Abstract
Pasireotide long-acting release (PAS-LAR) is a second-generation somatostatin receptor ligand (SRL) approved for acromegaly treatment. This meta-analysis aimed to evaluate the real-world effectiveness and safety of PAS-LAR in patients with acromegaly resistant to first-generation somatostatin receptor ligands (fgSRL). A systematic literature search was conducted in PubMed and Web of Science for real-world studies on PAS-LAR in acromegaly published between 2014 and 2023. Random-effects meta-analyses were performed on biochemical control rates, tumor shrinkage, and metabolic parameters. Twelve studies comprising 409 patients were included. The pooled rate of insulin-like growth factor 1 (IGF-1) control was 57.9% [95% CI: 48.4–66.8] and the percentage of patients with tumor shrinkage was 33.3% [95%CI: 19.7–50.4]. Significant reductions were observed in growth hormone standardized mean difference (SMD) 0.6 ng/mL [95% CI: 0.3 to 1.0] and IGF-1 levels SMD 0.9 ULN [95% CI: 0.4 to 1.4]. However, as expected, a worsening in glucose metabolism was noted as an increase in fasting glucose SMD − 0.8 mg/dL [95% CI: -1.0 to -0.5, p < 0.01], glycated hemoglobin SMD − 0.5% [95% CI: -0.7 to -0.2]. and type 2 diabetes mellitus prevalence SMD − 11.5% (95% CI: -17.5 to -5.5). PAS-LAR demonstrated higher effectiveness in real-world settings, with over 60% of patients achieving IGF-1 control compared to the around 30% efficacy observed in clinical trials. These findings suggest that PAS-LAR is an effective option for acromegaly patients resistant to fgSRL, but careful monitoring of glucose levels is essential. The high heterogeneity observed across studies emphasizes the need for identifying PAS-LAR response biomarkers to set-up individualized treatment approaches for optimizing patient outcomes.
Keywords: Pasireotide, Acromegaly, Real world, Effectiveness, Hyperglycemia, Pituitary adenoma
Introduction
Acromegaly is a rare disease with devastating consequences if biochemical control is not reached [1, 2]. The primary objective in managing acromegaly is achieving age-adjusted normalization of insulin-like growth factor-1 (IGF-1) levels, as a reliable indicator of disease activity [3]. Secondary, albeit also very important goals encompass tumor control while preserving pituitary function, alleviating symptoms, and reversing the associated morbidities, impaired quality of life, and increased mortality risk [4–7]. Transsphenoidal surgery is considered the treatment of choice as it offers curative potential. Remission rates following surgical intervention can reach 80–90% for patients with microadenomas, while macroadenomas, being the more prevalent lesion type, exhibit a lower remission rate of approximately 50% [8]. Consequently, nearly half of the patient population will require medical treatment due to persistent disease after surgery or contraindications to surgical intervention.
Pasireotide long-acting release (PAS-LAR) is a second-generation somatostatin multi-receptor ligand approved as a second-line medical therapy for acromegaly. In contrast to first generation somatostatin receptors ligands (fgSRL) (that is, lanreotide and octreotide long acting formulations) which mainly binds to SSTR-2, PAS-LAR exhibits binding affinity to four out of the five human somatostatin receptor subtypes, with the highest affinity for SSTR-2 and SSTR-5 (more than 40 fold higher than fgSRL) [9], which are the two main receptor subtypes expressed on somatotropinomas.
The efficacy of PAS-LAR has been evaluated in two main pivotal studies. A phase 3 study that directly compared PAS-LAR to octreotide LAR in 358 medically naive acromegaly patients [10] observed at month 12 that 31.3% of patients receiving PAS-LAR achieved biochemical control (GH < 2.5 µg/L and normal IGF-1) compared to 19.2% of those on octreotide LAR. The PAOLA trial [11], was the other main phase 3 study, in which 198 patients inadequately controlled with the higher doses of fgSRL were randomly assigned 1-1-1 to receive PAS-LAR 40 mg or PAS-LAR 60 mg every 28 days for 24 weeks, or continued treatment with fgSRL (active control group). At 24 weeks, ten (15%) patients in the PAS-LAR 40 mg group and 13 (20%) patients in the PAS-LAR 60 mg group achieved biochemical control, compared with no patients in the active control group.
Regarding tumor volume, in naive patients a clinically relevant (≥ 20%) tumor volume reduction was achieved by 80.8% and 77.4% of PAS-LAR and octreotide LAR patients respectively [10] and in previously treated patients in up to 18.5% [11].
The most frequent adverse effects observed in both studies, were glucose-related in up to 33% of patients [10, 11].
Interestingly, in emerging real-world studies [12–15], PAS-LAR demonstrated higher effectiveness when compared to randomized controlled trials (RCTs), as in general IGF1 was normalized in general in more than 50% of patients, with a clinically relevant tumor shrinkage, in more than 40% of previously treated patients [12–14] with mild side effects and a low rate of patients dropping out.
Given these initial promising real-world findings contrasting with the pivotal trial results, there is a clear need to systematically review and synthesize the growing body of real-world evidence on PAS-LAR’s effectiveness and safety profile in clinical practice settings. A comprehensive literature review and meta-analysis could provide more precise and generalizable estimates of PAS-LAR’s real-world performance and tolerability to better inform clinical decision-making.
Thus, with all the stated above, the aim of this meta-analysis was to evaluate the real-world experience (RWE) with PAS-LAR. Specifically, we will address the following questions using the Population, Intervention, Comparator, Outcome (PICO) framework: (1) In adult patients with acromegaly resistant to fgSRL(P), how effective is PAS-LAR treatment (I) compared to other treatments (C) in achieving biochemical disease control (O)? (2) What percentage of acromegaly patients treated with PAS-LAR experience clinically relevant tumor volume shrinkage? (3) Which is the impact on glucose metabolism, associated with PAS-LAR treatment in acromegaly patients? By synthesizing real-world data, this meta-analysis will provide a comprehensive overview of PAS-LAR’s effectiveness, safety profile, and tolerability in the management of acromegaly in regular clinical practice performed usually in specialized centers, thus, offering valuable and generalisable insights to guide clinical decision-making and patient care.
Methods
Protocol registration
This study was performed according to the Cochrane Collaboration and PRISMA statement [16]. The systematic review protocol was submitted to PROSPERO in December 2023, and registered in June 2024 (registration number CRD42024468603). At the time of registration, no other systematic review addressing PAS-LAR in RWE had been published or registered.
Eligibility criteria
Eligibility criteria for study selection included: (1) real-word studies; (2) adult acromegaly patients population uncontrolled with fgSRL; (3) PAS-LAR treatment with at least 6 months of follow-up; (4) assessment of biochemical efficacy, tumor volume shrinkage or glucose metabolism outcomes (as primary or secondary endpoints) before and after treatment with PAS-LAR.
We excluded clinical trials, reviews and animal studies. When studies of the same Country/Hospital showed overlap with the participants, the study with the lower number of participants or containing insufficient information was excluded.
Information sources and search strategy
A literature search was performed in January 2024 and covered studies published from 2014 (when the pivotal studies of PAS-LAR were published ) to December 31st, 2023. The research was limited to articles available in English. The systematic search was conducted using PUBMED and the Web Science of Knowledge (WoS). In PUBMED we used the terms: (“pasireotide“[Title/Abstract]) AND (2014:2024/5/10[pdat]). A total of 507 articles were retrieved, link access https://pubmed.ncbi.nlm.nih.gov/?term=pasireotide+%5BTIAB.
