Abstract
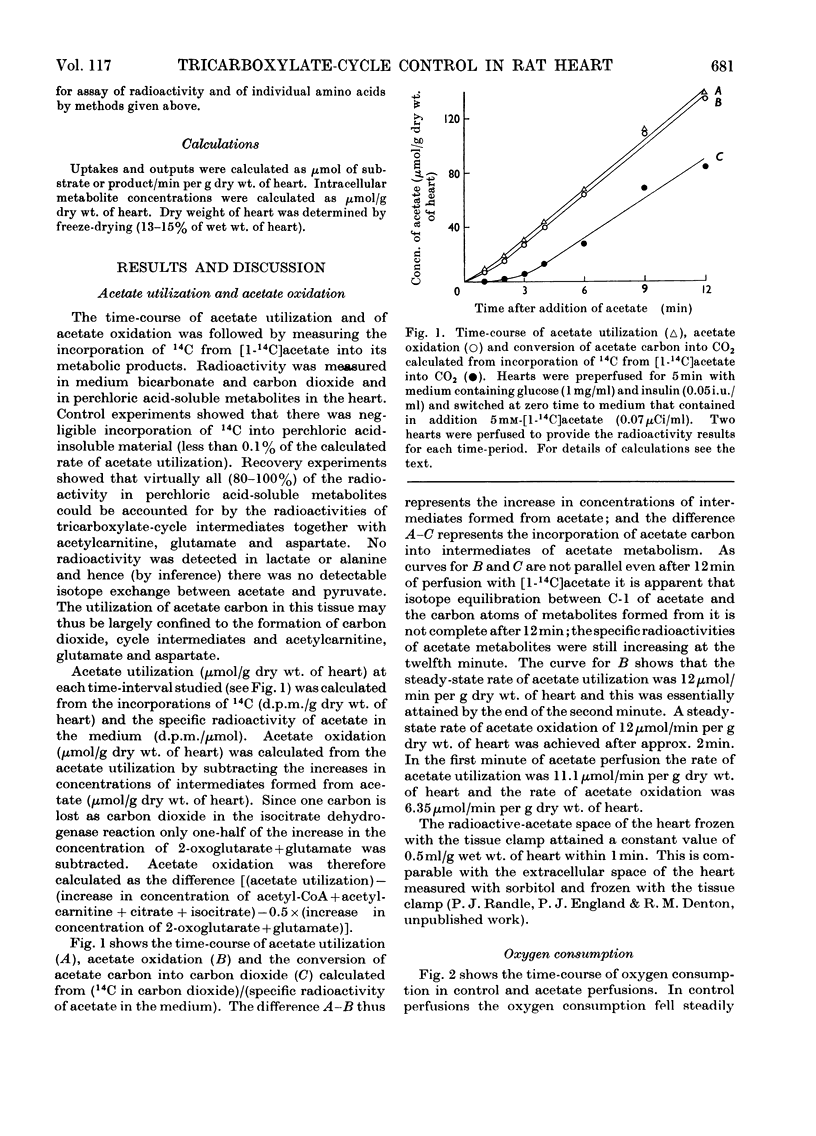
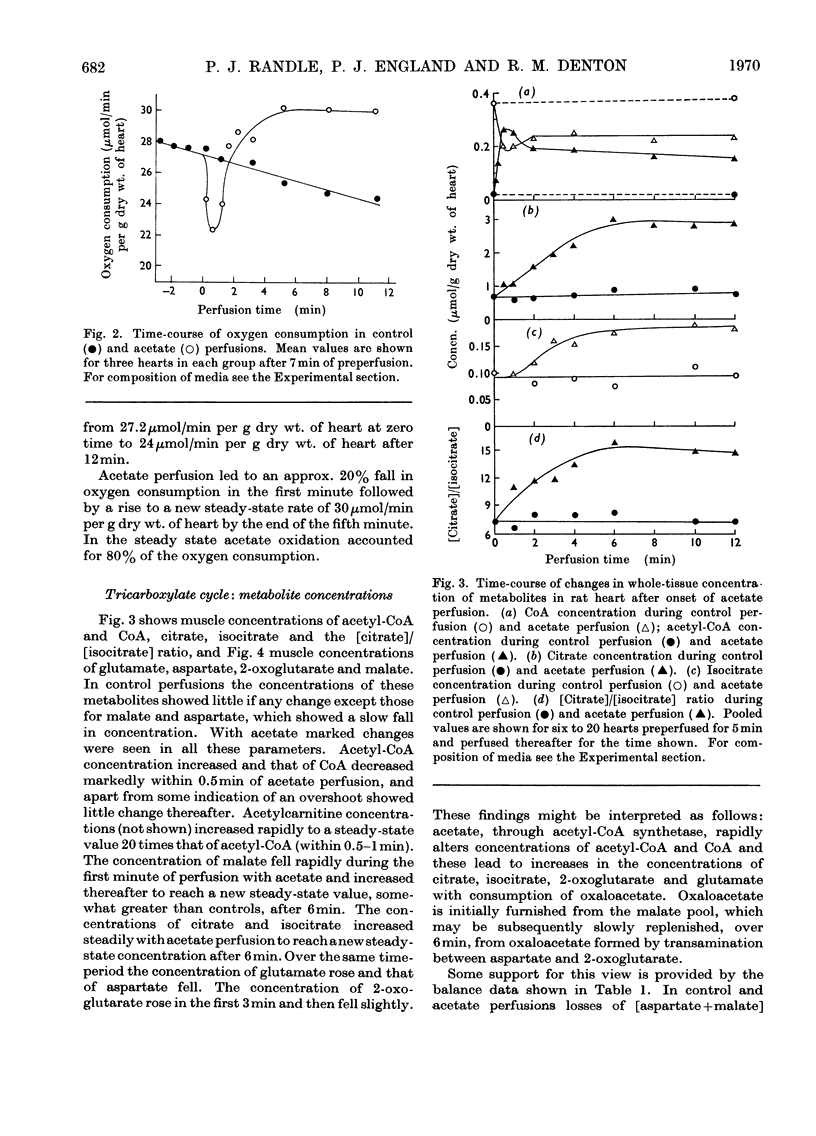
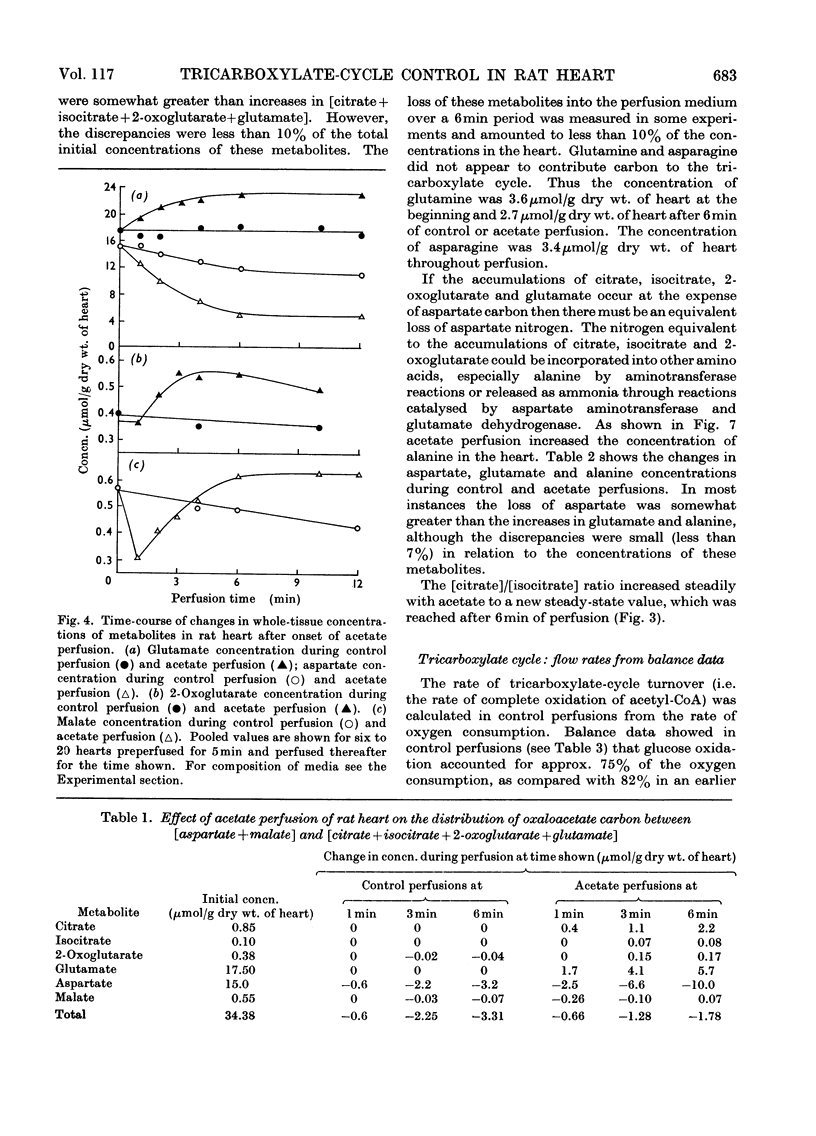
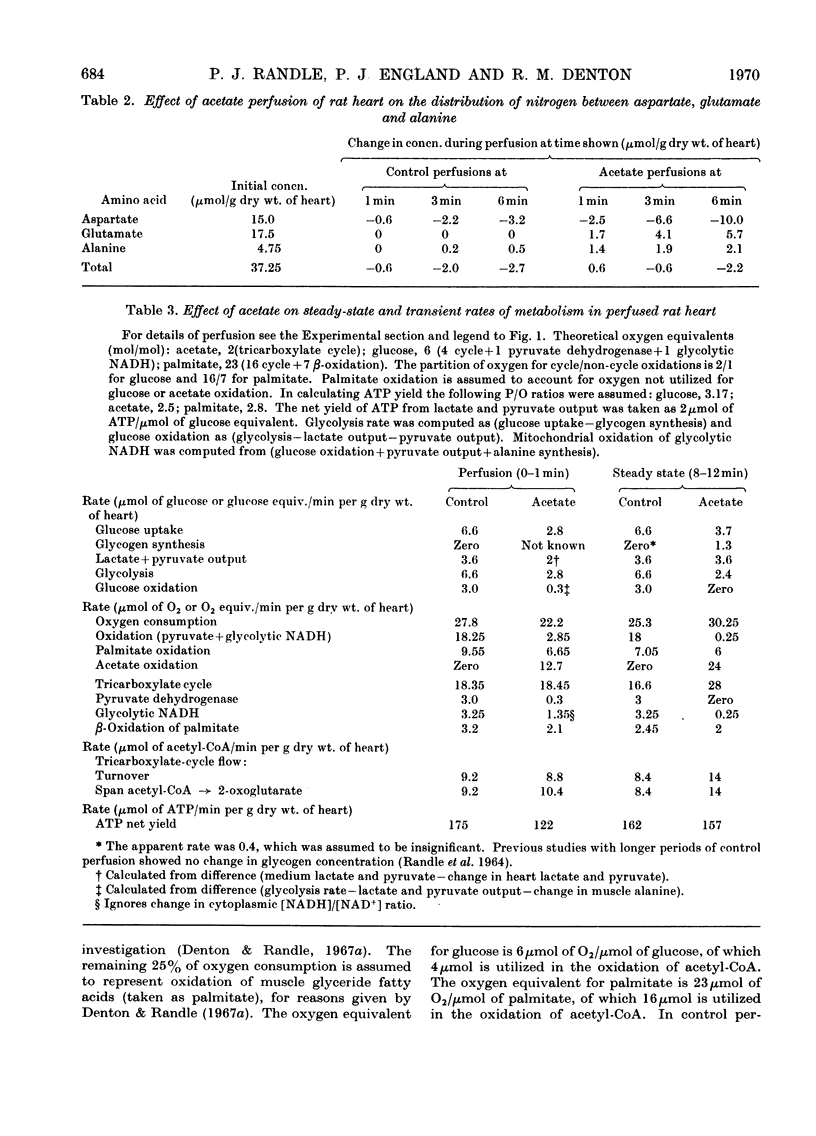
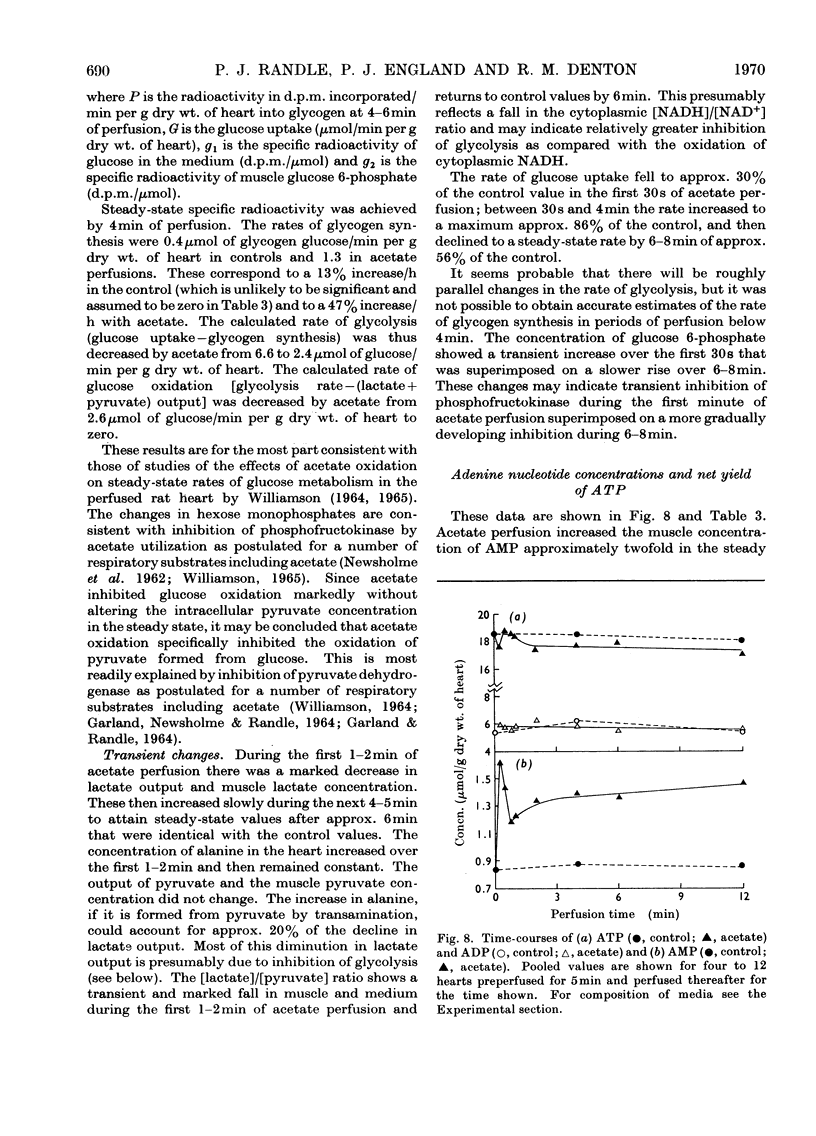
1. Transient and steady-state changes caused by acetate utilization were studied in perfused rat heart. The transient period occupied 6min and steady-state changes were followed in a further 6min of perfusion. 2. In control perfusions glucose oxidation accounted for 75% of oxygen utilization; the remaining 25% was assumed to represent oxidation of glyceride fatty acids. With acetate in the steady state, acetate oxidation accounted for 80% of oxygen utilization, which increased by 20%; glucose oxidation was almost totally suppressed. The rate of tricarboxylate-cycle turnover increased by 67% with acetate perfusion. The net yield of ATP in the steady state was not altered by acetate. 