Abstract
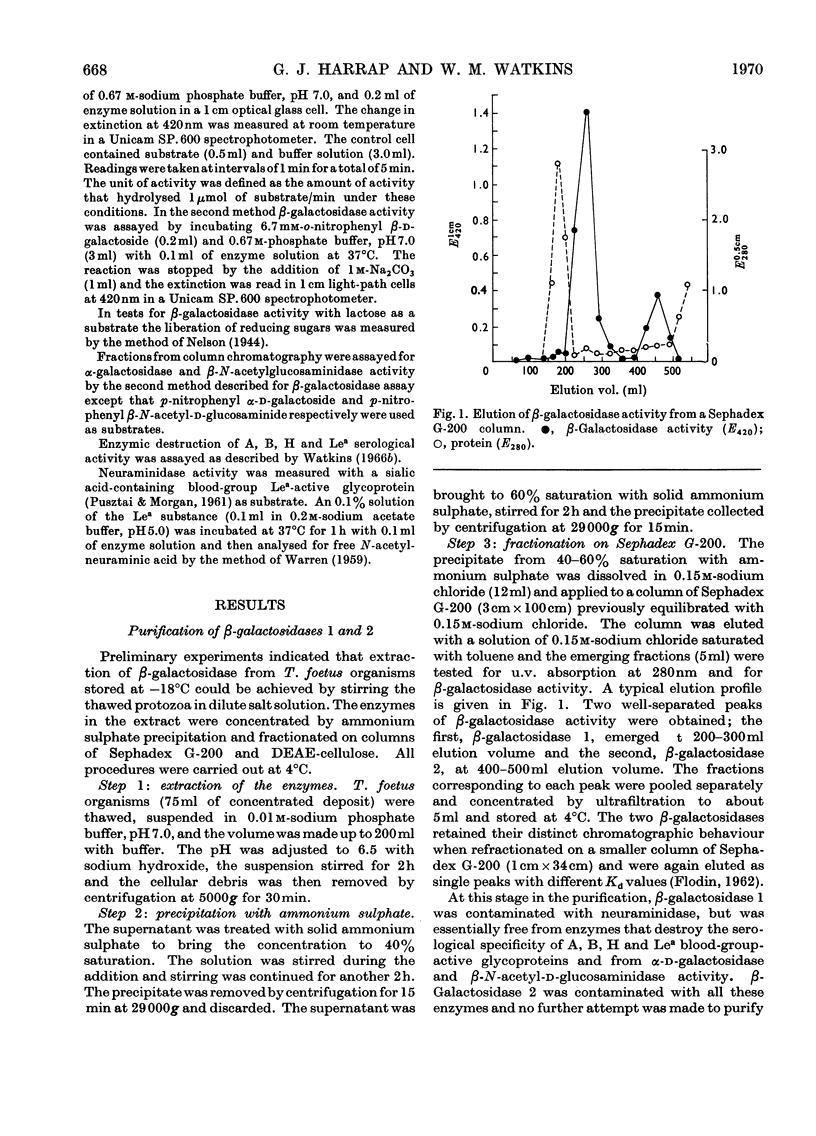
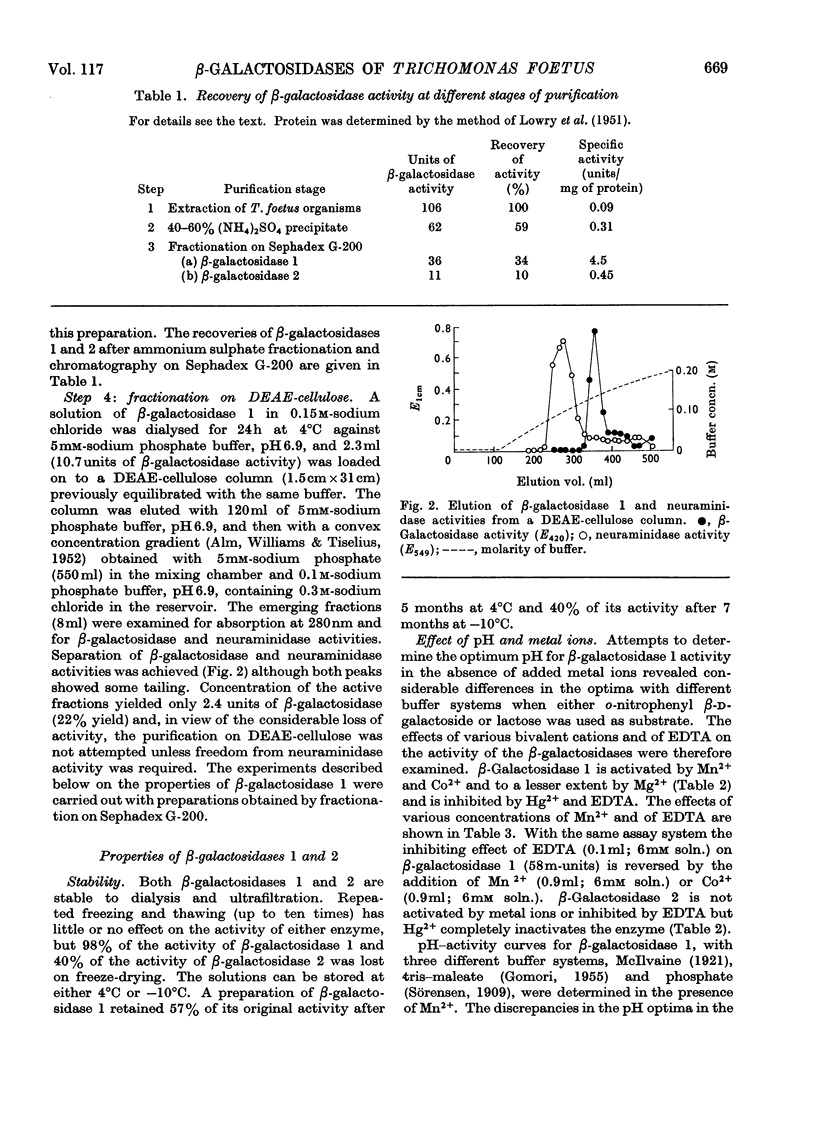
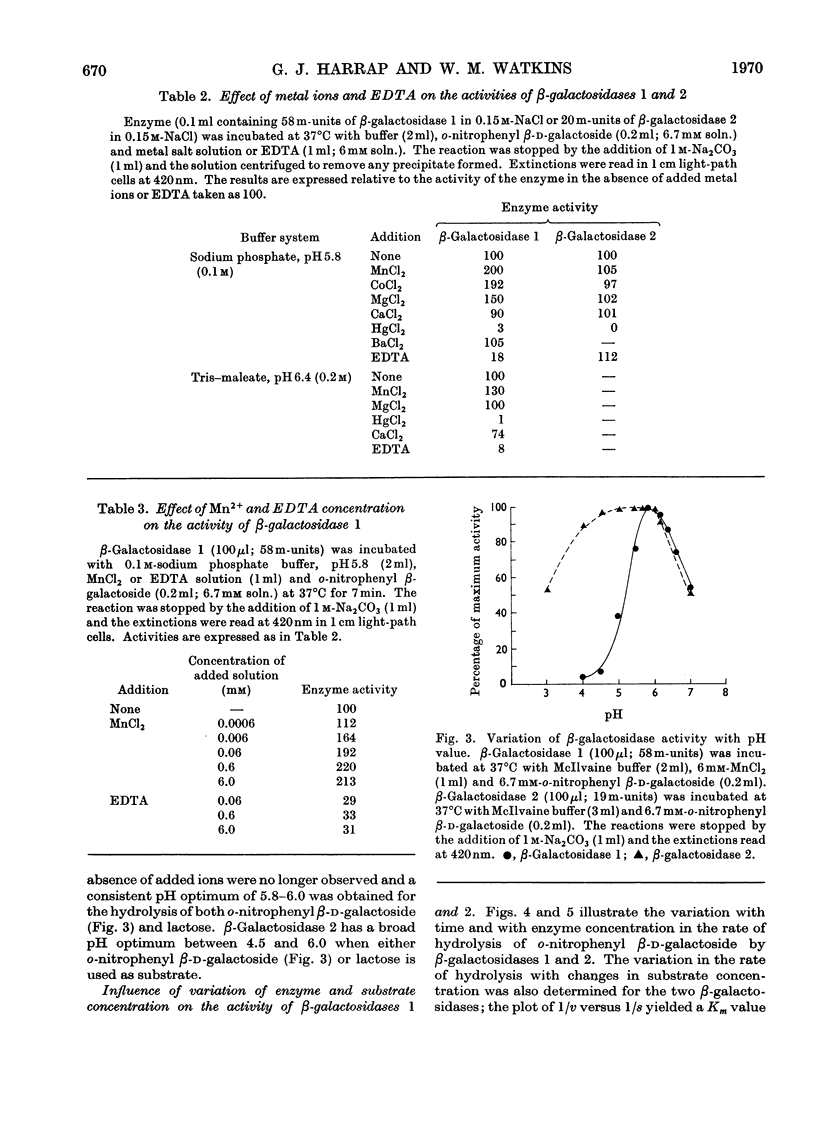
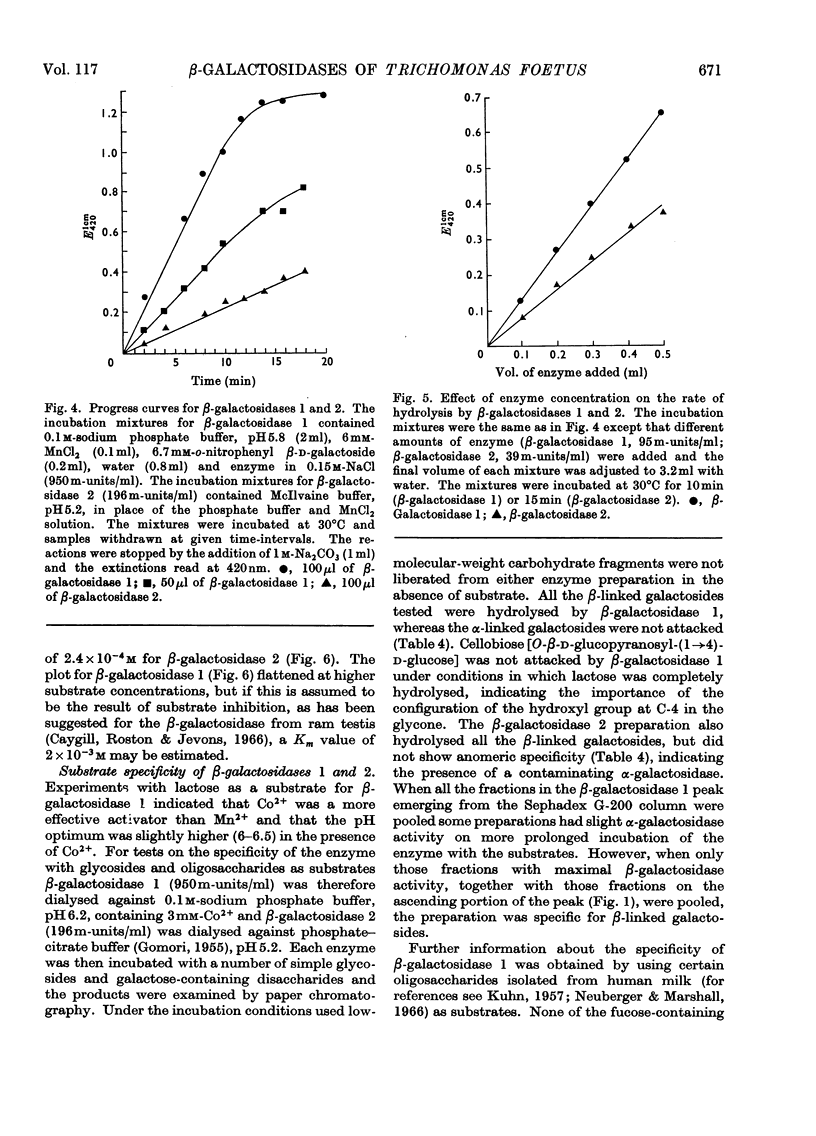
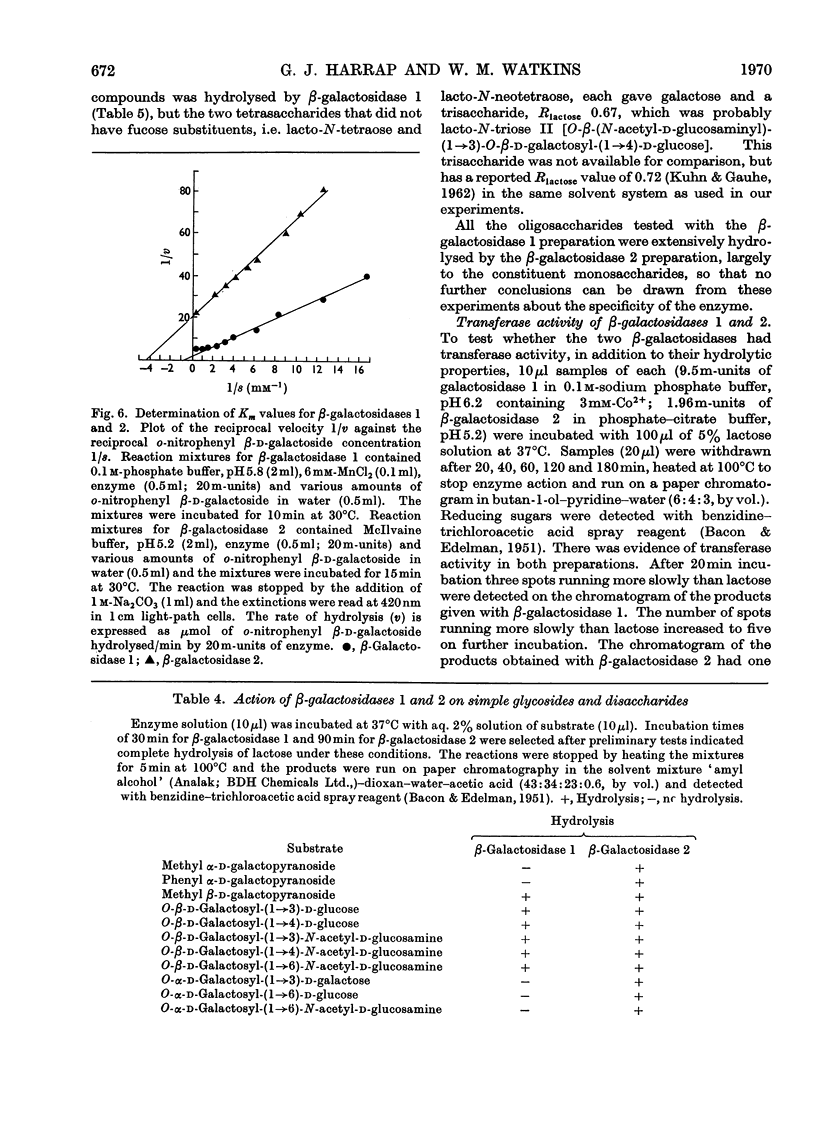
The β-galactosidase activity in extracts of Trichomonas foetus is separable into two fractions by gel filtration on Sephadex G-200. When o-nitrophenyl β-d-galactoside is used as substrate the first fraction to be eluted, β-galactosidase 1, has 50 times the activity (units per mg of protein) of the crude preparation. This fraction is activated by Mn2+ and Co2+ and inhibited by Hg2+ and EDTA. In the presence of Mn2+ the pH optimum for the hydrolysis of o-nitrophenyl β-d-galactoside or lactose is 5.8–6.0. β-Galactosidase 1 is an exoglycosidase that releases β-linked galactose joined to aliphatic and various carbohydrate aglycones. Hydrolysis is prevented, however, by a substituent on either the subterminal sugar