In WoS, with the terms: pasireotide (Abstract) AND Review Article (Exclude– Document Types) and 2006 or 2007 or 2008 or 2009 or 2010 or 2011 or 2012 or 2013 (Exclude– Publication Years), we retrieved 300 articles: link access: https://www.webofscience.com/wos/woscc/summary/1d796062-6b2e-4160-874d-6c06051bce8b-e9f1a818/relevance/1.
We updated the search in the 10th of May 2024, and 2 further studies were retrieved from PUBMED.
The initial screening process involved two team members (BB and MA). After excluding duplicated papers, both members independently examining the titles and abstracts of all retrieved studies. Articles that both reviewers considered potentially relevant were then selected for comprehensive evaluation. At both the preliminary and full-review stages, mutual agreement between the reviewers was necessary for a study to be excluded. In cases where there was disagreement about including or excluding a fully-reviewed study, all the researchers were consulted and decision taken after achieving full agreement. This approach ensured a through and unbiased selection process for the studies included in our analysis. A manual search in the references of the selected studies was also performed but not further articles were included.
Interobserver agreement was high (96.2%: 51 of the 53 studies selected for initial screening ). Figure 1 shows the flowchart of the eligibility assessment process.
Fig. 1.
Flowchart of literature eligibility assessment process. Illustrates the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram, detailing the process of study selection for the systematic review and meta-analysis. * Two studies by Chiloiro et al. were retained because, they report on different endpoints. One study was kept for general outcomes, while the other was included specifically for its data on carbohydrate metabolism, which was not available in the first study. This ensures that no duplication of results occurs, while maximizing the use of all relevant data
Data extraction and quality assessment
A clinical data extraction sheet was designed prior to data collection and the four independent investigators (B.B., M.A-C., M.M. and M.P-D) extracted data on (1) paper meta-data: authors’ names, year of publication, country (ies) of study conduction, study period, and study design (prospective or retrospective), (2) baseline characteristics: number of patients, gender, age, T2DM (%), blood glucose levels, GH and IGF-1 levels, (3) effectiveness post treatment: disease control as rate of patients with IGF-1 levels < 1 upper limit of normal (ULN) and/or < 1.3 ULN and percentage of patients with clinically relevant volume reduction (< 20 or 25%) as per investigator designation and (4) side effects: post treatment glucose, HbA1c and T2DM (%).
The quality of the papers was evaluated following the Strobe check list [17] to avoid bias. Studies were reviewed for funding support to minimize any potential bias.
Outcomes
We selected studies reporting at least one of the following parameters before and after PAS-LAR administration: rate of patients with IGF-1 control, clinically relevant volume reduction, T2DM before and after treatment. We excluded interim data and only the last follow-up assessment was considered.
Data synthesis and statistical analysis
Quantitative data reported as mean ± standard deviation (SD) or median and range at baseline and after PAS-LAR treatment were extracted for all outcomes. If reported, difference from baseline and percentage change were also extracted. Missing data were obtained from authors when possible. Pooled values of GH, IGF-1, glucose levels, HbA1c, T2DM, % of patients with clinically relevant tumor shrinkage and % IGF-1 control when available in the selected studies were estimated using the random effects meta-analysis model [18]. Pooled values were reported as standardised mean difference with 95% confidence intervals (CI), except for % IGF-1 control and % of patients with tumor shrinkage, which are reported as percentage with 95% CI. Forrest plots were presented to visualize the results. Statistical heterogeneity was assessed by the Q test and complemented with the I2 index. Leave-one-out sensitivity analysis was performed when high statistical heterogeneity was identified. A subgroup analysis was conducted to assess the overall percentage of patients achieving IGF-1 control and clinically significant tumor volume reduction. The analysis compared trials where patients had not received radiotherapy with those who had undergone radiotherapy. All analyses were performed using R software version 4.4.0 [19] and package meta version 7.0 [20].
Results
Study selection
We found 809 potentially related studies, 509 from Pubmed and 300 from WoS (Fig. 1). After removing duplicates 313 were screening. Of these 260 were excluded on the basis of title and abstract screening, and 28 were excluded after full-text revision. The remaining 25 studies were eligible [12–15, 21–41]. Of these 25 studies, a total of ten were excluded, four because they were clinical trials and not RWE [31, 32, 38, 39] and nine because of duplicated sample [22, 23, 26–30, 35, 36]. Of the 12 studies ultimately selected, two studies by Chiloiro S. et al., [24] and [25], could represent duplicate cohorts. To avoid duplication of results, the first study [25] was used for most of the outcomes, as it contains the majority of the relevant data. However, the second study [24] was used specifically for parameters related to carbohydrate metabolism, which are not available in study [25]. This approach ensures that data redundancy is avoided while incorporating all relevant information.
Study characteristics
Overall, 12 studies were selected and 11 were with independent samples. The papers showed homogeneous and good methodological quality as assessed by the Strobe checklist [17]. Table 1 summarizes the characteristics of the 11 included studies without duplicated samples. Publication years ranged from 2018 to 2024, with the majority (8/12) published in/or after 2021. All the selected studies except two [12, 37] were conducted in Europe, specifically in Italy (n = 3), France (n = 2), Poland (n = 2) and Spain (n = 2). Eight studies were multicentric, while three were single-center studies. All but one (Urbani C et al., 2023) [14] used PAS as monotherapy. This study used PAS-LAR in combination with cabergoline in two patients (one due to a mixed GH-PRL adenoma) and with pegvisomant in four patients.
Table 1.