3. Acetate oxidation increased muscle concentrations of acetyl-CoA, citrate, isocitrate, 2-oxoglutarate, glutamate, alanine, AMP and glucose 6-phosphate, and lowered those of CoA and aspartate; the concentrations of pyruvate, ATP and ADP showed no detectable change. The times for maximum changes were 1min, acetyl-CoA, CoA, alanine and AMP; 6min, citrate, isocitrate, glutamate and aspartate; 2–4min, 2-oxoglutarate. Malate concentration fell in the first minute and rose to a value somewhat greater than in the control by 6min. There was a transient and rapid rise in glucose 6-phosphate concentration in the first minute superimposed on the slower rise over 6min. 4. Acetate perfusion decreased the output of lactate, the muscle concentration of lactate and the [lactate]/[pyruvate] ratio in perfusion medium and muscle in the first minute; these returned to control values by 6min. 5. During the first minute acetate decreased oxygen consumption and lowered the net yield of ATP by 30% without any significant change in muscle ATP or ADP concentrations. 6. The specific radioactivities of cycle metabolites were measured during and after a 1min pulse of [1-14C]acetate delivered in the first and twelfth minutes of acetate perfusion. A model based on the known flow rates and concentrations of cycle metabolites was analysed by computer simulation. The model, which assumed single pools of cycle metabolites, fitted the data well with the inclusion of an isotope-exchange reaction between isocitrate and 2-oxoglutarate+bicarbonate. The exchange was verified by perfusions with [14C]bicarbonate. There was no evidence for isotope exchange between citrate and acetyl-CoA or between 2-oxoglutarate and malate. There was rapid isotope equilibration between 2-oxoglutarate and glutamate, but relatively poor isotope equilibration between malate and aspartate. 7. It is concluded that the citrate synthase reaction is displaced from equilibrium in rat heart, that isocitrate dehydrogenase and aconitate hydratase may approximate to equilibrium, that alanine aminotransferase is close to equilibrium, but that aspartate transamination is slow for reasons that have yet to be investigated. 8. The slow rise in citrate concentration as compared with the rapid rise in that of acetyl-CoA is attributed to the slow generation of oxaloacetate by aspartate aminotransferase. 9. It is proposed that the tricarboxylate cycle may operate as two spans: acetyl-CoA→2-oxoglutarate, controlled by citrate synthase, and 2-oxoglutarate→oxaloacetate, controlled by 2-oxoglutarate dehydrogenase; a scheme for cycle control during acetate oxidation is outlined. The initiating factors are considered to be changes in acetyl-CoA, CoA and AMP concentrations brought about by acetyl-CoA synthetase. 10. Evidence is presented for a transient inhibition of phosphofructokinase during the first minute of acetate perfusion that was not due to a rise in whole-tissue citrate concentration. The probable importance of metabolite compartmentation is stressed.
