Abstract
Immunotherapy represents an emergent and heterogeneous group of anticancer treatments harnessing the human immune-surveillance system, including immune-checkpoint inhibitor monoclonal antibodies (mAbs), Chimeric Antigen Receptor T Cells (CAR-T) therapy, cancer vaccines and lymphocyte activation gene-3 (LAG-3) therapy. While remarkably effective against several malignancies, these therapies, often in combination with other cancer treatments, have showed unforeseen toxicity, including cardiovascular complications. The occurrence of immuno-mediated adverse (irAEs) events has been progressively reported in the last 10 years. These irAEs present an extended range of severity, from self-limiting to life-threatening conditions. Although recent guidelines in CardioOncology have provided important evidence in managing cancer treatments, they often encompass general approaches. However, a specific focus is required due to the particular etiology, unique risk factors, and associated side effects of immunotherapy. This review aims to deepen the understanding of the prevalence and nature of cardiovascular issues in patients undergoing immunotherapy, offering insights into strategies for risk stratification and management.
Keywords: CardioOncology, Immunotherapy, Cardioprotection, Cardiovascular prevention, Heart failure, Cancer therapy
Background
Over recent years, advancements in supportive care and personalized anticancer treatments have significantly improved cancer patients’ prognoses, increasing the number of long-term survivors. Anticancer immunotherapy is a heterogeneous treatment modality based on the use of biological agents to enhance the immune system’s ability to fight tumors [59]. While early approaches often failed, the discovery of primary immune checkpoints led to the development of monoclonal antibodies (mAbs) able to bind and block these natural inhibitory pathways. The inhibitory immune-checkpoints represent physiological mechanisms in the host, that prevent immunological overreactions and that are able to abrogate the host natural immune response in case of chronic inflammatory processes and cancer. Specifically, mAbs targeting the Programmed cell death receptor 1 (PD-1)/PD-ligand 1 (PD-L1) immune checkpoint produce their staggering anticancer activity by restoring the cytolytic activity of specific immune-effectors in the tumors. These immune-effectors are usually represented by CD8+ T cells (cytotoxic T cells, CTLs) whose precursors have risen in the host, along the lengthy process of cancerogenesis [46]. Immune-check points, such as CTLA-4 (expressed on activated CTL precursors and that binds B7.1 in the lymph-nodes) and PD-1 (expressed on tumor infiltrating CTLs and which binds PD-L1 and or PD-L2 in the tumor) may, consequently, become opportunistic instruments of the tumor to escape the immuno-surveillance system [96]. Targeting these checkpoints has revolutionized the treatment for several malignancies, including non-small-cell lung cancer (NSCLC), head and neck carcinoma (HNC), urothelial/kidney cancer, malignant melanoma, triple-negative breast cancer (TNBC), and several gastrointestinal and gynecological malignancies. ICIs target specific immune checkpoints, reviving the immune response to target and erase cancer cells [52]. Additionally, cancer vaccines introduce antigens or immune-stimulating agents to prompt the immune system to recognize and attack cancer cells [69]. Finally, adoptive cell transfer therapies like chimeric antigen receptor T (CAR-T) cell therapy genetically modify a patient’s T cells to better recognize and eliminate cancer cells [37]. Leveraging the body’s immune system, these drugs have displayed remarkable efficacy in prolonging progression-free survival (PFS) and overall survival (OS) in clinical trials. However, they also pose risk of adverse events, particularly cardiovascular and hemodynamic disorders (CHDs) [8]. In fact, since their first use, these agents showed potential cardiovascular side effects, including myocarditis, pericardial diseases and arterial thromboembolic events [12, 82, 85]. The European Society of Cardiology (ESC) emphasize the importance of early detection and preventive measures for CHDs in cancer patients. Guidelines recommend multidisciplinary risk evaluations considering factors like age, lifestyle, type of anticancer treatment and existing cardiovascular conditions [49]. Over 70% of long-term cancer survivors develop CHDs, significantly impacting their quality of life and leading to increased medical expenses [71]. The Heart Failure Association (HFA) and the International Cardio-Oncology Society (ICOS) have developed a stratification tool to classify cancer patients into cardiovascular risk groups [7, 48]. The development of innovative molecular target therapy and immune-oncological drugs poses a significant challenge in preventing and managing adverse events, distinct from traditional antiblastic agents. Managing the cardiovascular side effects of innovative cancer treatments, especially when combined with traditional therapies, is challenging [29, 84]. This review examines the cardiovascular implications of various immunotherapy drugs.
Immune-oncological drugs
Table 1 provides comprehensive data on available immuno-oncological treatments.
Table 1.