Baseline demographic and patients characteristics
| Author and reference | Year | Country | M | Mono | N | Male n (%) |
Age (y) diagnosis |
Macro n (%) |
Surgery n (%) | RT n (%) | Time RT | Time PAS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corica G. et al. [21] | 2023 | Italy | Yes | Yes | 21 | 8 (38.1) | 37.0 | 19 (90.5) | 13 (61.9) | 3 (12.3) | * | 45.5 |
| Urbani C. et al. [14] | 2024 | Italy | Yes | No | 50 | 27 (54.0) | 43.0 | 46 (92.0) | 35 (70.0) | 7 (14.0) | 8.6 ± 10.6 years | 24.0 |
| Chiloiro S. et al. [25] | 2021 | Italy | No | Yes | 33 | 13 (39.4) | 36.0 | 33 (100) | 33 (100) | 0 | 54.0 | |
| Araujo-Castro M. et al. [15] | 2024 | Spain | Yes | Yes | 24 | 13 (54.2) | 38.5 | 22 (91.7) | 20 (83.3) | 8 (33.3) | 38 ± 29 months | 30.8 |
| Ruiz S. et al. [13] | 2023 | Spain | Yes | Yes | 81 | 42 (51.9) | 50.0 | NA | 65 (80.2) | 29 (35.8) | 70 ± 17 months | 12.0 |
| Stelmachowska-Banaś M. et al. [33] | 2022 | Poland | No | Yes | 28 | 14 (50.0) | 42.6 | NA | 25 (89.3) | 3 (10.7) | more than 3 years | 12.0 |
| Witek P. et al. [34] | 2021 | Poland | Yes | Yes | 39 | 23 (59.0) | 47.2 | 39 (100) | 33 (84.6) | 4 (10.3) | NA | 6.0 |
| Shimon I. et al. [37] | 2018 | Israel | Yes | Yes | 35 | 20 (57.1) | 40.8 | 28 (80.0) | 30 (85.7) | 6 (17.1) | 3–47 months | 12.0 |
| Gadelha M. et al. [12] | 2023 | Brazil | No | Yes | 50 | 22 (44.0) | 40.0 | NA | 11 (50.0) | 2 (4.0) | more than 10 years | 58.0 |
| Lasolle H. et al. [40] | 2019 | France | Yes | Yes | 15 | 5 (33.3) | 38.0 | 14 (93.3) | 15 (100) | 4 (26.7) | 2.8–14.1 years | 29.0 |
| Wolf P. et al. [41] | 2022 | France | No | Yes | 33 | 13 (39.4) | 46.0 | NA | 29 (87.8) | NA | NA | 19.0 |
Quantitative variables are expressed as mean or median. Abbreviatures: M: multicenter, Mono: monotherapy, N: number of patients, Macro: macroadenoma, RT: radiotherapy
*2 patients (5 and 2 years before PAS-LAR, respectively), while one patient was referred to radiotherapy after 18 months of PAS withdrawal
Demographics
Sample sizes ranged from 15 to 81 patients, encompassing a total of 409 patients with acromegaly treated with PAS-LAR. The mean ages across studies ranged from 36.0 to 50.0 years. A total of 200 were male (48.8%), indicating a relatively balanced gender distribution and the time in months with PAS-LAR range from 6 to 58 months (Table 1).
Disease characteristics
In the 7 studies reporting tumor size, 201 out of 217 patients (92.6%) had macroadenomas and tumor size (based on 5 studies), ranged from 9.3 to 37.5 mm. A substantial majority (75.8%, n = 310) had undergone previous surgery. History of radiotherapy (RT) was registered in 10 of the 11 selected studies. However, the number of patients who had received RT prior to starting PAS-LAR treatment varied significantly across the studies, ranging from 0 to 35.8% of the cohorts. Additionally, the time between RT and the initiation of PAS-LAR was highly heterogeneous, with some patients having undergone RT just a few months before starting PAS-LAR, while others received RT up to 10 years prior (Table 1).
Baseline biochemical disease activity and glucose metabolism
Table 2 summarize baseline clinical and biochemical parameters. The baseline GH levels ranged from 1.9 to 19.0 ng/mL and IGF-1, ranged from 1.3 to 3.3 times above ULN. The fasting blood glucose level was reported in 6 studies, with means ranging from 93.0 to 111.0 mg/dL and HbA1c levels were reported in 10 studies, ranging from 5.6 to 6.4%. Baseline T2DM prevalence, ranging from 10.3 to 42.1% of patients.
Table 2.
Baseline biochemical disease activity and glucose metabolism
| Author and reference | GH (ng/mL) |
IGF-1 (ULN) |
Glucose (mg/dL) | T2DM (%) | A1c (%) |
|---|---|---|---|---|---|
| Corica G. et al. [21] | 3.4 | 1.3 | 93.0 | 14.0 | 5.7 |
| Urbani C. et al. [14] | 4.3 | 1.9 | 104.0 | 38.0 | 5.8 |
| Chiloiro S. et al. [24] | 4.0 | 3.1 | 109.0 | 14.7 | 5.9 |
| Araujo-Castro M. et al. [15] | NA | 3.3 | 110.5 | 29.2 | 6.4 |
| Ruiz S. et al. [13] | 19.0 | 1.8 | NA | 14.0 | NA |
| Stelmachowska-Banaś M. et al. [33] | 3.9 | 2.3 | 111.0 | 15.4 | 5.9 |
| Witek P. et al. [34] | 3.1 | 1.9 | NA | 10.3 | 5.6 |
| Shimon I. et al. [37] | 1.9 | 1.8 | 109.0 | 31.4 | 6.1 |
| Gadelha M. et al. [12] | 4.0 | 2.4 | NA | 22.0 | 5.9 |
| Lasolle H. et al. [40] | NA | 1.7 | 101.0 | NA | 5.8 |
| Wolf P. et al. [41] | 7.8 | 1.6 | NA | 39.0 | 5.8 |
Abbreviatures: BMI: body mass index, GH: growth hormone, IGF-1: Insulin-like Growth Factor 1, ULN: upper limit of normal, T2DM, Type 2 diabetes mellitus
Metanalysis
Growth hormone levels
The meta-analysis of GH levels included 5 studies with 182 patients (Fig. 2). The random effects model showed a significant reduction in GH levels post-treatment, with a standardized mean difference (SMD) of 0.63ng/mL (95% CI: 0.25 to 1.0, p < 0.01). Heterogeneity was moderate to high (I² = 67.0%, p = 0.02).
Fig. 2.
Forrest plot including the five studies reporting GH levels before and after PAS-LAR treatment. This forest plot illustrates the standardized mean difference (SMD) in growth hormone (GH) levels before and after pasireotide treatment across the five studies. The random effects model shows a significant reduction in GH levels post-treatment, with an SMD 0.6ng/dL (95% CI: 0.3 to 1.0, p < 0.01). Moderate to high heterogeneity is indicated (I² = 66.8%, p = 0.02)
IGF-1- ULN levels
For IGF-1 levels, only 2 studies were included in the analysis. The random effects model demonstrated a significant decrease in IGF-1 levels, with an SMD of 0.9ULN (95% CI: 0.4 to 1.4, p < 0.01). However, heterogeneity was also moderately high (I² = 69.0%, p < 0.07.
Fasting glucose metabolism
The analysis of fasting glucose levels included 4 studies (Fig. 3a). The random effects model showed a significant increase in glucose levels post-treatment, with an SMD of -0.8 mg/dL (95% CI: -1.0 to -0.5, p < 0.01). There was no significant heterogeneity (I² = 0.0%, p = 0.54).
Fig. 3.
Forrest plot changes in glucose levels (a), HbA1c (b), and diabetes prevalence (c) Following pasireotide LAR treatment. It provides a comprehensive overview of the impact of Pasireotide LAR treatment on glucose metabolism in acromegaly patients. The figure is divided into three sections: (a) Glucose Levels (mg/dL) - This section illustrates the changes in pre-treatment and post-treatment glucose levels across multiple studies. The standardized mean difference (SMD) indicates a significant increase in glucose levels post-treatment. (b) HbA1c Levels (%) - This section displays the changes in HbA1c levels before and after treatment. The SMD demonstrates a notable increase in HbA1c levels. (c) Diabetes Prevalence (%) - This section highlights the prevalence of diabetes before and after treatment. The analysis shows a significant increase in diabetes prevalence
Eight studies were included in the HbA1c analysis (Fig. 3b). The random effects model revealed a significant increase in HbA1c levels, with an SMD of -0.5% (95% CI: -0.7 to -0.2, p < 0.01). Heterogeneity was low to moderate (I² = 420%, p = 0.10).
The analysis of T2DM prevalence included 8 studies, (Fig. 3). The random effects model showed a significant increase in T2DM prevalence post-treatment, with an SMD of -11.5% (95% CI: -17.5 to -5.5, p < 0.01). However, heterogeneity was very high (I² = 99.0%, p < 0.01).
