Abstract
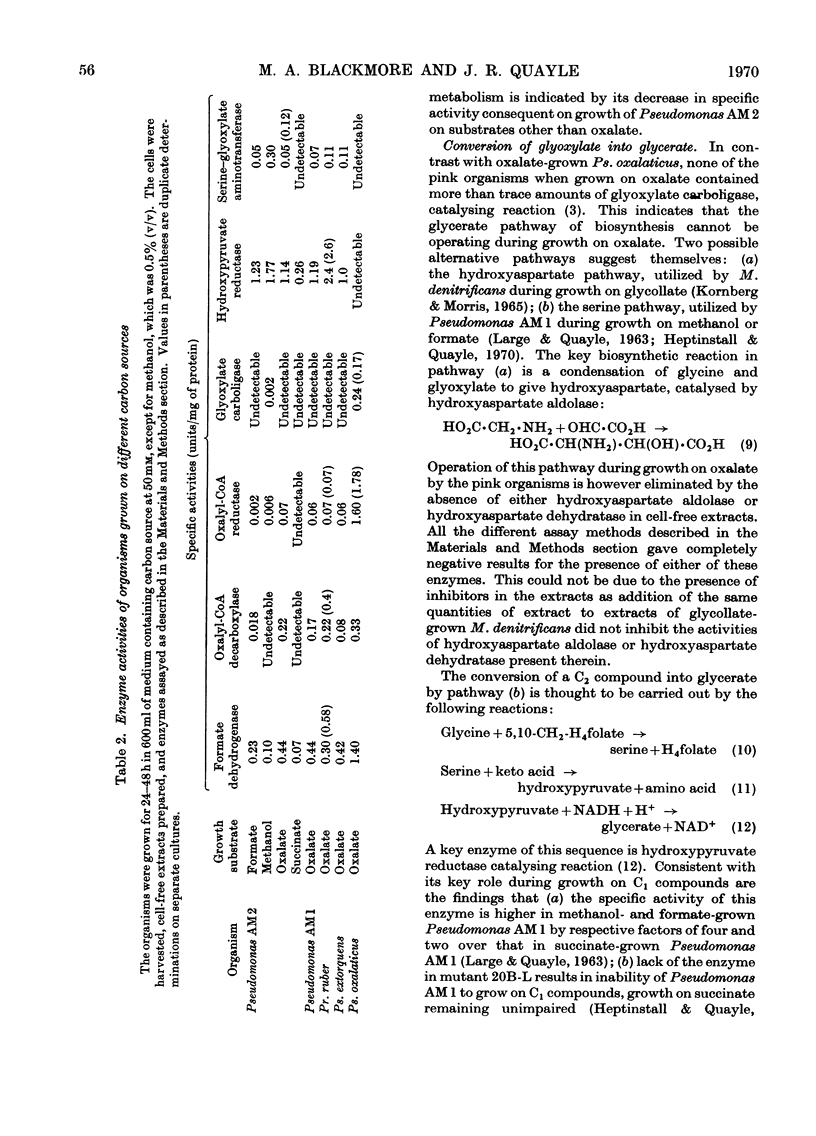
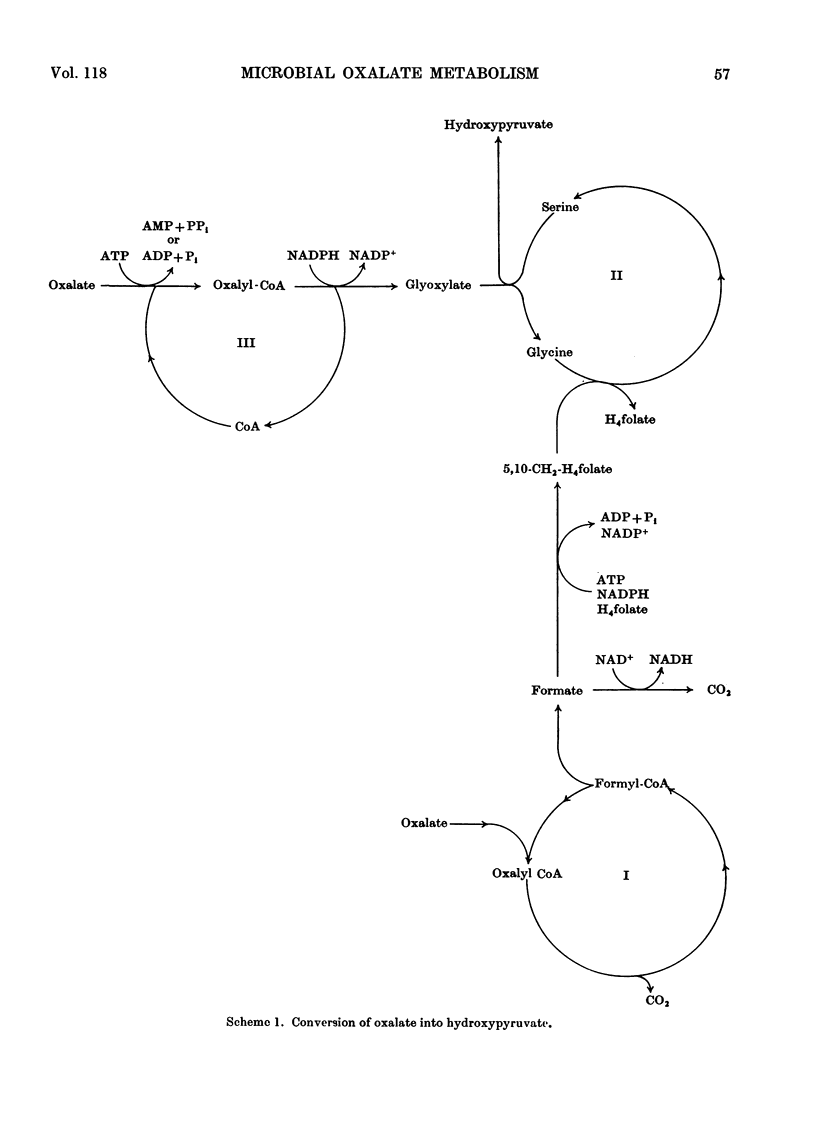
1. The metabolism of oxalate by the pink-pigmented organisms, Pseudomonas AM1, Pseudomonas AM2, Protaminobacter ruber and Pseudomonas extorquens has been compared with that of the non-pigmented Pseudomonas oxalaticus. 2. During growth on oxalate, all the organisms contain oxalyl-CoA decarboxylase, formate dehydrogenase and oxalyl-CoA reductase. This is consistent with oxidation of oxalate to carbon dioxide taking place via oxalyl-CoA, formyl-CoA and formate as intermediates, and also reduction of oxalate to glyoxylate taking place via oxalyl-CoA. 3. The pink-pigmented organisms, when grown on oxalate, contain l-serine–glyoxylate aminotransferase and hydroxypyruvate reductase but do not contain glyoxylate carboligase. The converse of this obtains in oxalate-grown Ps. oxalaticus. This indicates that, in contrast with Ps. oxalaticus, synthesis of C3 compounds from oxalate by the pink-pigmented organisms occurs by a variant of the `serine pathway' used by Pseudomonas AM1 during growth on C1 compounds. 4. Evidence in favour of this scheme is provided by the finding that a mutant of Pseudomonas AM1 that lacks hydroxypyruvate reductase is not able to grow on oxalate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackmore M. A., Quayle J. R. Choice between autotrophy and heterotrophy in Pseudomonas oxalaticus. Growth in mixed substrates. Biochem J. 1968 May;107(5):705–713. doi: 10.1042/bj1070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore M. A., Quayle J. R., Walker I. O. Choice between autotrophy and heterotrophy in Pseudomonas oxalaticus. Utilization of