Immuno-oncological treatments and randomized studies reporting CTR-CVT [3, 6, 9, 10, 31, 38, 66, 75, 80, 90]
| Treatment | Mechanism of Action | Approved Uses | Study reference | Cancer type | ICI arm | Comparison arm | CTR-CVT type | CTR-CVT incidence (%) |
|---|---|---|---|---|---|---|---|---|
| Ipilimumab (Yervoy) | CTLA-4 inhibitor | Advanced melanoma, renal cell carcinoma, metastatic colorectal cancer, hepatocellular carcinoma | Hodi et al., 2010 | Melanoma | Ipilimumab plus gp100/Ipilimumab alone | Gp 100 | Dyspnea | 12.7 |
| Nivolumab (Opdivo) | PD-1 inhibitor | Advanced melanoma, non-small cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, squamous cell carcinoma of the head and neck, urothelial carcinoma, hepatocellular carcinoma | Borghaei et al., 2015 | NSCLC | Nivolumab | Docetaxel | Dyspnea, Pericardial effusion | 0.7 |
| Pembrolizumab (Keytruda) | PD-1 inhibitor | Advanced melanoma, non-small cell lung cancer, head and neck squamous cell carcinoma, classical Hodgkin lymphoma, urothelial carcinoma, microsatellite instability-high cancer, gastric cancer | Robert et al., 2015 | Advanced Melanoma | Pembrolizumab | Ipilimumab | HTN | 0.7 |
| Atezolizumab (Tecentriq) | PD-L1 inhibitor | Non-small cell lung cancer, small cell lung cancer, urothelial carcinoma, triple-negative breast cancer | Socinski et al., 2018 | NSCLC | Atezolizumab + Bevacizumab + Carboplatin + Paclitaxel |
Bevacizumab + NSCLC + Carboplatin + Paclitaxel |
MI, HF, HTN | 0.5 |
| Durvalumab (Imfinzi) | PD-L1 inhibitor | Non-small cell lung cancer, extensive-stage small cell lung cancer | Antonia et al. (2017) | NSCLC | Durvalumab | Placebo | MI, AF, HF, Pericardial effusion, cardiogenic shock, VT, HTN | 3.3 |
| Avelumab (Bavencio) | PD-L1 inhibitor | Merkel cell carcinoma, urothelial carcinoma | Barlesi et al., 2018 | NSCLC | Avelumab | Docetaxel | MI, HF, Myocarditis, HTN | 1.5 |
|
Relatlimab (Opdualag) |
LAG-3 inhibitor | Melanoma | Tawbi et al., 2022 | Melanoma | Relatlimab + Nivolumab | Nivolumab | Myocarditis | 1.7 |
| Axicabtagene ciloleucel (Yescarta) | CAR T-cell therapy | Diffuse large B-cell lymphoma and high-grade B-cell lymphoma | Westin et al., 2023 | Diffuse large B-cell lymphoma | Axicabtagene ciloleucel | Two to three cycles of chemoimmunotherapy followed by high-dose chemotherapy with autologous stem-cell transplantation in patients who had a response | Hypotension; CRS; Cardiac arrest (1 patient) | 9; CRS 92%; |
| Tisagenlecleucel (Kymriah) | CAR T-cell therapy | B-cell acute lymphoblastic leukaemia; Diffuse large B-cell lymphoma and follicular lymphoma | Bishop et al., 2022 | Diffuse large B-cell lymphoma | Tisagenlecleucel with optional bridging therapy | Salvage chemotherapy and autologous hematopoietic stem-cell transplantation (HSCT) | CRS | CRS 61.3% |
| Lisocabtagene maraleucel (Breyanzi) | CAR T-cell therapy | Diffuse large B-cell lymphoma; high-grade B-cell lymphoma; Primary mediastinal large B-cell lymphoma; Follicular lymphoma grade 3B | Kamdar et al., 2022 | Relapsed or refractory large B-cell lymphoma | Lisocabtagene maraleucel | Three cycles of salvage immunochemotherapy | Hypotension; CRS | 22.8 CRS 50% |
NSCLC, non-small cell lung cancer; HTN, hypertension; MI, myocardial infarction; HF, heart failure; AF, atrial fibrillation; VT, ventricular tachycardia; CRS, cytokine release syndrome
Cytotoxic T lymphocyte antigen-4 (CTLA-4)
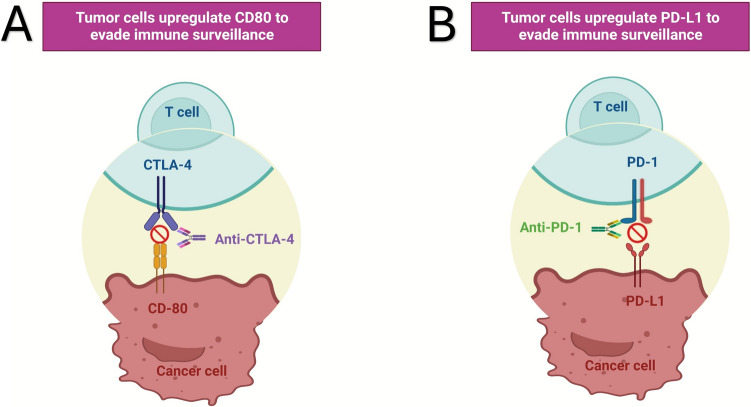
CTLA-4 pathway was the first inhibitory immune-checkpoint to be identified. CTLA-4 is a trans-membrane glycoprotein expressed on the surface of activated T cells, competing with CD28 for binding B7.1 and B7.2 on antigen-presenting cells (APCs) and B7H on tumors. CTLA-4 binding sends an inhibitory signal to T cell precursors, that become mostly anergic (Fig. 1A). Ipilimumab and tremelimumab were the first two mAbs against CTLA-4 approved for clinical use for the treatment of malignant melanoma and other malignant [55]. These mAbs restore the proliferative activity of T cell precursors in cancer patients [68]. However, early application of CTLA-4-ICI revealed significant challenges, including several immune-mediated adverse effects. Notably, these adverse events extended to the cardiovascular system, triggering episodes of pericarditis and myocarditis. Ipilimumab-related pericarditis has been reported as a late event, potentially self-limiting or rapidly progressing to cardiac tamponade [88, 92]. The use of CTLA-4 ICI has been sporadically associated to other CHDs including left ventricular dysfunction and myocardial fibrosis whose diagnosis and treatment remains mostly empiric [63, 67, 87]. Massive inflammatory cytokine release syndrome (CRS) may trigger cardiological, hemodynamic and respiratory failure [91]. The assessment of cancer-therapy related cardiovascular toxicity (CTR-CVT) presents challenges due to the differences in how cardiovascular and oncological outcomes are prioritized. Evaluation of CRS represents an example in these disparities. In fact, several scales have been developed to grade the severity of CRS, reflecting different perspectives from cardiology and oncology. More in detail, Lee criteria categorizes CRS based on clinical symptoms, focusing on the presence of fever, organ toxicity and the need for interventions like vasopressors or mechanical ventilation [43]. Penn criteria also include specific cardiovascular parameters such as hypotension and cardiac dysfunction, reflecting a broader assessment including cardiovascular impact [62]. Finally, the American Society for Transplantation and Cellular Therapy (ASTCT) grading for CRS includes clinical and laboratory markers, aligning with oncological practices while considering cardiovascular symptoms [44]. While oncological assessments prioritize managing CRS symptoms to continue cancer therapy, cardiology assessment emphasize detailed cardiovascular evaluation to prevent long-term complications. To close these gaps, multidisciplinary approach is essential. The incidence of irAEs notably increases when CTLA-4 inhibitors are combined with anti-PD-1 mAbs like nivolumab, emphasizing the need for patient monitoring [36]. Furthermore, despite high dosage of ICI could suggest an increased incidence of IrAEs, recent evidence did not show a significant impact of ICI dosage on the occurrence of cardiovascular events [54]. ECG changes, including ventricular arrhythmias, heart blocks, low voltages and pathological Q waves have shown to be related with ICI-related adverse cardiac events [64]. Therefore, ECG monitoring is essential for the surveillance of possible ICI-related cardiovascular toxicity.