IGF-1 control
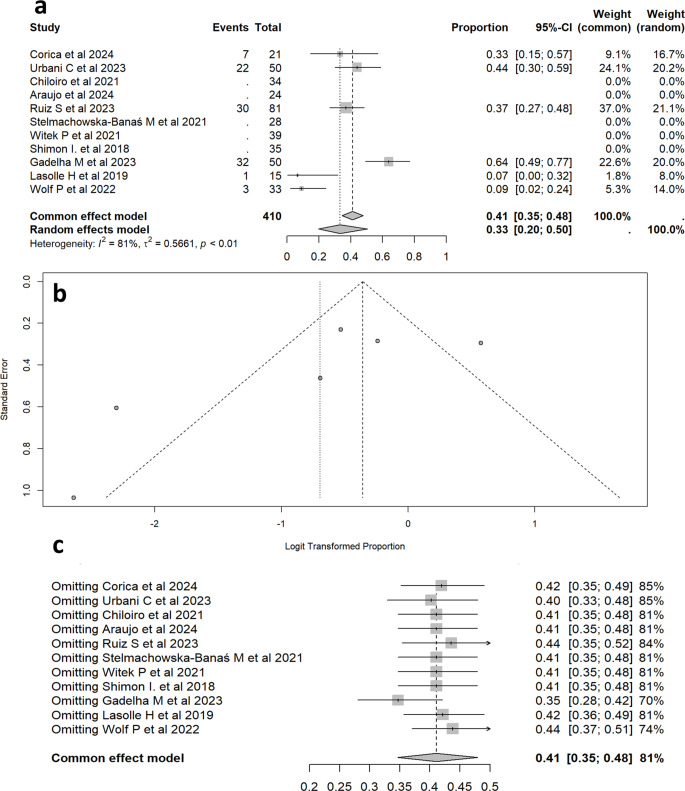
The proportion of patients achieving IGF-1 control was analyzed across all 11 studies. The Forrest plot in Fig. 4a summarizes the individual study results and the pooled proportion estimates for IGF-1 control. The random effects model estimated that 57.9% of patients (95% CI: 48.4–66.8%) achieved IGF-1 control. Heterogeneity was moderate to high (I² = 68.1%, p < 0.01).
Fig. 4.
Meta-analysis of the proportion of acromegaly patients achieving IGF-1 control with pasireotide treatment forrest plot (a), a funnel plot (b), and a sensitivity analysis (c). It consists of three parts: a forest plot (a), a funnel plot (b), and a sensitivity analysis (c). (a) Forest Plot - This plot illustrates the proportion of acromegaly patients achieving IGF-1 control in each included study, alongside the pooled proportion estimates. (b) Funnel Plot - The funnel plot assesses the potential for publication bias among the studies. The symmetrical distribution of points suggests minimal publication bias, although some outliers indicate variability in reported IGF-1 control rates. (c) Sensitivity Analysis - This analysis evaluates the robustness of the overall proportion estimate by sequentially omitting each study. The consistent range confirm the stability of the pooled estimate despite significant heterogeneity
The Funnel plot in Fig. 4b was employed to assess the potential for publication bias in the included studies. The plot, which displays the logit-transformed proportions against their standard errors, appears largely symmetrical, indicating minimal publication bias. However, the presence of some outliers accounts for an increased variability in reported IGF-1 control rates, contributing to the overall heterogeneity observed in the meta-analysis.
The sensitivity analysis in Fig. 4c demonstrates the stability of the pooled estimate, despite the exclusion of individual studies. The I2 values, which remained high (68%), confirm the presence of substantial heterogeneity.
Clinically relevant tumor shrinkage
Our meta-analysis included 6 studies with a total of 250 patients, of whom 95 experienced clinically relevant tumor shrinkage. The random effects model, estimated that 33.3% (95% CI: 19.7 − 50.4%) of patients achieved clinically relevant tumor volume reduction. There was significant heterogeneity among the studies (I² = 81.1%,p < 0.01). The Forrest plot Fig. 5 visually represents the proportion of patients with tumor shrinkage in each study and the overall effect. Individual study estimates ranged from 8.0 to 64.0%, with varying precision as indicated by the width of the confidence intervals.
Fig. 5.
Proportion of acromegaly patients achieving tumor volume shrinkage following pasireotide treatment. It presents a meta-analysis of the proportion of acromegaly patients who experienced clinically significant tumor volume reduction after treatment with PAS-LAR. (Panel a): Forest Plot - This section summarizes the individual study results and the pooled proportion estimates for tumor volume shrinkage. (Panel b): Funnel Plot - This panel assesses potential publication bias by plotting the logit-transformed proportions against their standard errors. The symmetrical distribution of points suggests minimal publication bias, though some asymmetry and outliers indicate variability in reported shrinkage rates. (Panel c): Sensitivity Analysis - This section demonstrates the stability of the overall proportion estimate by sequentially excluding each study. The pooled proportion remains consistent (35–45%) despite the exclusion of individual studies
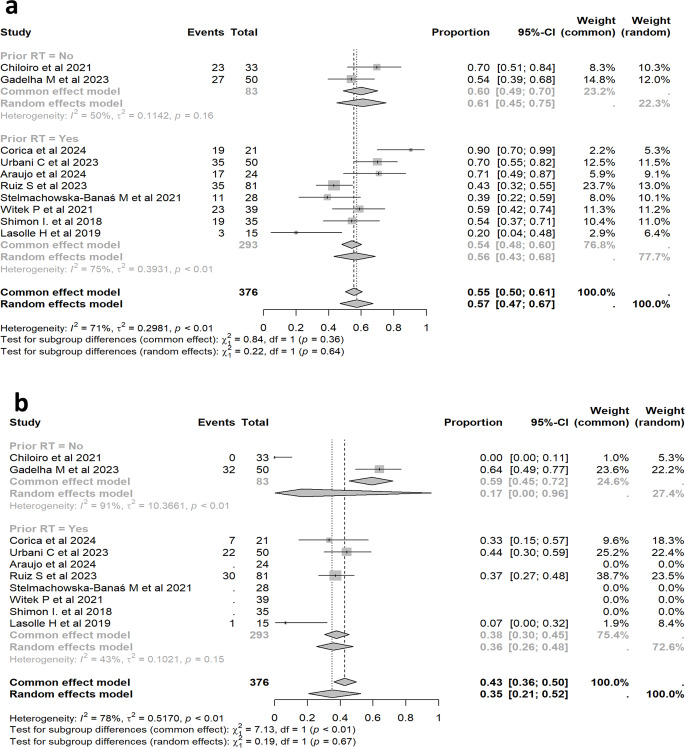
Sensitivity analysis on studies with radiotherapy
We performed this sensitivity analysis to evaluate the potential impact of prior RT on IGF-1 control and tumor shrinkage in patients treated with PAS-LAR. Figure 6 shows the sensitivity analysis for patients stratified into two groups: nine studies with prior RT and two studies, who had either not received RT [25] or had undergone RT more than 10 years prior [12], making it unlikely that RT influenced the treatment outcomes. The analysis examines two primary outcomes: (a) IGF-1 control and (b) tumor volume reduction. Despite the stratification, the CI for the overall IGF-1 control effect remained overlapping across the different subgroups. For all studies combined (Fig. 6a), IGF-1 control was 57.9% (95% CI: 48.4–66.8%). In the non-RT group, IGF-1 control was slightly higher at 61.0% [95% CI: 45.0–75.0%], whereas in the RT group, it was 57.0% [95% CI: 47.0–67.0%]. These results indicate that prior RT does not have a major differential impact on our results on IGF-1 control in patients on PAS-LAR treatment.