Full text
PDF




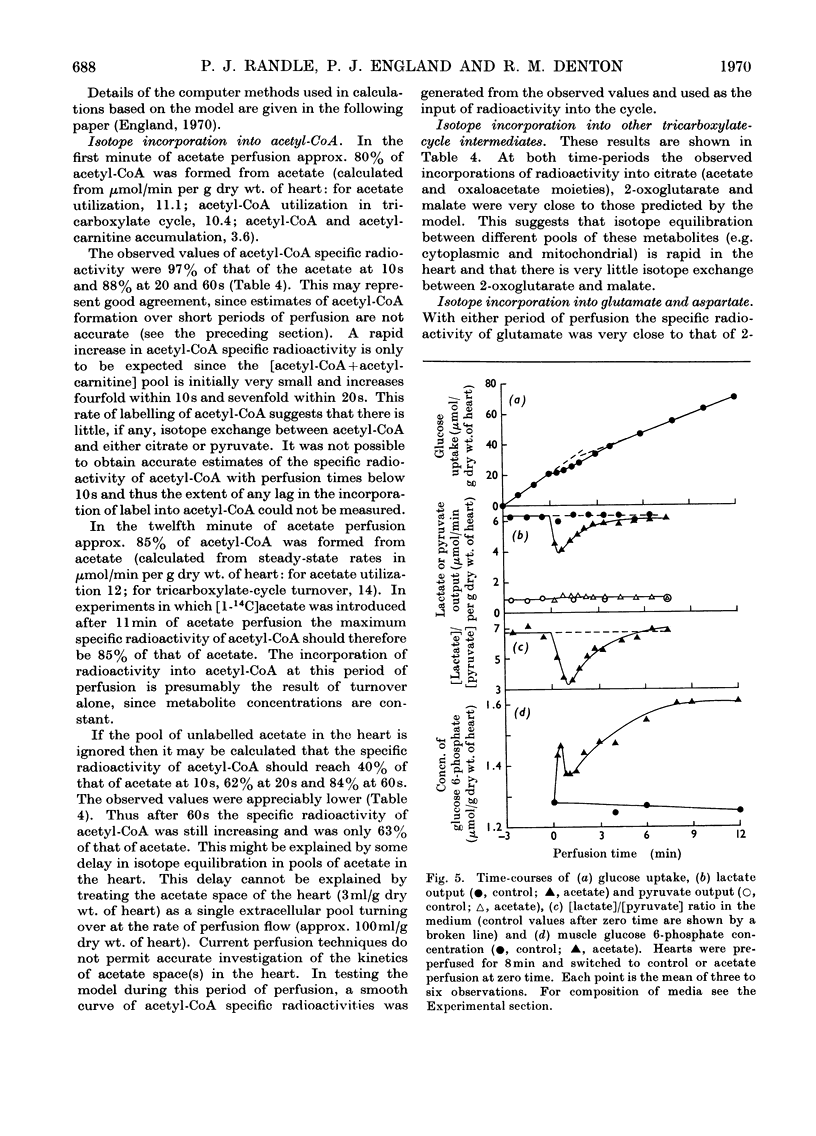
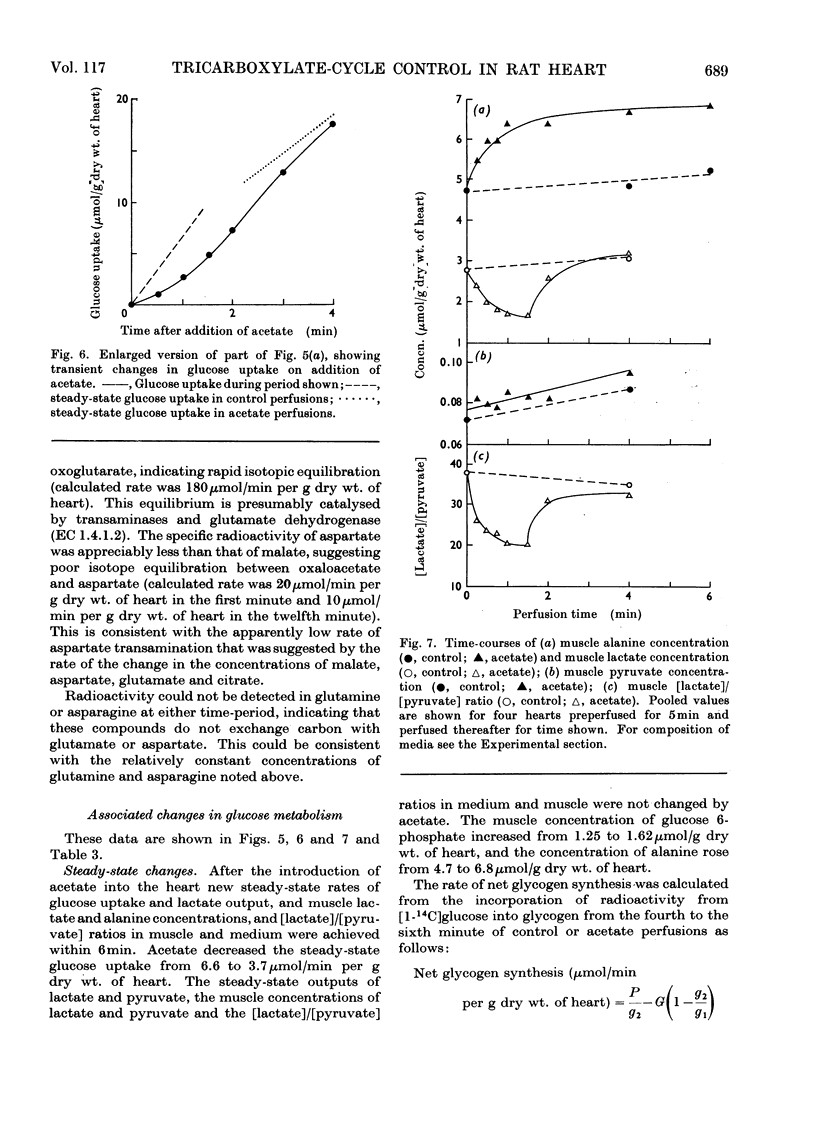





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
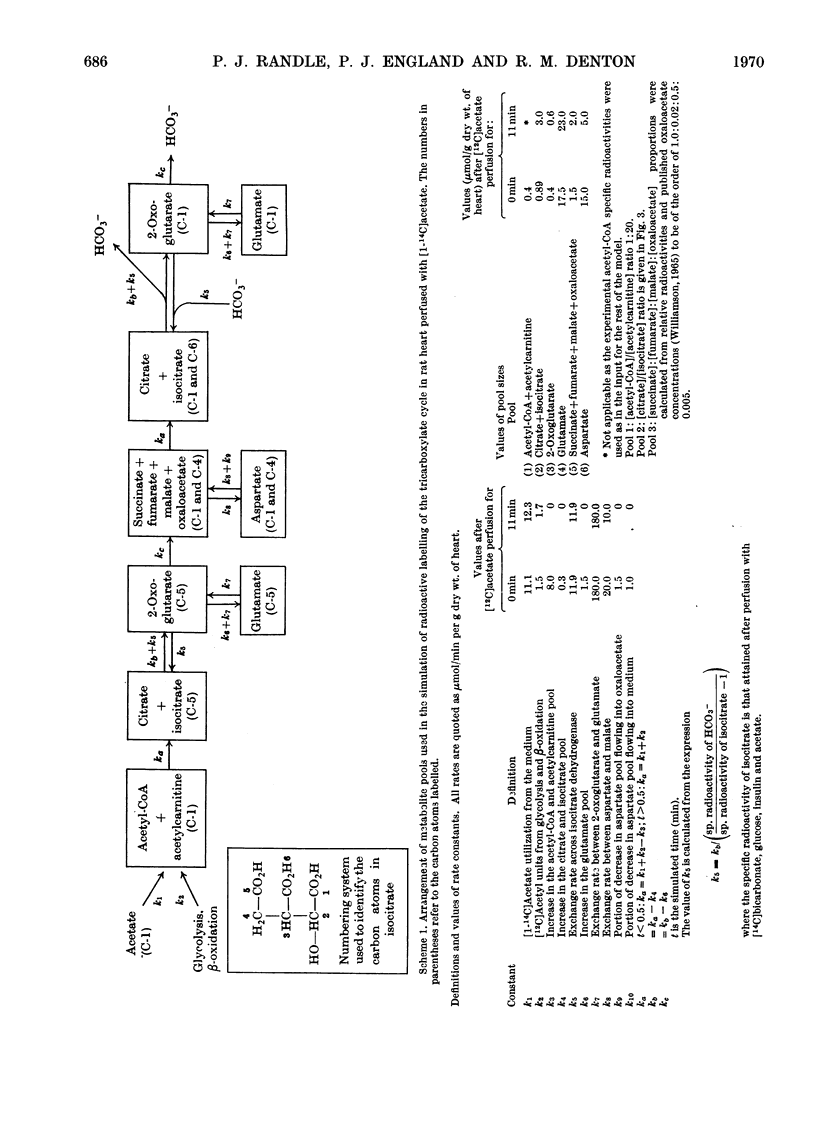
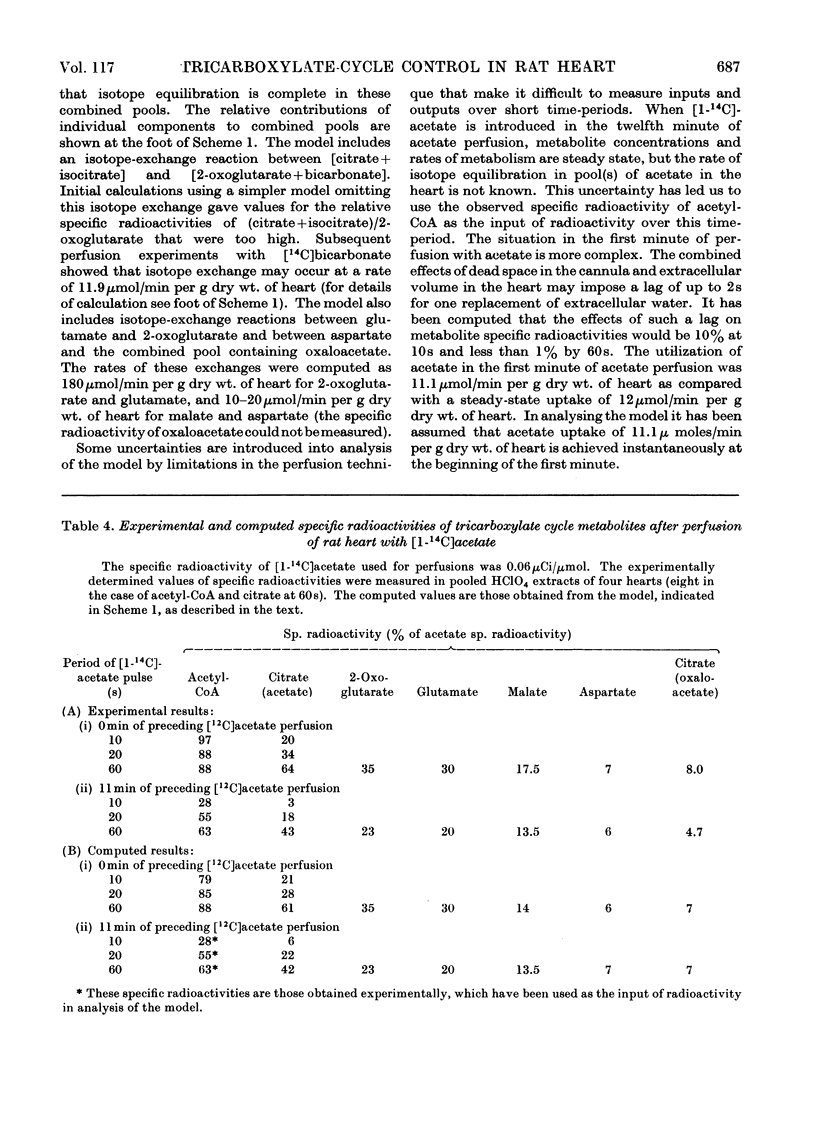
- Bowman R. H. Effects of diabetes, fatty acids, and ketone bodies on tricarboxylic acid cycle metabolism in the perfused rat heart. J Biol Chem. 1966 Jul 10;241(13):3041–3048. [PubMed] [Google Scholar]
- CAMPAGNARI F., WEBSTER L. T., Jr Purification and properties of acetyl coenzyme A synthetase from bovine heart mitochondria. J Biol Chem. 1963 May;238:1628–1633. [PubMed] [Google Scholar]
- Chase J. F. pH-dependence of carnitine acetyltransferase activity. Biochem J. 1967 Aug;104(2):503–509. doi: 10.1042/bj1040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J. Concentrations of glycerides and phospholipids in rat heart and gastrocnemius muscles. Effects of alloxan-diabetes and perfusion. Biochem J. 1967 Aug;104(2):416–422. doi: 10.1042/bj1040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J. Measurement of flow of carbon atoms from glucose and glycogen glucose to glyceride glycerol and glycerol in rat heart and epididymal adipose tissue. Effects of insulin, adrenaline and alloxan-diabetes. Biochem J. 1967 Aug;104(2):423–434. doi: 10.1042/bj1040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J. Computer