Fig. 1.
Immunotherapy drugs mechanisms; A CTLA-4 mAbs; B PD-1/PD-L1 mAbs
Programmed cell death receptor 1 (PD-1) and PD-ligand 1 (PD-L1) immune checkpoint inhibitor mAbs
The advent of PD-1 and PD-L1 ICIs marked a significant breakthrough in cancer therapy. These inhibitors act on peripheral CTLs, modulating immune responses in chronic inflammation and cancer settings. PD-1, a transmembrane receptor on activated CTLs, binds to PD-L1 and PD-L2 on inflammatory and tumor cells (Fig. 1B). Clinical evaluations have shown that PD-1 and PDL-1 mAbs can reactivate anergic tumor-infiltrating CTLs, restoring robust antitumor activity [79]. However, their use is associated with irAEs, including autoimmune responses, leading to severe cardiac complications like myocarditis and pericarditis. Recent studies have identified a correlation between irAE risk and specific HLA alleles [16]. On these bases, it cannot be excluded a potential link between specific high-risk HLA alleles and the risk of ICI-related cardiovascular events, supported by preclinical studies where genetically engineered mice expressing high-risk HLA-DQ8.abo developed spontaneous autoimmune myocarditis, hinting at regulatory CD4 + T cell dysfunction [77, 86]. Moreover, certain high-risk HLA alleles correlate with irAEs occurrences, including HLA-B35 and HLA-DRB1.11 associated with pneumonitis [78]. PD-1 inhibitors such as nivolumab, pembrolizumab, cemiplimab and dostarlimab, along with PD-L1 inhibitors atezolizumab, avelumab, and durvalumab, have shown variable degrees of cardiotoxicity, including cardiac ischemia and myocardial infarction [56]. Preclinical studies suggest PD-L1’s role in post-ischemic tissue repair, indicating that PD-L1 mAbs might affect these mechanisms, increasing cardiovascular risk [47]. A meta-analysis involving more than 20,000 patients receiving different ICI combinations, particularly double ICI blockade (Ipilimumab and Nivolumab), highlights a significantly elevated risk of irAEs with ICI combinations, particularly, ipilimumab and nivolumab [32]. Moreover, combined PD-1/PD-L1 mAbs with cardiotoxic chemotherapy showed an increased risk of severe cardiac arrhythmias and myocardial damage [73]. IrAEs can also be influenced by the kinetics of mABs. Anti-PD-1 mAbs target PD-1 + T cells directly, while anti-PD-L1 mAbs bind to PD-L1 expressing tissues, including cardiac muscle cells, potentially causing damage [60]. Recent preclinical studies show that PD-L1 is over-expressed in normal tissues during post-inflammatory repair or post-ischemic recovery, exerting protective anti-apoptotic effects. Anti-PD-L1 mAbs may disrupt these properties, increasing the risk of cardiovascular adverse events in patients with preexisting or subclinical ischemic disease [15, 89]. The incidence of ICI-related cardiac adverse events varies widely among tumor entities. More in detail, thymic epithelial tumors, in particular thymoma, are more frequently associated with ICI-related cardiotoxicity [20]. Immuno-related arrhythmias, which pose significant mortality risks, may result from concurrent irAEs or preexisting conditions. These arrhythmias include conduction delays and ventricular and supraventricular arrhythmias, with immune-related arrhythmias involving severe cardiac dysfunction presenting worse prognosis [33]. The occurrence of ICI related auto-immunity can directly affect the cardiac tissues and the cardiovascular system resulting in the occurrence of myocarditis, pericarditis, heart failure, arrhythmias, Tako-Tsubo syndrome (TTS), dyslipidemia, and myocardial infarction (central illustration). Additionally, ICI induced systemic inflammatory syndrome and associated irAEs involving endocrine glands, may indirectly affect heart function, increasing arterial and thromboembolic risk. These findings highlight the importance of baseline evaluations and rigorous cardiac monitoring, especially in patients undergoing PD-1 blockade, chemotherapy, or combined immunotherapy.
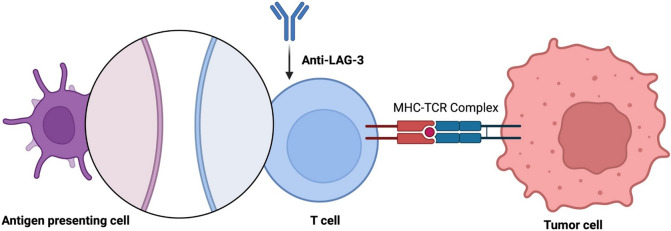
Lymphocyte activation gene-3 (LAG-3) Therapy
LAG-3 is an immune checkpoint molecule that regulates both CD4 + and CD8 + T-cell activation. Its primary function is to bind MHC class II molecules, acting as a negative regulator. By reducing T cell activation and proliferation, LAG-3 helps to prevent hyperactive immune responses and promotes immune tolerance [1]. LAG-3’s role in maintaining immunological homeostasis is further highlighted by its co-expression with other immune checkpoints, most notably PD-1, and its interaction with APCs (Fig. 2). Due to these mechanisms, LAG-3 is a therapeutic target for regulating immune responses in various clinical situations, including cancer [18]. Preclinical evidence showed that mice deficient in both LAG-3 and PD-1 developed severe myocarditis, suggesting that LAG-3 acts synergistically with PD-1 to prevent autoimmunity [57]. In a phase 3 study with a LAG-3 inhibitor (Relatlimab) in association with a PD-1 inhibitor (nivolumab), myocarditis occurred in 1.7% of the patients [80].
Fig. 2.
