Abstract
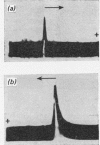
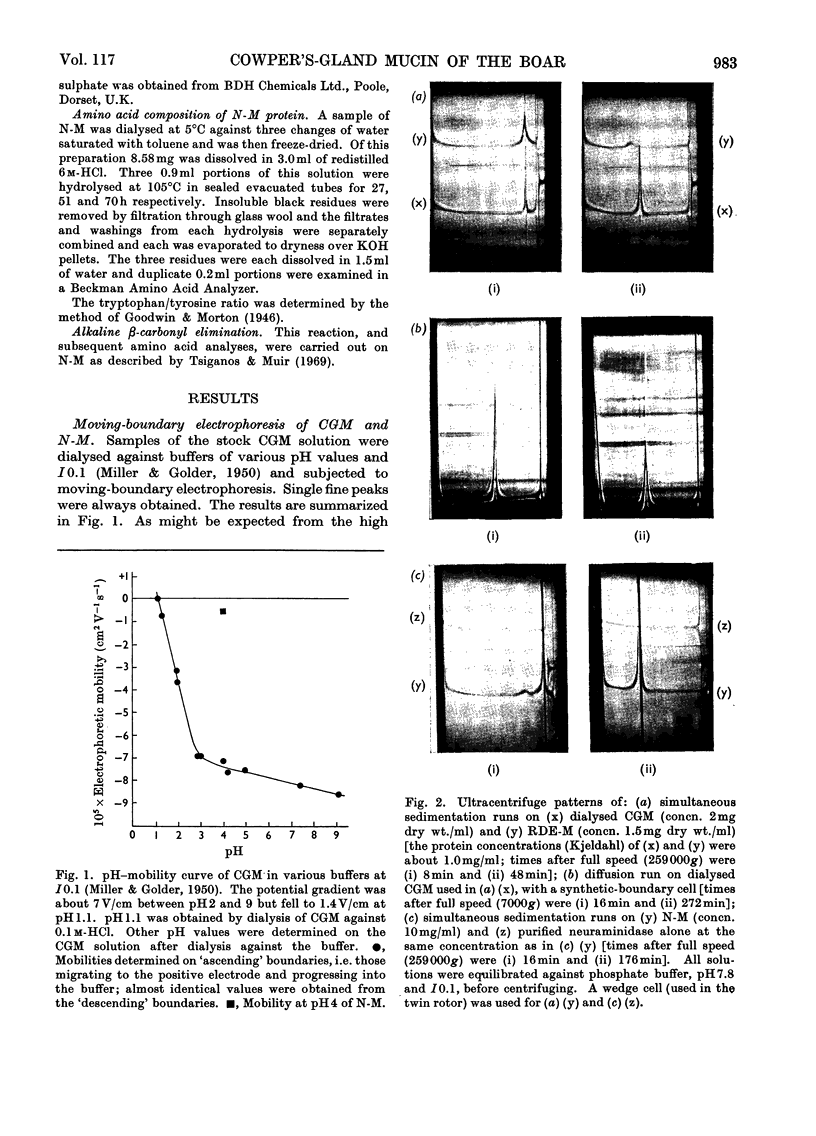
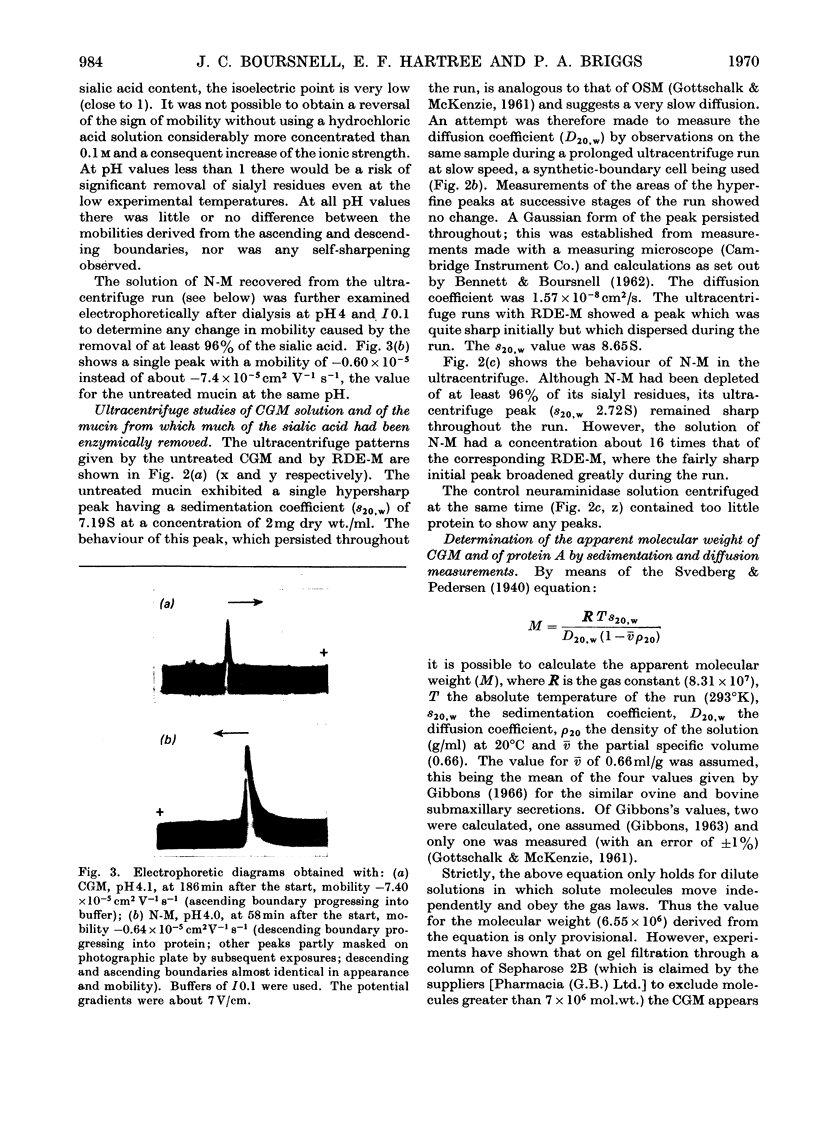
1. Moving-boundary electrophoresis of the mucin from the Cowper's gland of the boar revealed a sharp single peak at pH values from 1.1 to 9.0 and an isoelectric point of 1.1. 2. Neuraminidase treatment of the mucin, which removed at least 96% of the sialic acid groups, decreased the electrophoretic mobility at pH4 from −7.4×10−5 (for the mucin) to −0.64×10−5cm2V−1s−1. 3. Ultracentrifugal sedimentation values of s20,w showed a marked dependence on concentration. A hyperfine peak, similar to that given by ovine submaxillary secretion, persisted throughout the run at higher concentrations. Ultracentrifugal studies further showed a very low value for the diffusion coefficient (D20,w −1.57×10−8cm2/s). 4. Calculation of the approximate molecular weight from comparable s20,w and D20,w values gave a provisional value of 6.5×106. 5. Two proteins present in the boar vesicular secretion known as protein A and protein H (the haemagglutinating protein) were shown to promote the swelling of the mucin to form the characteristic rigid elastic gel of boar semen. It is suggested that protein A molecules particularly (mol.wt. 2.8×104) cross-link with the long molecules of the mucin to form the seminal gel.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertolini M., Pigman W. Action of alkali on bovine and ovine submaxillary mucins. J Biol Chem. 1967 Sep 10;242(17):3776–3781. [PubMed] [Google Scholar]
- Boursnell J. C., Briggs P. A. Boar seminal plasma proteins. II. Electrophoretic identification of the haemagglutinin. J Reprod Fertil. 1969 Jun;19(1):157–166. doi: 10.1530/jrf.0.0190157. [DOI] [PubMed] [Google Scholar]
- Boursnell J. C., Cole D. M., Briggs P. A. Boar seminal plasma proteins. Further observations on the haemagglutinating protein and on the coagulation by heat of some seminal plasma proteins. J Reprod Fertil. 1968 Dec;17(3):533–541. doi: 10.1530/jrf.0.0170533. [DOI] [PubMed] [Google Scholar]