simulation of the non-steady-state radioactive labelling of a system of metabolite pools with constant rates of influx and efflux. Biochem J. 1970 May;117(4):697–698. doi: 10.1042/bj1170697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J., Robinson B. H. The permeability of rat heart mitochondria to citrate. Biochem J. 1969 Mar;112(1):8P–8P. doi: 10.1042/bj1120008p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J., NEWSHOLME E. A. CITRATE AS AN INTERMEDIARY IN THE INHIBITION OF PHOSPHOFRUCTOKINASE IN RAT HEART MUSCLE BY FATTY ACIDS, KETONE BODIES, PYRUVATE, DIABETES, AND STARVATION. Nature. 1963 Oct 12;200:169–170. doi: 10.1038/200169a0. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Shepherd D., Yates D. W. Steady-state concentrations of coenzyme A, acetyl-coenzyme A and long-chain fatty acyl-coenzyme A in rat-liver mitochondria oxidizing palmitate. Biochem J. 1965 Nov;97(2):587–594. doi: 10.1042/bj0970587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSICKI G. W., SRERE P. A. Kinetic studies on the citrate-condensing enzyme. J Biol Chem. 1961 Oct;236:2560–2565. [PubMed] [Google Scholar]
- KREBS H. A. Equilibria in transamination systems. Biochem J. 1953 Apr;54(1):82–86. doi: 10.1042/bj0540082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosicki G. W., Lee L. P. Effect of divalent metal ions on nucleotide inhibition of pig heart citrate synthase. J Biol Chem. 1966 Aug 10;241(15):3571–3574. [PubMed] [Google Scholar]