Lymphocyte activation gene-3 (LAG-3) therapy
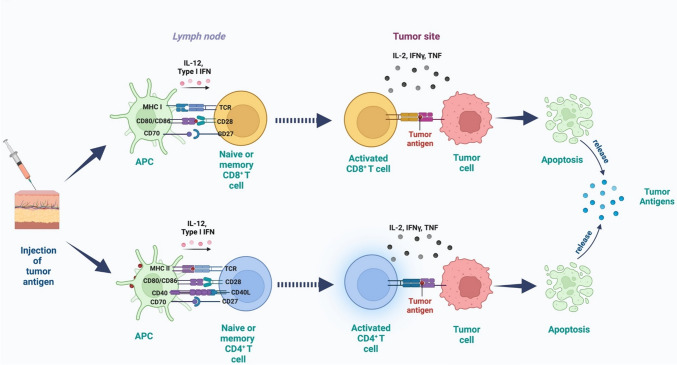
Active specific immunotherapy: cancer vaccines
Active specific immunotherapy, also known as “cancer vaccines”, operates on the premise that cancer cells express altered genes products known as tumor specific antigens (TSA) and tumor associate antigens (TAA), recognized by sensitized CTLs. These immune cells act as “magic bullets” to identify and kill cancer cells while sparing healthy tissues. Cancer vaccines mimic the natural immunization mechanisms and deliver constructs with target antigens to lymphoid organs, sensitizing specific T cell precursors and triggering an efficient T cell-mediated immune-response (Fig. 3) [72].
Fig. 3.
Cancer vaccines injection and pathways generating immune response against cancer cells
Cancer vaccines are classified in: (1) multi-antigen cell-based vaccines; (2) mono or poli-gene engineered viruses-based vaccines, (3) mono or poly-antigen gene engineered nucleic acids-based and finally, (4) peptide/epitope -based vaccines [81]. Currently, no cancer vaccine is approved yet for standard treatment in Europe. The multi-epitope Sipuleucel-T vaccine resulted efficacious in clinical trials and received FDA approval for the treatment of advanced prostate cancer patients over a decade ago, showing immuno-response and safety, and it was rarely associated to autoimmunity or cardiovascular adverse events, including myocardial infarction and thrombo-embolism [30]. In the most recent years, a great impulse to the development of cancer vaccines has followed the recent COVID-19 pandemics [34]. Newly developed nucleic acid (DNA/RNA) and engineered viruses-based vaccines effectively trigger immune-response against tumor antigens, mimicking a viral infection in the host. The first vaccines belonging to this family used viral DNA and RNA backbones (poxvirus, adenovirus, Castle virus, etc.) engineered to express the genes of known tumor target antigens/co-accessory molecules’ (B7.1, ICAM-1 LFA-3, CD40, TRICOM- PSA and TRICOM CEA) and/or proinflammatory cytokines (GM-CSF, IL12). Vaccines like prostate-specific antigen (PSA)-directed vaccine PROSTVAC (Poxoviruses engineered to express the genes of PSA, B7-1, ICAM-1, and LFA-3) showed controversial results in prostate cancer patients [25]. More recently, recombinant mRNA vaccines have been proposed as potential anticancer drugs for colorectal cancer, malignant melanoma, and non-small cell lung cancer [93]. The main safety risk associated with mRNA vaccines is their ability to trigger potent immune reactions and inflammatory syndromes, potentially leading to cardiovascular damage. Cancer vaccines might become a viable therapeutic option for patients with cold tumors, or those refractory to ICIs. However, more clinical trial data are needed to assess the benefits and risks of combining vaccines with ICIs.
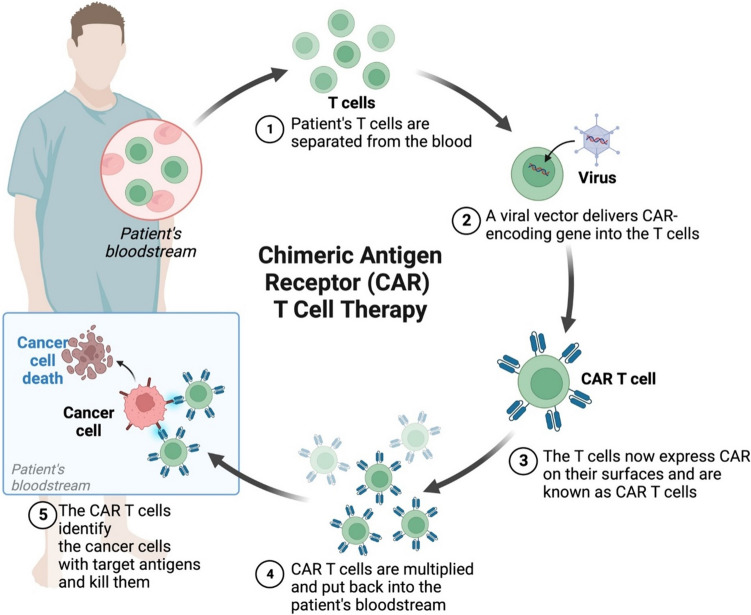
Chimeric antigen receptor T Cells (CAR-T) and cardiovascular damage
CAR-T cell therapy has gained prominence in treating selected blood cell malignancies. This approach relies on sensitized antigen-specific CTLs that recognize and eliminate tumors by engaging a trimeric complex involving the T cell receptor and MHC-presented antigens [76]. Despite decades of research into adoptive therapy using autologous antigen-specific CTLs, challenges like ex vivo culture, unpredictable effector homing, and declining cytolytic activity have limited its clinical translation [95]. However, advances in gene engineering have enabled the clinical development of CAR-T therapies, which involve infusing engineered T cells expressing selected TCR genes or other recognition molecules to target tumor cells with specific antigens (Fig. 4). Although promising, particularly for refractory lymphoblastic lymphoma and certain acute leukemias, CAR-T therapy for solid malignancies remains in its early stages. Current EMA and FDA approvals for CAR-T therapies in hematological malignancies are based on promising early trial results. However, their widespread use is restricted to specialized centers due to high costs, significant toxicity requiring close monitoring, and associations with severe adverse events, including cardiovascular complications and inflammatory syndromes [35]. Adoptive CAR-T therapy can lead to severe acute and delayed adverse events, primarily associated with compromised immunity and pathogen spread. These events include hemodynamic alterations, cardiovascular complications, and inflammatory lung syndromes, often with high lethality. Cardiovascular toxicity after CAR-T infusion is linked to triggering inflammatory (CRS), resulting in condition like acute respiratory distress syndrome (ARDS), heart failure, and multi-organ failure. Risk factors such as age, coronary artery disease, and renal impairment are used to assess CAR-T related cardiovascular events [83]. Vascular leak syndrome (VLS), left ventricular dysfunction, and myocardial ischemia have been reported, driven by inflammatory cytokines, causing acute systemic inflammation and vascular impairment [13, 19]. The majority of CAR-T cardiovascular adverse events stem from a massive release of inflammatory cytokines like IL-6, TNF-α and IFN-γ. These cytokines activate the prostaglandin pathway, causing systemic acute inflammatory syndrome with symptoms like fever, tachycardia, hypotension, multi-organ dysfunction, and cardiac damage. Persistent high levels of IL-6 contribute to chronic inflammation leading to cardiovascular impairments like atherosclerosis, arteria hypertension, heart failure, and myocardial ischemia [65]. Retrospective studies on cancer patients receiving CAR-T therapy showed cardiological events including HF, arrhythmias, ACS, myocardial injury, stroke, prolonged QTc and sudden cardiovascular death [2]. Similarly, another study highlighted a direct correlation between CRS onset and risk of MACE, emphasizing the need for continuous cardiovascular monitoring [45]. Finally, in a retrospective study including 116 patients treated with CD19-directed CAR T-cells, 10% experienced new or worsening cardiomyopathy, with a significant decline in left ventricular ejection fraction occurring after treatment initiation [22]. However, these data should be interpreted with caution. In fact, the study by Lefebvre et al. included de novo cardiac arrhythmias in the definition of MACE, which are not typically included in other studies. Furthermore, these retrospective data often report events from the “early days” of CAR-T therapy, where consequently tocilizumab, an IL-6 inhibitor which significantly reduces the clinical impact of CRS, was not used. Additionally, patients treated with CAR T cells are heavily pretreated, which could affect their prognosis. Finally, prospective data reported cardiac decompensation and atrial fibrillation episodes but did not show MACE (defined as a composite of myocardial infarction, stroke, revascularization, ventricular arrhythmias, and arrhythmias requiring implantable devices), contrasting with previous retrospective studies [39]. These cardiovascular events are not exclusive to adults; pediatric patients also showed CRS episodes, left ventricular dysfunction, and hypotension needing vaso-active agents [74]. On these bases, effectively monitoring, diagnosing, and treating CAR-T related cardiotoxicity remains challenging.