oxalate by cells after adaptation from growth on formate to growth on oxalate. Biochem J. 1968 May;107(5):699–704. doi: 10.1042/bj1070699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS R. G., MORRIS J. G. ASSAY AND PROPERTIES OF BETA-HYDROXYASPARTATE ALDOLASE FROM MICROCOCCUS DENITRIFICANS. Biochim Biophys Acta. 1964 Jun 1;85:501–503. doi: 10.1016/0926-6569(64)90318-9. [DOI] [PubMed] [Google Scholar]
- Heptinstall J., Quayle J. R. Pathways leading to and from serine during growth of Pseudomonas AM1 on C1 compounds or succinate. Biochem J. 1970 Apr;117(3):563–572. doi: 10.1042/bj1170563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON P. A., JONES-MORTIMER M. C., QUAYLE J. R. USE OF A PURIFIED BACTERIAL FORMATE DEHYDROGENASE FOR THE MICRO-ESTIMATION OF FORMATE. Biochim Biophys Acta. 1964 Aug 26;89:351–353. doi: 10.1016/0926-6569(64)90225-1. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., MORRIS J. G. Enzymic formation of oxaloacetate from erythro beta-hydroxyaspartate. Biochim Biophys Acta. 1962 Dec 17;65:537–540. doi: 10.1016/0006-3002(62)90467-5. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., MORRIS J. G. THE UTILIZATION OF GLYCOLLATE BY MICROCOCCUS DENITRIFICANS: THE BETA-HYDROXYASPARTATE PATHWAY. Biochem J. 1965 Jun;95:577–586. doi: 10.1042/bj0950577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Large P. J., Quayle J. R. Microbial growth on C(1) compounds. 5. Enzyme activities in extracts of Pseudomonas AM1. Biochem J. 1963 May;87(2):386–396. doi: 10.1042/bj0870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. I. Isolation and characterization of Pseudomonas AM 1. Biochem J. 1961 Dec;81:465–469. doi: 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAYLE J. R. CARBON ASSIMILATION BY PSEUDOMONAS OXALATICUS (OX1). 