- Moellering H., Gruber W. Determination of citrate with citrate lyase. Anal Biochem. 1966 Dec;17(3):369–376. doi: 10.1016/0003-2697(66)90172-2. [DOI] [PubMed] [Google Scholar]
- NEWSHOLME E. A., RANDLE P. J., MANCHESTER K. L. Inhibition of the phosphofructokinase reaction in perfused rat heart by respiration of ketone bodies, fatty acids and pyruvate. Nature. 1962 Jan 20;193:270–271. doi: 10.1038/193270a0. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. Biochem J. 1964 Dec;93(3):641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARMEGGIANI A., BOWMAN R. H. REGULATION OF PHOSPHOFRUCTOKINASE ACTIVITY BY CITRATE IN NORMAL AND DIABETIC MUSCLE. Biochem Biophys Res Commun. 1963 Aug 1;12:268–273. doi: 10.1016/0006-291x(63)90294-8. [DOI] [PubMed] [Google Scholar]
- Pearson D. J., Tubbs P. K. Carnitine and derivatives in rat tissues. Biochem J. 1967 Dec;105(3):953–963. doi: 10.1042/bj1050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson C. I., Randle P. J. The control of rat-heart phosphofructokinase by citrate and other regulators. Biochem J. 1966 Sep;100(3):683–693. doi: 10.1042/bj1000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J., Denton R. M., England P. J. Citrate as a metabolic regulator in muscle and adipose tissue. Biochem Soc Symp. 1968;27:87–103. [PubMed] [Google Scholar]