Fig. 4.
Chimeric antigen receptor (CAR) T cell therapy mechanisms
Immune effector cell–associated neurotoxicity syndrome (ICANS) should be strictly monitored for their potential ability to sustain the general risk of cardiovascular dysfunctions. Although no data from randomized clinical trials suggest that early intervention reduces the risk of CAR T-associated cardiovascular morbidity, indirect evidence suggest that cardiovascular protection may be achieved when early treatment of CRS takes place with corticosteroids and anti-IL-6 mAbs (tocilizumab) [42].
Immune-related cardiovascular events
ICI-related myocarditis and pericarditis
Table 2 summarizes all immune-related cardiovascular side effects with the relative treatment strategies [26, 50]. Myocarditis and pericarditis deserve a special mention since they are the most frequent and severe cardiac irAEs. Pericarditis, myocarditis and myopericarditis are severe immune-related complications, mandating immediate ICI discontinuation, anti-inflammatory therapies and potential pericardiocentesis [41]. The onset of ICI IR-myocarditis generally occurs within 3 months of treatment initiation (80% of cases), though symptoms can appear a few days to years later [11]. Clinical phenotypes of this condition range from smoldering (subclinical) to severe cases presenting with heart failure, arrhythmias or cardiogenic shock [28]. Moreover, cases of myocarditis with myositis and/or myasthenia gravis overlap syndrome (IM3OS) have been reported, with significant mortality and morbidity [61]. The pathogenic mechanisms of ICI-related CTR-CVT are multifaceted. According to Axelrod et al., myocardial immune infiltrate in ICI-related myocarditis is characterized by clonally expanded CD8 + T cells. Depleting these cells in mice significantly improved survival, showing that CD8 + cells are essential for the development of myocarditis. The last-mentioned study identified α-myosin, a heart-specific protein, as the main autoantigen recognized by CD8 + cells [5]. Furthermore, Michel et al. evaluated the direct effects of ICIs on cardiac cells, showing that these drugs can induce cardiomyocyte apoptosis and necrosis through immune-mediated mechanisms. They identified specific pathways, including endothelial dysfunction and increased expression of pro-apoptotic factors, which contribute to cardiotoxicity. Finally, they found that cardiolipins, component of the inner mitochondrial membrane, were significantly higher in anti-PD-1 treated mice, identifying mitochondrial damage and dysfunction as additional pathogenetic pathway in ICI-related CTR-CVT. This mitochondrial impairment affects energy production and increases oxidative stress [53]. Diagnosis should be pursued promptly upon symptom presentation using ECG monitoring, serial troponin and natriuretic peptide (BNP) assays and, progressively, echocardiography, cardiac imaging (cMRI and/or Cardio CT scan) and endomyocardial biopsy if highly suspected. Data indicate that 89% of positive cases present recognizable ECG abnormalities [51]. Elevated troponin (94%) and BNP (66%) levels are reliable early markers of ICI-related myocarditis and increased risk of Major Adverse Cardiac Events (MACE). Echocardiography is the first-line imaging modality, though left ventricular ejection fraction (LVEF) anomalies are undetectable in 50% of cases at the onset [50]. Diagnosis may be confirmed by detecting high troponin and BNP levels alongside LVEF decline and decreased global longitudinal strain (GLS) [4]. Cardiac magnetic resonance imaging (cMRI) has limited sensitivity in ICI-associated myocarditis as reported in an international registry of ICI-associated myocarditis especially if performed at the beginning of the symptoms [24, 94]. Recently, fibroblast activation protein inhibitor (FAPI) PET/CT has been proposed for early detection [21]. Endomyocardial biopsy, while being the definitive diagnostic standard, is invasive nature and risky, limiting its use in cancer patients. Treatment guidelines recommend early administration of corticosteroids, supportive therapy, and permanent interruption of the immune-oncological treatment. Rapid severe onset may necessitate plasmapheresis to remove the excess of ICI mAbs from the bloodstream, timed-according to the half-life of the specific drugs (e.g., ipilimumab 14.5 days, pembrolizumab 25.0 days, nivolumab 26.7 days and atezolizumab 27.0 days) [27]. Severe cases may require immunosuppressant like abatacept [14]. Inpatient management is recommended, including hemodynamic and arrhythmia monitoring. Advanced mechanical circulatory support, including intra-aortic balloon pump, Impella (Abiomed, Danvers, Massachusetts, USA) or extracorporeal membrane oxygenation, may also be required in case of cardiogenic shock [58]. Pericarditis, myopericarditis and pericardial effusion are further immune-related adverse events, mainly occurring in NSCLC patients receiving PD-1/PD-L1 mAbs alone or in combination. Clinical onset includes chest pain and dyspnea with pericarditis-related ECG changes (low voltages and tachycardia) and pericardial effusion on imaging. These immuno-related complications have a high rate of lethality (21%), higher than other pericarditis forms [17]. Treatment requires immediate ICI therapy discontinuation, anti-inflammatories drugs, steroids, supportive therapy, and eventually pericardiocentesis. Proactive cardiac care with close cardiovascular monitoring and prompt therapeutic intervention is essential for cancer patients receiving ICI mAbs, whether alone or in combination.