- Boursnell J. C., Nelson M., Cole D. M. Studies on boar seminal plasma proteins. 3. Fractionation by gel filtration, ion exchange and other means. Biochim Biophys Acta. 1966 Mar 28;117(1):134–143. doi: 10.1016/0304-4165(66)90160-7. [DOI] [PubMed] [Google Scholar]
- Boursnell J. C., Nelson M. Studies on boar seminal plasma proteins. II. An investigation on the heat-coagulable component ("fraction A"). Biochim Biophys Acta. 1965 Jun 15;104(1):181–188. doi: 10.1016/0304-4165(65)90233-3. [DOI] [PubMed] [Google Scholar]
- Carlson D. M. Oligosaccharides isolated from pig submaxillary mucin. J Biol Chem. 1966 Jun 25;241(12):2984–2986. [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Conway E. J., O'malley E. Microdiffusion methods. Ammonia and urea using buffered absorbents (revised methods for ranges greater than 10mug. N). Biochem J. 1942 Sep;36(7-9):655–661. doi: 10.1042/bj0360655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. A. THE SENSITIVITY OF THE NEURAMINOSIDIC LINKAGE IN MUCOSUBSTANCES TOWARDS ACID AND TOWARDS NEURAMINIDASE. Biochem J. 1963 Nov;89:380–391. doi: 10.1042/bj0890380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK A. Correlation between composition, structure, shape and function of a salivary mucoprotein. Nature. 1960 Jun 18;186:949–951. doi: 10.1038/186949a0. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A., McKENZIE H. A. Studies on mucoproteins. VIII. On the molecular size and shape of ovine sub-maxillary gland mucoprotein. Biochim Biophys Acta. 1961 Dec 9;54:226–235. doi: 10.1016/0006-3002(61)90361-4. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A., SIMMONDS D. H. Studies on mucoproteins. II. Analysis of the protein moiety of ovine submaxillary gland mucoprotein. Biochim Biophys Acta. 1960 Jul 29;42:141–146. doi: 10.1016/0006-3002(60)90760-5. [DOI] [PubMed] [Google Scholar]