Fig. 6.
Sensitivity analysis of Pasireotide effectiveness on IGF-1 control (a) and tumor volume shrinkage (b) in Patients with and without prior radiotherapy. Figure 6 shows the sensitivity analysis comparing the effectiveness of pasireotide in achieving IGF-1 control (a) and tumor volume reduction (b) between patients with and without prior radiotherapy. The analysis highlights differences in heterogeneity and treatment response between the two groups
Regarding tumor volume reduction: in one study, no tumor volume reduction was observed in any patient, while the other study reported some degree of reduction (Fig. 6b). This discrepancy between the two studies contributes to the high heterogeneity observed in the non-RT group (I² = 91.0%, p < 0.01). In contrast, the studies involving patients with prior RT showed significantly lower heterogeneity (I² = 42.0%, p < 0.15), indicating more consistent results. The extreme variability in the non-RT group, as indicated by the wide CI, suggests that tumor volume reduction in these patients may be more inconsistent. This could be due to differences in baseline tumor characteristics or the absence of the stabilizing effect of prior RT. In contrast, the lower heterogeneity and narrower CI in the RT group suggest that prior RT may have contributed to a more predictable and uniform response to PAS-LAR treatment.
Discussion
Our meta-analysis, encompassing 12 studies (11 without duplicated sample) with a total of 409 patients, revealed that the overall IGF-1 control rate in acromegaly patients treated with PAS-LAR was 57.9% (95% CI: 48.4–66.8%) indicating better outcomes in clinical practice than previously reported in the pivotal RCTs [10, 11], which was of about 30%. This finding is striking and surprising as, RCTs tend to show better results than clinical practice, rigorous follow-up of patients and frequent visits. In addition, one-third of patients experience clinically relevant tumor shrinkage. On the other hand, not surprisingly, these outcomes were accompanied by significant increases in glucose levels, HbA1c, and T2DM prevalence, as previously reported [10, 11], which reflects PAS-LAR predominant action at SSTR5 and the dampening of insulin and incretin hormone secretion [42, 43].
Biochemical control
Our study reports higher effectiveness in the real-world setting than in RCTs [10, 11]. This discrepancy can be attributed to several factors as, pivotal trials often employ strict biochemical thresholds for IGF-1 and GH levels to define control, in contrast, in clinical practice IGF-1 is the preferred parameter to establish disease control [3]. In addition, variations in the specific cut-off values for IGF-1 and the methods used to measure hormone levels can lead to differences in reported control rates. However, this did not affect our findings, as the leave-out-one study confirmed the robustness of the results. The sensitivity analysis also revealed that previous radiotherapy did not significantly alter the outcomes for IGF-1 control, as both groups—those with and without prior radiotherapy—exhibited overlapping confidence intervals, suggesting consistent effectiveness across patient populations. Also, the timing of outcome assessments, and the duration of follow-up can contribute to the discrepancies observed. In this regard, longer follow-up periods and extended timeframe can allow for better dose titration and potentially improve IGF-1 control over time. The individualized treatment approaches used in clinical practice, where physicians have the flexibility to tailor treatment regimens to each patient’s specific needs, comorbidities, and response patterns may account in part to explain these discrepancies. In this regard this more personalized approach, may include a pre-treatment selection of patients that have a better response to PAS-LAR such as e.g. younger patients, with T2 magnetic resonance imaging hyperintensity [13, 29], sparsely granulated tumors [26], and/or a high SSTR5/SSTR2 ratio [25, 29, 44, 45]. However, probably all factors previously mentioned do not explain completely these findings, and it could not be discarded that some special cases have also been treated with other second line compounds, such as pegvisomant, thus avoiding a therapeutic PAS-LAR failure. This could be the case in patients with a previous diagnosis of T2DM, in which case the treating clinician may have avoided PAS-LAR, thus preselecting the cases.
While real-world effectiveness was higher than RCTs, metanalysis heterogeneity was also high, which could be attributed to factors such as differences in patient populations, treatment protocols, or follow-up durations across studies. Also, individual responses to PAS-LAR in specific subgroup of patients that may be concentrated in certain centers could also contribute to the differences and relatively high variability observed between the meta-analysis and pivotal studies. Patients with more aggressive or refractory disease may respond less favourably, and this subtype of patients may had been more prominent in early pivotal studies, thus concentrating patients with highly resistant acromegaly, while those with milder forms of resistant acromegaly or partial responders may have acceded to PAS-LAR showing better outcomes, contributing to higher control rates in the present meta-analysis of real-world data. The sensitivity analysis further highlighted that variability in treatment response was not significantly explained by prior radiotherapy, as heterogeneity persisted even when this factor was considered.
The analysis of GH levels, based on 5 studies, showed also a significant reduction with PAS-LAR treatment. This is a crucial finding with several implications for patients with acromegaly as GH control is intimately linked to improved patient outcomes and symptoms amelioration. Notably, the normalization of GH levels has been consistently associated with a reduction in mortality rates [46, 47]. Elevated GH levels have been associated with increased mortality rates, particularly due to cardiovascular [48], respiratory diseases [48], and malignancies [49].
Percentage of patients with clinically relevant tumor shrinkage
The results of our meta-analysis indicate that during PAS-LAR therapy approximately one third of patients experience clinically relevant tumor volume reduction. This percentage underline the therapeutic efficacy of PAS-LAR in managing tumor burden, particularly in patients unresponsive to fgSRL. However, this efficacy rate should be viewed in the context of the significant heterogeneity observed among the studies (I2 = 81.1%), indicating a substantial variability in patient populations, treatment protocols, and study designs. The sensitivity analysis for tumor volume reduction also revealed significant heterogeneity, particularly in the non-RT group, where variability was higher. This suggests that tumor shrinkage in patients without prior radiotherapy may be less predictable. The mechanism underlying PAS-LAR effectiveness involves its high affinity for somatostatin receptor subtypes 1, 2, 3, and particularly 5, which is more prevalent in somatotroph adenomas. This broad receptor affinity contributes to its superior efficacy in inhibiting GH secretion and tumor cell proliferation, although it has proposed that binding to SSTR3 maybe a principal driver of tumor reduction although not the only one [50–52]. Nonetheless, the variable response rates highlight the need for personalized treatment approaches and further research to elucidate the predictors of tumor response.