Table 2.
Immuno-related cardiovascular events with treatment strategies, adapted from ESMO guidelines [26]
| Cardiotoxicity | Clinical presentation | Biomarkers and Instrumental Diagnosis | ICI strategy | Immunosuppression | Cardiac treatment |
|---|---|---|---|---|---|
| Myocarditis |
Chest pain Dyspnea Pulmonary edema Cardiogenic shock |
Troponin NT-proBNP ECG Echocardiography CMR imaging (modified Lake Louis criteria) FAPI-PET-CT Endomyocardial biopsy (Multifocal inflammatory cell infiltrates with overt cardiomyocyte loss by light microscopy) Coronary angiography (exclude ACS) |
Stop ICI |
First-line: i.v. methylprednisolone 500–1000 mg daily for 3 days or until clinically stable, Follow with oral prednisolone 1 mg/kg o.d. with tapering schedule of 10 mg/week with troponin monitoring High-risk myocarditis: abatacept and ruxolitinib |
i.v. diuretics ± nitrates if pulmonary edema ACE inhibitors, Beta blockers |
| Pericarditis complicated by cardiac tamponade |
Chest pain Dyspnea Cardiogenic shock |
ECG Echocardiography CMR imaging (for concomitant myocarditis) |
Interrupt ICI Consider ICI re-administration when stable and no evidence of ongoing pericarditis |
Colchicine 500 μg b.i.d i.v. methylprednisolone 500–1000 mg daily until clinically stable, follow with oral prednisolone 1 mg/kg o.d. with tapering 10 mg/week |
Emergency pericardiocentesis Colchicine |
| Acute pericarditis (with or without effusion but without cardiac tamponade) |
Chest pain Dyspnea |
ECG Echocardiography CMR imaging (for concomitant myocarditis) |
Interrupt ICI Consider ICI re-administration when stable and no evidence of ongoing pericarditis |
Colchicine 500 μg b.i.d. and oral prednisolone 0.5 mg/kg o.d. with tapering 10 mg/week | |
| New advanced conduction disease (second- or third- degree heart block) |
Syncope Lipothymia |
ECG Holter ECG Serum electrolytes count |
Multidisciplinary approach for optimal management of immunological treatment | Consider i.v. methylprednisolone if progressive PR prolongation or any evidence of co-existing myocarditis e.g. elevated troponin, CMR evidence | Emergency pacing |
| Acute MI |
Chest pain Dyspnea Pulmonary edema Cardiogenic shock |
Troponin NT pro-BNP ECG Echocardiography CMR Coronary angiography |
Multidisciplinary approach for optimal management of immunological treatment | Consider i.v. methylprednisolone if evidence of coronary vasculitis on angiography |
Follow ESC/ACC/AHA guidelines for STEMI or NSTEMI Consider vasculitis if atherosclerosis is excluded by coronary angiography |
| New onset AF |
Palpitations Dyspnea Weakness Asymptomatic |
ECG Holter ECG Exclude myocarditis |
Multidisciplinary approach for optimal management of immunological treatment |
Follow ESC guideline for AF Anticoagulation unless contraindication or limited life expectancy |
|
| VT or VF |
Syncope Hypotension Palpitations Cardiac arrest |
ECG Holter ECG |
Multidisciplinary approach for optimal management of immunological treatment | First-line: i.v. methylprednisolone 500–1000 mg daily if myocarditis evident until clinically stable and troponin-negative followed by oral prednisolone 1 mg/kg o.d. with tapering 10 mg/week |
Emergency defibrillation Beta blockers and/or antiarrhythmics |
| Takotsubo syndrome |
Chest pain Dyspnea Pulmonary edema Cardiogenic shock |
Troponin NT pro-BNP ECG Echocardiography CMR ± biopsy Exclusion of ACS according to AHA and ESC guidelines |
Multidisciplinary approach for optimal management of immunological treatment | HFA position statement management algorithm | |
| New early conduction abnormality on ECG |
Asymptomatic Palpitations Weakness |
Troponin NT pro-BNP ECG Holter ECG |
Multidisciplinary approach for optimal management of immunological treatment | If high-grade heart block excluded, monitoring with ECG before each cycle | |
| New asymptomatic rise in BNP or NT-proBNP | Asymptomatic |
BNP or NT-pro-BNP Troponin ECG Echocardiogram CMR if suspected myocarditis |
Multidisciplinary approach for optimal management of immunological treatment |
Periodic monitoring Myocarditis treatment if diagnosed |
|
| New onset Left Ventricle Systolic Dysfunction (LVSD) |
Asymptomatic Dyspnea Pulmonary edema Cardiogenic shock |
BNP or NT-pro-BNP Troponin ECG Echocardiogram |
Multidisciplinary approach for optimal management of immunological treatment | AHA/ACC/ESC guidelines for heart failure |
Future perspective
Immunotherapy, especially involving ICIs and mAbs, is revolutionizing cancer treatment, expanding its use in managing various malignances. Recently, ICIs have been extended to adjuvant and neoadjuvant settings for early-stage disease, potentially aiding radical surgery and emphasizing the imperative of long-term quality of life and adverse event prevention. Despite fast-track approvals, the comprehensive understanding of ICIs’ short and long-term toxicity and impact on patient’ quality of life remains unclear [40]. Several approaches have been identified to mitigate the cardiotoxic effects of ICIs. Recent evidence supports the use of IL-6 inhibitors, such as tocilizumab, which have shown efficacy in reducing CRS severity without affecting CAR-T cell efficacy [42]. Ruxolitinib, a JAK1/2 inhibitor, is another promising agent in ICI-related CTR-CVT. By inhibiting the JAK-STAT pathway, ruxolitinib mitigates inflammation, thereby reducing the risk of cardiotoxicity. In a recent study of patients with ICI-related myocarditis, treatment with abatacept and ruxolitinib significantly reduced the incidence of MACE [70]. Co-therapies that combine ICIs with cardio-protective agents are another promising approach.
