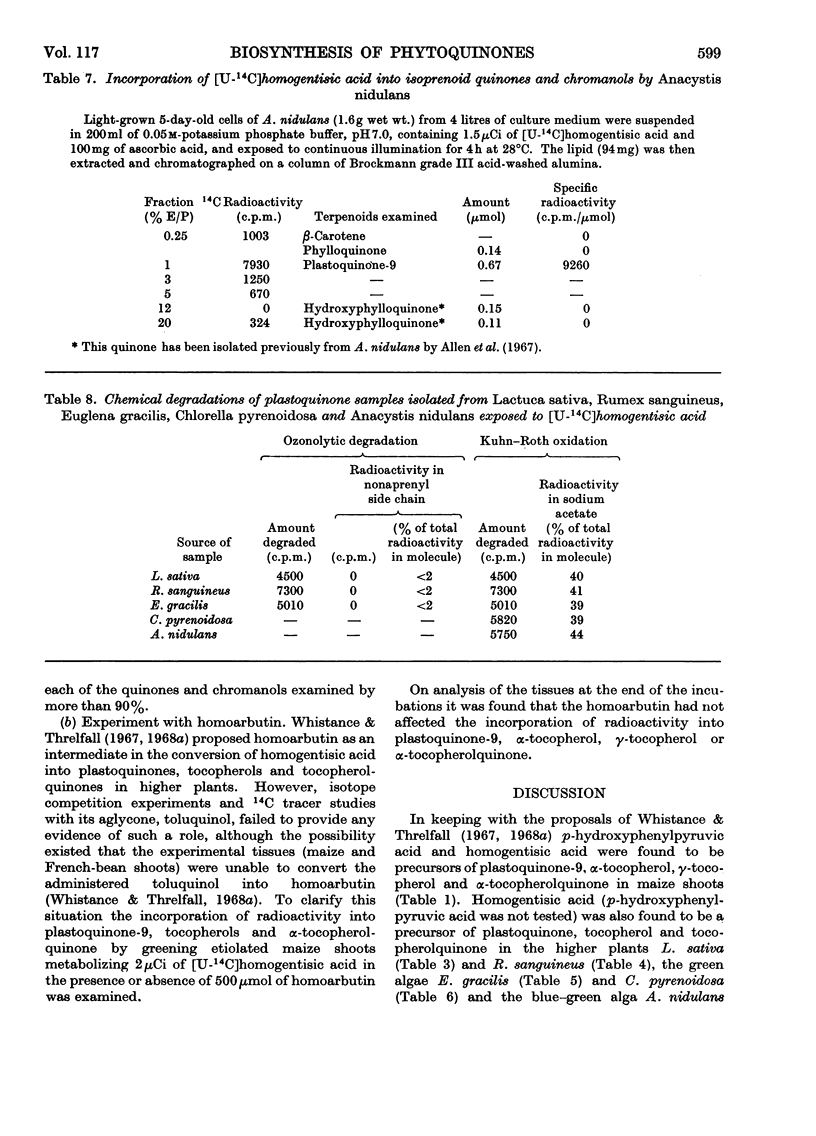
1. By means of 14C tracer experiments and isotope competition experiments the roles of d-tyrosine, p-hydroxyphenylpyruvic acid, p-hydroxyphenylacetic acid, phenylacetic acid, homogentisic acid and homoarbutin (2-methylquinol 4-β-d-glucoside) in the biosynthesis of plastoquinones, tocopherols and α-tocopherolquinone by maize shoots was investigated. It was established that d-tyrosine, p-hydroxyphenylpyruvic acid and homogentisic acid can all be utilized for this purpose, whereas p-hydroxyphenylacetic acid, phenylacetic acid and homoarbutin cannot. Studies on the mode of incorporation of d-tyrosine, p-hydroxyphenylpyruvic acid and homogentisic acid showed that their nuclear carbon atoms and the side-chain carbon atom adjacent to the nucleus give rise (as a C6-C1 unit) to the p-benzoquinone rings and nuclear methyl groups (one in each case) of plastoquinone-9 and α-tocopherolquinone and the aromatic nuclei and nuclear methyl groups (one in each case) of γ-tocopherol and α-tocopherol. 2. By using [14C]-homogentisic acid it has been shown that homogentisic acid is also a precursor of plastoquinone, tocopherols and α-tocopherolquinone in the higher plants Lactuca sativa and Rumex sanguineus, the green algae Chlorella pyrenoidosa and Euglena gracilis and the blue–green alga Anacystis nidulans.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C. F., Franke H., Hirayama O. Identification of a plastoquinone and two naphthoquinones in Anacystis nidulans by NMR and mass spectroscopy. Biochem Biophys Res Commun. 1967 Mar 9;26(5):562–568. doi: 10.1016/0006-291x(67)90102-7. [DOI] [PubMed] [Google Scholar]
- BERGMEYER H. U. Zur Messung von Katalase-Aktivitäten. Biochem Z. 1955;327(4):255–258. [PubMed] [Google Scholar]
- Dada O. A., Threlfall D. R., Whistance G. R. Biosynthesis of phytoquinones. Stereospecific biosynthesis of the polyprenyl side chains of terpenoid quinones and chromanols in maize shoots. Eur J Biochem. 1968 Apr;4(3):329–333. doi: 10.1111/j.1432-1033.1968.tb00214.x. [DOI] [PubMed] [Google Scholar]
- EVANS W. C. THE MICROBIOLOGICAL DEGRADATION OF AROMATIC COMPOUNDS. J Gen Microbiol. 1963 Aug;32:177–184. doi: 10.1099/00221287-32-2-177. [DOI] [PubMed] [Google Scholar]