Glucose metabolism
The analysis of fasting glucose levels, HbA1c and percentage of T2DM showed a generalized negative impact of PAS-LAR on glucose parameters. The deleterious effect of PAS-LAR in glucose metabolism is largely attributable to the mechanism of action of the drug. Understanding this mechanism helps to explain it to the patient and how we can reduce and treat it more appropriately. As we previously stated in the Introduction section, PAS-LAR is a multi-receptor-targeted somatostatin receptor ligand with high affinity for four of the five somatostatin receptor subtypes (SSTR-1, SSTR-2, SSTR-3, and SSTR-5). Importantly, it has a particularly high affinity for SSTR-5, which is more prevalent on GH-secreting pituitary adenomas than the SSTR-2 [53, 54] targeted by fg-SRLs. While the SSTR-5 binding is beneficial for GH suppression, it also affects pancreatic islet cells. Somatostatin receptors, particularly SSTR-5, are expressed on pancreatic α and β cells [55] and pasireotide binding leads to impaired insulin secretion from β cells, suppression of glucagon-like peptide-1 (GLP-1) secretion and reduction in glucose-dependent insulinotropic polypeptide (GIP) levels [56]. The hyperglycemic effect of PAS-LAR seems to be primarily caused by impaired insulin secretion, while insulin sensitivity remains unaffected, making it crucial to monitor patients’ glycemic control during treatment [41]. This side effect often manifests early in treatment [33] and factors such as age, baseline HbA1c levels, fasting plasma glucose, and pre-existing prediabetes or diabetes conditions are associated with an increased risk while PAS-LAR is initiated [57]. However, It is worth noting that these metabolic effects are often partially reversible upon discontinuation of PAS-LAR [58], highlighting the direct relationship between the drug’s mechanism and the observed glucose abnormalities. Understanding this mechanism has led to propose specific management strategies for PAS-LAR-induced hyperglycemia, including the use of GLP-1 receptor agonists or DPP-4 inhibitors, which can help counteract the reduction in incretin hormones [59].
Implications for clinical practice
The remarkable combined IGF-1 control and volume shrinkage rate observed suggest that PAS-LAR use in a real world setting is an effective treatment option for acromegaly patients resistant to or intolerant to fgSRL. The dual impact on both GH hypersecretion and tumor size highlights the therapeutic value of PAS-LAR in improving long-term prognosis and quality of life for individuals with a difficult acromegaly condition. However, the significant heterogeneity observed in most of the outcomes indicates that treatment response may vary among patients, emphasizing the need to discover and implement PAS-LAR response biomarkers in personalized treatment approaches. In addition, the substantial impact on glucose metabolism highlights the need for careful patient selection, close monitoring of glucose levels, and proactive management of hyperglycemia.
Limitations
Several limitations should be considered when interpreting our results. Although our meta-analysis shows consistent improved IGF-1 control and tumor shrinkage rates, it is important to acknowledge that real-world studies inherently have limitations, such as potential selection bias and less stringent monitoring. The Gadelha et al. Study [12], while originally initiated as an RCT, may have had a marginal impact on the outcomes due to initially a potentially more strict patient selection an follow-up. However, the 10-year extended roll-over period of real-life follow-up likely mitigated this bias and provides invaluable insights into the long-term effects and management of PAS-LAR in a real-world context. In fact, as we previously suggested, the results of the present study suggest that patients’ selection may explain the differences in outcomes compared to RCTs. Nevertheless, the higher IGF-1 control rates in real-world settings are encouraging and suggest that the management of acromegaly in clinical practice may be more effective than previously thought based on RCTs alone. The high heterogeneity in many outcomes also suggests that factors influencing treatment response need further investigation.
Future directions
Future research should focus on: (a) Larger, long-term studies to confirm the efficacy and safety profile of PAS-LAR. (b) Investigation of factors contributing to heterogeneity in treatment response. (c) Comprehensive assessment of metabolic effects. (d) Evaluation of quality of life outcomes to better understand the overall impact of PAS-LAR treatment on patient’s lives and (e) Studies comparing PAS-LAR with other treatment options for acromegaly to establish the best positioning of this compound in the treatment algorithm.
In conclusion, our meta-analysis supports the efficacy of PAS-LAR in controlling IGF-1, GH levels and tumor volume in acromegaly patients, while highlighting the importance of careful glucose metabolism monitoring. These findings contribute to the growing body of evidence supporting the use of PAS-LAR in acromegaly management, particularly in patients who do not respond adequately to first-line treatments. Additionally, the sensitivity analysis suggests that previous radiotherapy does not significantly impact the overall effectiveness of PAS-LAR, though it may influence tumor volume reduction in certain cases. Therefore, RT should be considered when interpreting the real-world effectiveness of pasireotide, particularly regarding its impact on tumor volume reduction. However, the significant heterogeneity observed across studies emphasizes the need for individualized treatment approaches and further research to optimize patient outcomes. As clinicians gain more experience with newer treatments over time, their ability to optimize dosing, manage side effects, and select appropriate candidates for specific therapies improves. This accumulated expertise will contribute to better outcomes in real-world settings compared to the earlier RCTs.
Abbreviations
- ADA
American Diabetes Association
- fgSRL
First-Generation Somatostatin Receptor Ligands
- GH
Growth Hormone
- GLP-1
Glucagon-Like Peptide 1
- GIP
Glucose-Dependent Insulinotropic Polypeptide
- HbA1c
Glycated Hemoglobin
- HOMA-IR
Homeostasis Model Assessment of Insulin Resistance
- I²
I-squared (a measure of heterogeneity in meta-analyses)
- IGF-1
Insulin-like Growth Factor 1
- PAS-LAR
Pasireotide Long-Acting Release
- PICO
Population, Intervention, Comparator, Outcome
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
- Q
Q test (test for heterogeneity in meta-analysis)
- RCT
Randomized Controlled Trial
- RWE
Real-World Evidence
- SMD
Standardized Mean Difference
- SSTR
Somatostatin Receptor Subtype
- T2DM
Type 2 Diabetes Mellitus
- ULN
Upper Limit of Normal
- WHO
World Health Organization
Author contributions
Conceptualization and literature search: [Betina Biagetti, Marta Araujo-Castro, Mónica Marazuela and Manel Puig-Domingo], the formal analysis was performed by [Cristian Tebe]. The first draft of the manuscript was written by [Betina Biagetti] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability
Access data supporting the results and analysis in the article, are publicly archived in ZENODO https://zenodo.org/records/13118437.
Declarations
Institutional review board statement
Not applicable.
Informed consent
Not applicable.
Conflict of interest
B.B., MA, MM and M.P.D. have received consulting fees, research support, or participated in clinical trials supported by Pfizer, Ipsen, and/or Recordati. C.T. has received fees for speaker lectures from Gedeon Richte.
Footnotes
The original online version of this article was revised to update Figure 4, parts (a) and (b) overlap, resulting in the lower section of Figure 4a being hidden.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/19/2024
To update Figure 4, parts (a) and (b) overlap, resulting in the lower section of Figure 4a being hidden.
Change history
11/27/2024
A Correction to this paper has been published: 10.1007/s11154-024-09935-4
Contributor Information
Betina Biagetti, Email: betinaloys.biagetti@vallhebron.cat.
Manel Puig-Domingo, Email: mpuigd@igtp.cat.