Some irAEs significantly impact the cardiovascular system, affecting patient outcomes, treatment discontinuation, and overall survival in immune-oncological therapies [23]. Approximately, one-third of new cancer cases annually warrant complex immune-oncological treatments, escalating the risk of irAEs. This knowledge acquires a dramatic meaning while considering that a third of the 1.8 million of the new cancer cases per year will have indication for complex immuno-oncological treatments with high risk of irAEs. This review provides detailed, updated evidence on current immunotherapy drugs, including cancer vaccines, highlighting how their side effects differ profoundly from standard chemotherapy in both etiopathogenesis and risk factors. Given these substantial differences, it is essential to conduct specific patient risk assessment based on immunotherapy treatments. In the future, the development of validated scores could help to assess the risk of cardiac complications related to immune-oncological treatments.
Conclusions
Given the high risk of cardiovascular complications with immuno-oncological treatments, their detrimental impact on patients’ lives, and the lack of recognized clinical or laboratory biomarkers able to predict the risk of IR-cardiovascular events, it is crucial to support the use of these new anticancer drugs with a structurally organized multidisciplinary team. The increased use of immunotherapy and immune checkpoint blockade as standard cancer treatments introduces new challenges in managing cardiovascular immune-related adverse events. This necessitates a new line of research and a tailored approach focused on preventing and treating these severe and potentially lethal complications.
Funding
Open access funding provided by Università degli studi "Magna Graecia" di Catanzaro within the CRUI-CARE Agreement.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Conflict of interest
The authors have nothing to declare.
Footnotes
Giuseppe Panuccio and Pierpaolo Correale have equally contributed to this work.
Contributor Information
Giuseppe Panuccio, Email: panuccio@unicz.it.
Daniele Torella, Email: dtorella@unicz.it.
References
- 1.Aggarwal V, Workman CJ, Vignali DAA (2023) LAG-3 as the third checkpoint inhibitor. Nat Immunol 24:1415–1422. 10.1038/s41590-023-01569-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvi RM, Frigault MJ, Fradley MG, Jain MD, Mahmood SS, Awadalla M, Lee DH, Zlotoff DA, Zhang L, Drobni ZD, Hassan MZO, Bassily E, Rhea I, Ismail-Khan R, Mulligan CP, Banerji D, Lazaryan A, Shah BD, Rokicki A, Raje N, Chavez JC, Abramson J, Locke FL, Neilan TG (2019) Cardiovascular events among adults treated with chimeric antigen receptor T-Cells (CAR-T). J Am Coll Cardiol 74:3099–3108. 10.1016/j.jacc.2019.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim Y-C, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro CJ, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M, PACIFIC Investigators (2017) Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377:1919–1929. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 4.Awadalla M, Mahmood SS, Groarke JD, Hassan MZO, Nohria A, Rokicki A, Murphy SP, Mercaldo ND, Zhang L, Zlotoff DA, Reynolds KL, Alvi RM, Banerji D, Liu S, Heinzerling LM, Jones-O’Connor M, Bakar RB, Cohen JV, Kirchberger MC, Sullivan RJ, Gupta D, Mulligan CP, Shah SP, Ganatra S, Rizvi MA, Sahni G, Tocchetti CG, Lawrence DP, Mahmoudi M, Devereux RB, Forrestal BJ, Mandawat A, Lyon AR, Chen CL, Barac A, Hung J, Thavendiranathan P, Picard MH, Thuny F, Ederhy S, Fradley MG, Neilan TG (2020) Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol 75:467–478. 10.1016/j.jacc.2019.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axelrod ML, Meijers WC, Screever EM, Qin J, Carroll MG, Sun X, Tannous E, Zhang Y, Sugiura A, Taylor BC, Hanna A, Zhang S, Amancherla K, Tai W, Wright JJ, Wei SC, Opalenik SR, Toren AL, Rathmell JC, Ferrell PB, Phillips EJ, Mallal S, Johnson DB, Allison JP, Moslehi JJ, Balko JM (2022) T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature 611:818–826. 10.1038/s41586-022-05432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, Özgüroğlu M, Szczesna A, Polychronis A, Uslu R, Krzakowski M, Lee J-S, Calabrò L, Arén Frontera O, Ellers-Lenz B, Bajars M, Ruisi M, Park K (2018) Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 19:1468–1479. 10.1016/S1470-2045(18)30673-9 [DOI] [PubMed] [Google Scholar]