- Randle P. J., Garland P. B., Hales C. N., Newsholme E. A., Denton R. M., Pogson C. I. Interactions of metabolism and the physiological role of insulin. Recent Prog Horm Res. 1966;22:1–48. doi: 10.1016/b978-1-4831-9825-5.50004-x. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose I. A. The state of magnesium in cells as estimated from the adenylate kinase equilibrium. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1079–1086. doi: 10.1073/pnas.61.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANADI D. R., LITTLEFIELD J. W., BOCK R. M. Studies on alpha-ketoglutaric oxidase. II. Purification and properties. J Biol Chem. 1952 May;197(2):851–862. [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. Effect of epinephrine on rat diaphragm. J Biol Chem. 1950 Dec;187(2):769–776. [PubMed] [Google Scholar]
- WILLIAMSON J. R. GLYCOLYTIC CONTROL MECHANISMS. I. INHIBITION OF GLYCOLYSIS BY ACETATE AND PYRUVATE IN THE ISOLATED, PERFUSED RAT HEART. J Biol Chem. 1965 Jun;240:2308–2321. [PubMed] [Google Scholar]
- Williamson J. R. Effects of insulin and starvation on the metabolism of acetate and pyruvate by the perfused rat heart. Biochem J. 1964 Oct;93(1):97–106. doi: 10.1042/bj0930097. [DOI] [PMC free article] [PubMed] [Google Scholar]