7. DECARBOXYLATION OF OXALYL-COENZYME A TO FORMYL-COENZYME A. Biochem J. 1963 Dec;89:492–503. doi: 10.1042/bj0890492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAYLE J. R. Carbon assimilation by Pseudomonas oxalaticus (OX1). 6. Reactions of oxalyl-coenzyme A. Biochem J. 1963 May;87:368–373. doi: 10.1042/bj0870368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAYLE J. R., TAYLOR G. A. Carbon assimilation by Pseudomonas oxalaticus (OXI). 5. Purification and properties of glyoxylic dehydrogenase. Biochem J. 1961 Mar;78:611–615. doi: 10.1042/bj0780611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle J. R., Keech D. B., Taylor G. A. Carbon assimilation by Pseudomonas oxalaticus (OXI). 4. Metabolism of oxalate in cell-free extracts of the organism grown on oxalate. Biochem J. 1961 Feb;78(2):225–236. doi: 10.1042/bj0780225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAFFORD H. A., MAGALDI A., VENNESLAND B. The enzymatic reduction of hydroxypyruvic acid to D-glyceric acid in higher plants. J Biol Chem. 1954 Apr;207(2):621–629. [PubMed] [Google Scholar]
- STOCKS P. K., MCCLESKEY C. S. IDENTITY OF THE PINK-PIGMENTED METHANOL-OXIDIZING BACTERIA AS VIBRIO EXTORQUENS. J Bacteriol. 1964 Oct;88:1065–1070. doi: 10.1128/jb.88.4.1065-1070.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYNGAARDEN J. B., ASHTON D. M. The regulation of activity of phosphoribosyl-pyrophosphate amidotransferase by purine ribonacleotides: a potential feedback control of purine biosyntoesis. J Biol Chem. 1959 Jun;234(6):1492–1496. [PubMed] [Google Scholar]