- GOODWIN T. W. Some observations on carotenoid synthesis by the alga Chlorella vulgaris. Experientia. 1954 May 15;10(5):213–214. doi: 10.1007/BF02159276. [DOI] [PubMed] [Google Scholar]
- Griffiths W. T., Threlfall D. R., Goodwin T. W. Nature, intracellular distribution and formation of terpenoid quinones in maize and barley shoots. Biochem J. 1967 May;103(2):589–600. doi: 10.1042/bj1030589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LA DU B. N., ZANNONI V. G. The tyrosine oxidation system of liver. II. Oxidation of p-hydroxyphenylpyruvic acid to homogentisic acid. J Biol Chem. 1955 Dec;217(2):777–787. [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall D. R., Goodwin T. W. Nature, intracellular distribution and formation of terpenoid quinones in Euglena gracilis. Biochem J. 1967 May;103(2):573–588. doi: 10.1042/bj1030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall D. R., Whistance G. R., Goodwin T. W. Biosynthesis of phytoquinones. Incorporation of L-[Me-14C,3H]methionine into terpenoid quinones and chromanols in maize shoots. Biochem J. 1968 Jan;106(1):107–112. doi: 10.1042/bj1060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R. Biosynthesis of phytoquinones. Biosynthetic origins of the nuclei and satellite methyl groups of plastoquinone, tocopherols and tocopherolquinones in maize shoots, bean shoots and ivy leaves. Biochem J. 1968 Oct;109(4):577–595. doi: 10.1042/bj1090577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R. Biosynthesis of phytoquinones: an outline of the biosynthetic sequences involved in terpenoid quinone and chromanol formation by higher plants. Biochem Biophys Res Commun. 1967 Aug 7;28(3):295–301. doi: 10.1016/0006-291x(67)90308-7. [DOI] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R. Biosynthesis of phytoquinones: utilization of homogentisic acid by maize shoots for the biosynthesis of plastoquinone. Biochem J. 1968 Sep;109(3):482–483. doi: 10.1042/bj1090482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R., Goodwin T. W. Observations on the biosynthesis of phytoterpenoid quinone and chromanol nuclei. Biochem J. 1967 Oct;105(1):145–154. doi: 10.1042/bj1050145. [DOI] [PMC free article] [PubMed] [Google Scholar]


