Abstract
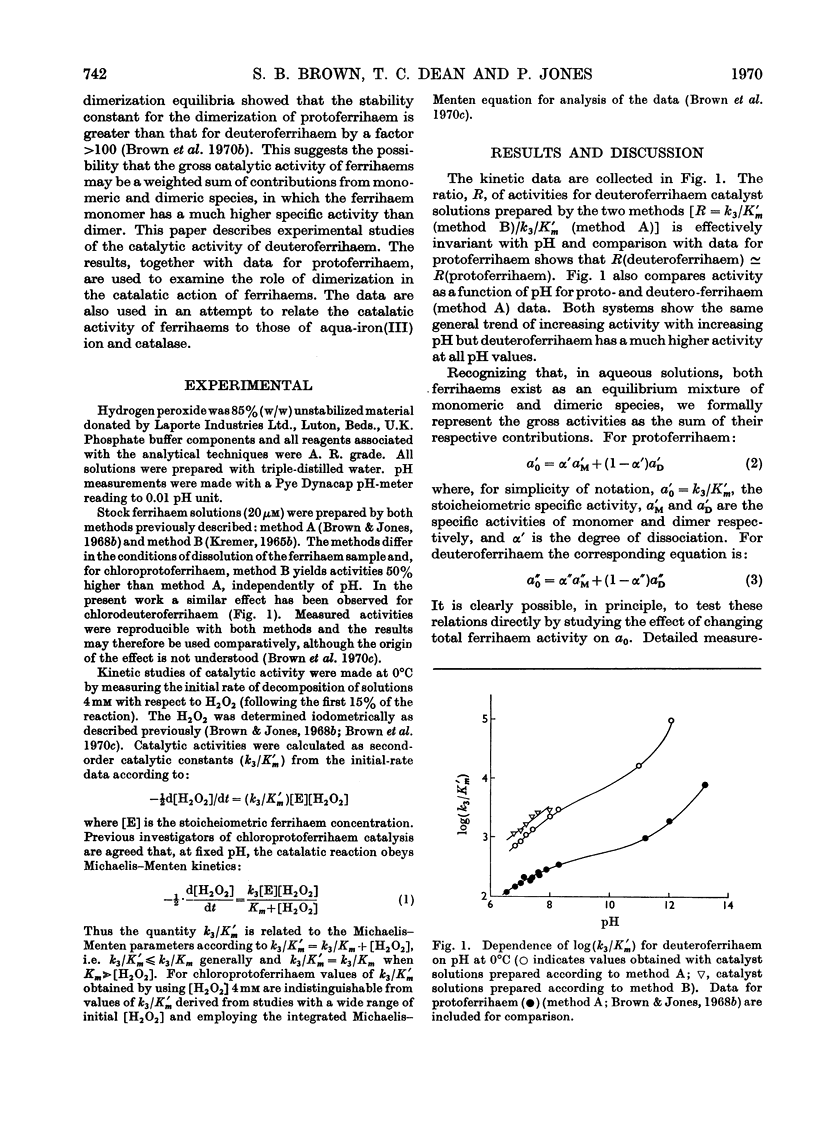
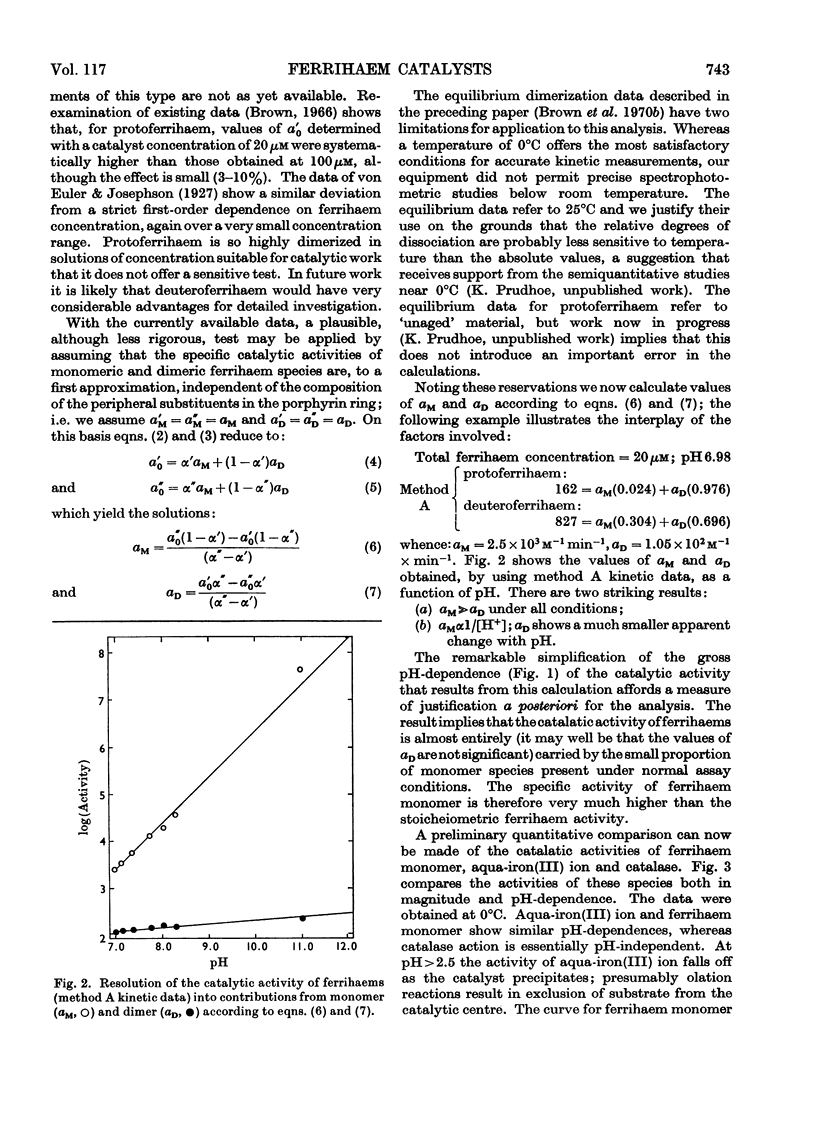
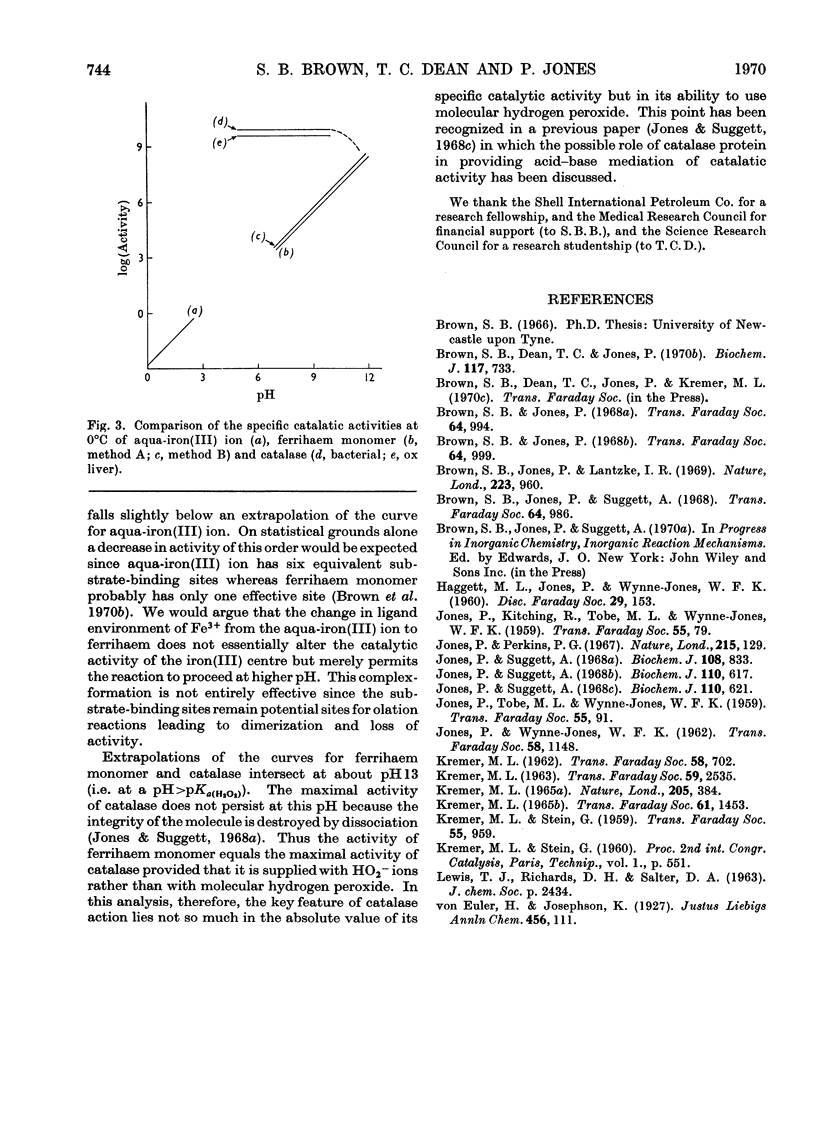
1. The specific stoicheiometric catalatic activity of deuteroferrihaem is 10–100-fold greater than that for protoferrihaem, depending on pH. It is suggested that the difference in activity may be related to quantitative differences in the extent of dimerization in aqueous solutions of proto- and deutero-ferrihaem (Brown, Dean & Jones, 1970b). 2. A quantitative comparison of the kinetic and equilibrium data implies that the catalytic activities of ferrihaems are determined by the proportion of monomer present. The specific activity of ferrihaem monomer calculated varies inversely with H+ ion concentration and attains a value equal to the maximal activity of catalase at pH>pKa(H2O2). 3. A comparison of catalatic behaviour in the series of iron(III)-centred catalysts aqua-iron(III) ion, ferrihaem monomer and catalase suggests that the unique feature of catalase action resides in the pH-independence of the reaction.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. B., Dean T. C., Jones P. Aggregation of ferrihaems. Dimerization and protolytic equilibria of protoferrihaem and deuteroferrihaem in aqueous solution. Biochem J. 1970 May;117(4):733–739. doi: 10.1042/bj1170733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Jones P., Lantzke I. R. Infrared evidence for an oxo-bridged (Fe-O-Fe) haemin dimer. Nature. 1969 Aug 30;223(5209):960–961. doi: 10.1038/223960a0. [DOI] [PubMed] [Google Scholar]
- Jones P., Suggett A. The catalase-hydrogen peroxide system. A theoretical appraisal of the mechanism of catalase action. Biochem J. 1968 Dec;110(4):621–629. doi: 10.1042/bj1100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P., Suggett A. The catalase-hydrogen peroxide system. Role of sub-units in the thermal deactivation of bacterial catalase in the absence of substrate. Biochem J. 1968 Aug;108(5):833–838. doi: 10.1042/bj1080833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P., Suggett A. The catalse-hydrogen peroxide system. Kinetics of catalatic action at high substrate concentrations. Biochem J. 1968 Dec;110(4):617–620. doi: 10.1042/bj1100617. [DOI] [PMC free article] [PubMed] [Google Scholar]