References
- 1.Gatto F, Campana C, Cocchiara F, Corica G, Albertelli M, Boschetti M, et al. Current perspectives on the impact of clinical disease and biochemical control on comorbidities and quality of life in acromegaly. Rev Endocr Metab Disord. 2019;20:365–81. [DOI] [PubMed] [Google Scholar]
- 2.Esposito D, Ragnarsson O, Johannsson G, Olsson DS. Prolonged diagnostic delay in acromegaly is associated with increased morbidity and mortality. Eur J Endocrinol. 2020;182:523–31. [DOI] [PubMed] [Google Scholar]
- 3.Giustina A, Biermasz N, Casanueva FF, Fleseriu M, Mortini P, Strasburger C, et al. Consensus on criteria for acromegaly diagnosis and remission. Pituitary. 2024;27:7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass JAH, et al. A Consensus Statement on acromegaly therapeutic outcomes. Nat Reviews Endocrinol. 2018;14:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C et al. A Consensus on the diagnosis and treatment of Acromegaly comorbidities: an update. J Clin Endocrinol Metab. 2019. [DOI] [PubMed]
- 6.Giustina A, Barkhoudarian G, Beckers A, Ben-Shlomo A, Biermasz N, Biller B, et al. Multidisciplinary management of acromegaly: a consensus. Rev Endocr Metab Disord. 2020;21:667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleseriu M, Langlois F, Lim DST, Varlamov EV, Melmed S. Acromegaly: pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol. 2022;10:804–26. [DOI] [PubMed] [Google Scholar]
- 8.Antunes X, Ventura N, Camilo GB, Wildemberg LE, Guasti A, Pereira PJM, et al. Predictors of surgical outcome and early criteria of remission in acromegaly. Endocrine. 2018;60:415–22. [DOI] [PubMed] [Google Scholar]
- 9.Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146:707–16. [DOI] [PubMed] [Google Scholar]
- 10.Colao A, Bronstein MD, Freda P, Gu F, Shen C-C, Gadelha M, et al. Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab. 2014;99:791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadelha MR, Bronstein MD, Brue T, Coculescu M, Fleseriu M, Guitelman M, et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:875–84. [DOI] [PubMed] [Google Scholar]
- 12.Gadelha M, Marques NV, Fialho C, Scaf C, Lamback E, Antunes X et al. Long-term efficacy and safety of pasireotide in patients with acromegaly: 14 years’ single-center real-world experience. J Clin Endocrinol Metab. 2023;dgad378. [DOI] [PMC free article] [PubMed]
- 13.Ruiz S, Gil J, Biagetti B, Venegas E, Cámara R, Garcia-Centeno R, et al. Magnetic resonance imaging as a predictor of therapeutic response to pasireotide in acromegaly. Clin Endocrinol (Oxf). 2023;99:378–85. [DOI] [PubMed] [Google Scholar]
- 14.Urbani C, Dassie F, Zampetti B, Mioni R, Maffei P, Cozzi R et al. Real-life data of Pasireotide LAR in Acromegaly: a long-term follow-up. J Endocrinol Invest. 2024. [DOI] [PMC free article] [PubMed]
- 15.Araujo-Castro M, Biagetti B, Menedez-Torre E, Novoa-Testa I, Cordido F, Pascual-Corrales E et al. Pegvisomant or pasirotide in PRL and GH co-secreting vs GH-secreting Pit-NETs. Endocr Relat Cancer. 2024;ERC-24-0043. [DOI] [PubMed]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13:S31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 19.R: The R Project for Statistical Computing [Internet]. [cited 2024 Jul 19]. https://www.r-project.org/
- 20.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corica G, Pirchio R, Milioto A, Nista F, Arecco A, Mattioli L et al. Pasireotide effects on biochemical control and glycometabolic profile in acromegaly patients switched from combination therapies or unconventional dosages of somatostatin analogs. J Endocrinol Invest. 2023. [DOI] [PubMed]
- 22.Favero V, Zampetti B, Carioni EI, Dalino Ciaramella P, Grossrubatscher E, Dallabonzana D, et al. Efficacy of pasireotide LAR for acromegaly: a prolonged real-world monocentric study. Front Endocrinol (Lausanne). 2024;15:1344728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiloiro S, Costa D, Lauretta R, Mercuri V, Sbardella E, Samperi I, et al. Partial response to first generation SSA guides the choice and predict the outcome of second line therapy in acromegaly. Endocrine. 2022;78:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiloiro S, Giampietro A, Visconti F, Rossi L, Donfrancesco F, Fleseriu CM, et al. Glucose metabolism outcomes in acromegaly patients on treatment with pasireotide-LAR or pasireotide-LAR plus Pegvisomant. Endocrine. 2021;73:658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiloiro S, Giampietro A, Mirra F, Donfrancesco F, Tartaglione T, Mattogno PP, et al. Pegvisomant and Pasireotide LAR as second line therapy in acromegaly: clinical effectiveness and predictors of response. Eur J Endocrinol. 2021;184:217–29. [DOI] [PubMed] [Google Scholar]
- 26.Iacovazzo D, Carlsen E, Lugli F, Chiloiro S, Piacentini S, Bianchi A, et al. Factors predicting pasireotide responsiveness in somatotroph pituitary adenomas resistant to first-generation somatostatin analogues: an immunohistochemical study. Eur J Endocrinol. 2016;174:241–50. [DOI] [PubMed] [Google Scholar]
- 27.Pirchio R, Auriemma RS, Vergura A, Pivonello R, Colao A. Long-term pasireotide therapy in acromegaly: extensive real-life experience of a referral center. J Endocrinol Invest. 2024. [DOI] [PMC free article] [PubMed]
- 28.Coopmans EC, El-Sayed N, Frystyk J, Magnusson NE, Jørgensen JOL, van der Lely A-J, et al. Soluble Klotho: a possible predictor of quality of life in acromegaly patients. Endocrine. 2020;69:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coopmans EC, Schneiders JJ, El-Sayed N, Erler NS, Hofland LJ, van der Lely A-J, et al. T2-signal intensity, SSTR expression, and somatostatin analogs efficacy predict response to pasireotide in acromegaly. Eur J Endocrinol. 2020;182:595–605. [DOI] [PubMed] [Google Scholar]
- 30.Muhammad A, Coopmans EC, Delhanty PJD, Dallenga AHG, Haitsma IK, Janssen JAMJL, et al. Efficacy and safety of switching to Pasireotide in Acromegaly patients controlled with pegvisomant and somatostatin analogues: PAPE extension study. Eur J Endocrinol. 2018;179:269–77. [DOI] [PubMed] [Google Scholar]
- 31.Muhammad A, Van der Janssen LAJ, Neggers J. S. Efficacy and safety of switching to pasireotide LAR alone or in combination with pegvisomant in acromegaly patients controlled with combination treatment of first-generation somatostatin analogues and weekly pegvisomant (PAPE study): a prospective open-label 48 week study, preliminary results 24 weeks. 2017 [cited 2017 Sep 24]; http://www.endocrine-abstracts.org/ea/0049/ea0049GP174.htm