- GRAHAM E. R., GOTTSCHALK A. Studies on mucoproteins. I. The structure of the prosthetic group of ovine submaxillary gland mucoprotein. Biochim Biophys Acta. 1960 Mar 11;38:513–524. doi: 10.1016/0006-3002(60)91286-5. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTREE E. F. Sialic acid in the bulbo-urethral glands of the boar. Nature. 1962 Nov 3;196:483–484. doi: 10.1038/196483a0. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO Y., TSUIKI S., QUINTARELLI G., PIGMAN W. A comparison of submaxillary glands of humans, cattle, dogs and rats. Biochim Biophys Acta. 1961 Apr 1;48:404–406. doi: 10.1016/0006-3002(61)90495-4. [DOI] [PubMed] [Google Scholar]
- KLENK E., UHLENBRUCK G. Uber die Abspaltung von N-Glykolyl-neuraminsäure (P-Sialinsäure) aus dem Schweine-Submaxillaris-mucin durch das Receptor Destroying Enzyme. Hoppe Seylers Z Physiol Chem. 1957;307(2-6):266–271. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER G. L., GOLDER R. H. Buffers of pH 2 to 12 for use in electrophoresis. Arch Biochem. 1950 Dec;29(2):420–423. [PubMed] [Google Scholar]
- Markham R. A steam distillation apparatus suitable for micro-Kjeldahl analysis. Biochem J. 1942 Dec;36(10-12):790–791. doi: 10.1042/bj0360790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRIVASTAVA P. N., ADAMS C. E., HARTREE E. F. ENZYMIC ACTION OF ACROSOMAL PREPARATIONS ON THE RABBIT OVUM IN VITRO. J Reprod Fertil. 1965 Aug;10:61–67. doi: 10.1530/jrf.0.0100061. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Heterogeneity, fractionation and characterization. Biochem J. 1969 Aug;113(5):885–894. doi: 10.1042/bj1130885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WARREN L. Unbound sialic acids in fish eggs. Biochim Biophys Acta. 1960 Nov 4;44:347–351. doi: 10.1016/0006-3002(60)91571-7. [DOI] [PubMed] [Google Scholar]
- WERNER I., ODIN L. On the presence of sialic acid in certain glycoproteins and in gangliosides. Acta Soc Med Ups. 1952;57(3-4):230–241. [PubMed] [Google Scholar]