or the terminal non-reducing β-galactosyl residue in an oligosaccharide. The second fraction, β-galactosidase 2, is not activated by metal ions or inhibited by EDTA and has a broad pH optimum from 4.5 to 6.0.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F., MORGAN W. T. J. Studies in immunochemistry. X. The isolation and properties of Lewis (Lea) human blood group substance. Biochem J. 1952 Feb;50(4):460–471. doi: 10.1042/bj0500460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston W. P., Donald A. S., Morgan W. T. A trisaccharide, O-beta-d-galactopyranosyl-(1-->3)-O-(N- acetyl-beta-d-glucosaminopyranosyl)-(1-->4)-d- galactose, obtained from human blood-group H substance. Biochem J. 1968 May;107(6):861–863. doi: 10.1042/bj1070861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACON J. S. D., EDELMAN J. The carbohydrates of the Jerusalem artichoke and other Compositae. Biochem J. 1951 Jan;48(1):114–126. doi: 10.1042/bj0480114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., MONOD J. Purification et proprietes de la beta-galactosidase (lactase) d'Escherichia coli. Biochim Biophys Acta. 1951 May;7(1):153–174. doi: 10.1016/0006-3002(51)90013-3. [DOI] [PubMed] [Google Scholar]
- Caygill J. C., Roston C. P., Jevons F. R. Purification of beta-acetylglucosaminase and beta-galactosidase from ram testis. Biochem J. 1966 Feb;98(2):405–409. doi: 10.1042/bj0980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOELL R. G., KRETCHMER N. Studies of small intestine during development. I. Distribution and activity of beta-galactosidase. Biochim Biophys Acta. 1962 Aug 13;62:353–362. doi: 10.1016/0006-3002(62)90097-5. [DOI] [PubMed] [Google Scholar]
- Furth A. J., Robinson D. Specificity and multiple forms of beta-galactosidase in the rat. Biochem J. 1965 Oct;97(1):59–66. doi: 10.1042/bj0970059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PUSZTAI A., MORGAN W. T. Studies in immunochemistry. 18. The isolation and properties of a sialomucopolysaccharide possessing blood-group Le-a specificity and virus-receptor activity. Biochem J. 1961 Jan;78:135–146. doi: 10.1042/bj0780135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing S. R., Crawford I. P. Purification and characterization of the beta-galactosidase of Aeromonas formicans. J Bacteriol. 1966 Mar;91(3):1085–1097. doi: 10.1128/jb.91.3.1085-1097.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WATKINS W. M. Changes in blood group specificity induced by enzymes. Bull Soc Chim Biol (Paris) 1960;42:1599–1605. [PubMed] [Google Scholar]
- WATKINS W. M. Enzymes of Trichomonas foetus; the action of cell-free extracts on blood-group substances and low-molecular-weight glycosides. Biochem J. 1959 Feb;71(2):261–274. doi: 10.1042/bj0710261. [DOI] [PMC free article] [PubMed] [Google Scholar]