- 32.Fleseriu M, Rusch E, Geer EB, ACCESS Study Investigators. Safety and tolerability of pasireotide long-acting release in acromegaly-results from the acromegaly, open-label, multicenter, safety monitoring program for treating patients who have a need to receive medical therapy (ACCESS) study. Endocrine. 2017;55:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stelmachowska-Banaś M, Czajka-Oraniec I, Tomasik A, Zgliczyński W. Real-world experience with pasireotide-LAR in resistant acromegaly: a single center 1-year observation. Pituitary. 2022;25:180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witek P, Bolanowski M, Szamotulska K, Wojciechowska-Luźniak A, Jawiarczyk-Przybyłowska A, Kałużny M. The Effect of 6 months’ treatment with pasireotide LAR on glucose metabolism in patients with resistant acromegaly in real-world clinical settings. Front Endocrinol (Lausanne). 2021;12:633944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akirov A, Gorshtein A, Dotan I, Khazen NS, Pauker Y, Gershinsky M, et al. Long-term safety and efficacy of long-acting pasireotide in acromegaly. Endocrine. 2021;74:396–403. [DOI] [PubMed] [Google Scholar]
- 36.Masri-Iraqi H, Akirov A, Shimon I. Medical Treatment Landscape for active acromegaly in a Pituitary Center in Israel. Endocr Pract. 2020;26:1298–303. [DOI] [PubMed] [Google Scholar]
- 37.Shimon I, Adnan Z, Gorshtein A, Baraf L, Saba Khazen N, Gershinsky M, et al. Efficacy and safety of long-acting pasireotide in patients with somatostatin-resistant acromegaly: a multicenter study. Endocrine. 2018;62:448–55. [DOI] [PubMed] [Google Scholar]
- 38.Tahara S, Murakami M, Kaneko T, Shimatsu A. Efficacy and safety of long-acting pasireotide in Japanese patients with acromegaly or pituitary gigantism: results from a multicenter, open-label, randomized, phase 2 study. Endocr J. 2017;64:735–47. [DOI] [PubMed] [Google Scholar]
- 39.Gadelha M, Bex M, Colao A, Pedroza García EM, Poiana C, Jimenez-Sanchez M, et al. Evaluation of the efficacy and safety of switching to Pasireotide in patients with acromegaly inadequately controlled with First-Generation somatostatin analogs. Front Endocrinol (Lausanne). 2019;10:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lasolle H, Ferriere A, Vasiljevic A, Eimer S, Nunes M-L, Tabarin A. Pasireotide-LAR in acromegaly patients treated with a combination therapy: a real-life study. Endocr Connect. 2019;8:1383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf P, Dormoy A, Maione L, Salenave S, Young J, Kamenický P, et al. Impairment in insulin secretion without changes in insulin resistance explains hyperglycemia in patients with acromegaly treated with pasireotide LAR. Endocr Connect. 2022;11:e220296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silverstein JM. Hyperglycemia induced by pasireotide in patients with Cushing’s disease or acromegaly. Pituitary. 2016;19:536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jepsen SL, Albrechtsen NJW, Windeløv JA, Galsgaard KD, Hunt JE, Farb TB et al. Antagonizing somatostatin receptor subtype 2 and 5 reduces blood glucose in a gut- and GLP-1R–dependent manner. JCI Insight [Internet]. 2021 [cited 2024 Jul 20];6. https://insight.jci.org/articles/view/143228 [DOI] [PMC free article] [PubMed]
- 44.Bolanowski M, Kałużny M, Witek P, Jawiarczyk-Przybyłowska A. Pasireotide-a novel somatostatin receptor ligand after 20 years of use. Rev Endocr Metab Disord. 2022;23:601–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gatto F, Feelders RA, Franck SE, van Koetsveld PM, Dogan F, Kros JM, et al. In Vitro Head-to-head comparison between Octreotide and Pasireotide in GH-Secreting pituitary adenomas. J Clin Endocrinol Metab. 2017;102:2009–18. [DOI] [PubMed] [Google Scholar]
- 46.Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008;159:89–95. [DOI] [PubMed] [Google Scholar]
- 47.Varadhan L, Reulen RC, Brown M, Clayton RN. The role of cumulative growth hormone exposure in determining mortality and morbidity in acromegaly: a single centre study. Pituitary. 2016;19:251–61. [DOI] [PubMed] [Google Scholar]
- 48.Orme S, McNally R, James PW, Davis J, Ayuk J, Higham C, et al. Increased mortality in acromegaly is due to vascular and respiratory disease and is normalised by control of GH levels-A retrospective analysis from the UK Acromegaly Register 1970–2016. Clin Endocrinol (Oxf). 2024;100:558–64. [DOI] [PubMed] [Google Scholar]
- 49.Mercado M, Gonzalez B, Vargas G, Ramirez C, de los Monteros ALE, Sosa E, et al. Successful mortality reduction and control of comorbidities in patients with acromegaly followed at a highly specialized multidisciplinary clinic. J Clin Endocrinol Metab. 2014;99:4438–46. [DOI] [PubMed] [Google Scholar]
- 50.Theodoropoulou M, Stalla GK. Somatostatin receptors: from signaling to clinical practice. Front Neuroendocrinol. 2013;34:228–52. [DOI] [PubMed] [Google Scholar]
- 51.Liu D, Martino G, Thangaraju M, Sharma M, Halwani F, Shen S-H, et al. Caspase-8-mediated intracellular acidification precedes mitochondrial dysfunction in somatostatin-induced Apoptosis*. J Biol Chem. 2000;275:9244–50. [DOI] [PubMed] [Google Scholar]
- 52.Lee M, Lupp A, Mendoza N, Martin N, Beschorner R, Honegger J et al. SSTR3 is a putative target for the medical treatment of gonadotroph adenomas of the pituitary. 2015 [cited 2024 Jul 25]; https://erc.bioscientifica.com/view/journals/erc/22/1/111.xml [DOI] [PubMed]
- 53.Taboada GF, Luque RM, Bastos W, Guimarães RFC, Marcondes JB, Chimelli LMC, et al. Quantitative analysis of somatostatin receptor subtype (SSTR1-5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur J Endocrinol. 2007;156:65–74. [DOI] [PubMed] [Google Scholar]
- 54.Taboada GF, Luque RM, Neto LV, Machado E, de O, Sbaffi BC, Domingues RC, et al. Quantitative analysis of somatostatin receptor subtypes (1–5) gene expression levels in somatotropinomas and correlation to in vivo hormonal and tumor volume responses to treatment with octreotide LAR. Eur J Endocrinol. 2008;158:295–303. [DOI] [PubMed] [Google Scholar]
- 55.Jaquet P, Ouafik L, Saveanu A, Gunz G, Fina F, Dufour H, et al. Quantitative and functional expression of somatostatin receptor subtypes in human prolactinomas. J Clin Endocrinol Metab. 1999;84:3268–76. [DOI] [PubMed] [Google Scholar]
- 56.Henry RR, Ciaraldi TP, Armstrong D, Burke P, Ligueros-Saylan M, Mudaliar S. Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab. 2013;98:3446–53. [DOI] [PubMed] [Google Scholar]
- 57.Samson SL, Bolanowski M, Zhang S-L, Yu Y, Witek P, Kietsiriroje N, et al. THU054 a Post Hoc analysis of the phase IV B2219 study to determine predictive factors for Hyperglycemia during Treatment with Pasireotide. J Endocr Soc. 2023;7:bvad1141134. [Google Scholar]
- 58.Gadelha MR, Gu F, Bronstein MD, Brue TC, Fleseriu M, Shimon I, et al. Risk factors and management of pasireotide-associated hyperglycemia in acromegaly. Endocr Connect. 2020;9:1178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Störmann S, Meyhöfer SM, Groener JB, Faust J, Schilbach K, Seufert J, et al. Management of pasireotide-induced hyperglycemia in patients with acromegaly: an experts’ consensus statement. Front Endocrinol (Lausanne). 2024;15:1348990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access data supporting the results and analysis in the article, are publicly archived in ZENODO https://zenodo.org/records/13118437.