- 7.Battisti NML, Andres MS, Lee KA, Ramalingam S, Nash T, Mappouridou S, Senthivel N, Asavisanu K, Obeid M, Tripodaki E-S, Angelis V, Fleming E, Goode EF, John S, Rosen SD, Allen M, Stanway S, Lyon AR, Ring A (2021) Incidence of cardiotoxicity and validation of the Heart Failure Association-International Cardio-Oncology Society risk stratification tool in patients treated with trastuzumab for HER2-positive early breast cancer. Breast Cancer Res Treat 188:149–163. 10.1007/s10549-021-06192-w [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu E, Spanjaart A, Roes A, Rachet B, Dalle S, Kersten MJ, Maucort-Boulch D, Jalali MS (2022) Health-related quality of life in cancer immunotherapy: a systematic perspective, using causal loop diagrams. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 31:2357–2366. 10.1007/s11136-022-03110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, Kato K, Sureda A, Greil R, Thieblemont C, Morschhauser F, Janz M, Flinn I, Rabitsch W, Kwong Y-L, Kersten MJ, Minnema MC, Holte H, Chan EHL, Martinez-Lopez J, Müller AMS, Maziarz RT, McGuirk JP, Bachy E, Le Gouill S, Dreyling M, Harigae H, Bond D, Andreadis C, McSweeney P, Kharfan-Dabaja M, Newsome S, Degtyarev E, Awasthi R, Del Corral C, Andreola G, Masood A, Schuster SJ, Jäger U, Borchmann P, Westin JR (2022) Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med 386:629–639. 10.1056/NEJMoa2116596 [DOI] [PubMed] [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, Gerber DE, Hamad L, Hansen E, Johnson DB, Lacouture ME, Masters GA, Naidoo J, Nanni M, Perales M-A, Puzanov I, Santomasso BD, Shanbhag SP, Sharma R, Skondra D, Sosman JA, Turner M, Ernstoff MS (2021) Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 9:e002435. 10.1136/jitc-2021-002435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buehning F, Lerchner T, Vogel J, Hendgen-Cotta UB, Totzeck M, Rassaf T, Michel L (2024) Preclinical models of cardiotoxicity from immune checkpoint inhibitor therapy. Basic Res Cardiol. 10.1007/s00395-024-01070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns EA, Gentille C, Trachtenberg B, Pingali SR, Anand K (2021) Cardiotoxicity associated with Anti-CD19 chimeric antigen receptor T-cell (CAR-T) therapy: recognition, risk factors, and management. Dis Basel Switz 9:20. 10.3390/diseases9010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cautela J, Zeriouh S, Gaubert M, Bonello L, Laine M, Peyrol M, Paganelli F, Lalevee N, Barlesi F, Thuny F (2020) Intensified immunosuppressive therapy in patients with immune checkpoint inhibitor-induced myocarditis. J Immunother Cancer 8:e001887. 10.1136/jitc-2020-001887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Jiang CC, Jin L, Zhang XD (2016) Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol Off J Eur Soc Med Oncol 27:409–416. 10.1093/annonc/mdv615 [DOI] [PubMed] [Google Scholar]
- 16.Correale P, Saladino RE, Giannarelli D, Sergi A, Mazzei MA, Bianco G, Giannicola R, Iuliano E, Forte IM, Calandruccio ND, Falzea AC, Strangio A, Nardone V, Pastina P, Tini P, Luce A, Caraglia M, Caracciolo D, Mutti L, Tassone P, Pirtoli L, Giordano A, Tagliaferri P (2020) HLA expression correlates to the risk of immune checkpoint inhibitor-induced pneumonitis. Cells 9:1964. 10.3390/cells9091964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Souza M, Nielsen D, Svane IM, Iversen K, Rasmussen PV, Madelaire C, Fosbøl E, Køber L, Gustafsson F, Andersson C, Gislason G, Torp-Pedersen C, Schou M (2021) The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J 42:1621–1631. 10.1093/eurheartj/ehaa884 [DOI] [PubMed] [Google Scholar]
- 18.Durham NM, Nirschl CJ, Jackson CM, Elias J, Kochel CM, Anders RA, Drake CG (2014) Lymphocyte activation gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo. PLoS ONE 9:e109080. 10.1371/journal.pone.0109080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettinghausen SE, Puri RK, Rosenberg SA (1988) Increased vascular permeability in organs mediated by the systemic administration of lymphokine-activated killer cells and recombinant interleukin-2 in mice. J Natl Cancer Inst 80:177–188. 10.1093/jnci/80.3.177 [DOI] [PubMed] [Google Scholar]
- 20.Fenioux C, Abbar B, Boussouar S, Bretagne M, Power JR, Moslehi JJ, Gougis P, Amelin D, Dechartres A, Lehmann LH, Courand P-Y, Cautela J, Alexandre J, Procureur A, Rozes A, Leonard-Louis S, Qin J, International ICI-Myocarditis Registry, Caheynier R, Charmeteau-De Muylder B, Redheuil A, Tubach F, Cadranel J, Milon A, Ederhy S, Similowski T, Johnson DB, Pizzo I, Catalan T, Benveniste O, Hayek SS, Allenbach Y, Rosenzwajg M, Dolladille C, Salem J-E (2023) Thymus alterations and susceptibility to immune checkpoint inhibitor myocarditis. Nat Med 29:3100–3110. 10.1038/s41591-023-02591-2 [DOI] [PubMed] [Google Scholar]
- 21.Finke D, Heckmann MB, Herpel E, Katus HA, Haberkorn U, Leuschner F, Lehmann LH (2021) Early detection of checkpoint inhibitor-associated myocarditis using 68Ga-FAPI PET/CT. Front Cardiovasc Med 8:614997. 10.3389/fcvm.2021.614997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganatra S, Redd R, Hayek SS, Parikh R, Azam T, Yanik GA, Spendley L, Nikiforow S, Jacobson C, Nohria A (2020) Chimeric antigen receptor T-cell therapy-associated cardiomyopathy in patients with refractory or relapsed non-Hodgkin lymphoma. Circulation 142:1687–1690. 10.1161/CIRCULATIONAHA.120.048100 [DOI] [PubMed] [Google Scholar]
- 23.Gergely TG, Drobni ZD, Sayour NV, Ferdinandy P, Varga ZV (2024) Molecular fingerprints of cardiovascular toxicities of immune checkpoint inhibitors. Basic Res Cardiol. 10.1007/s00395-024-01068-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goitein O, Matetzky S, Beinart R, Di Segni E, Hod H, Bentancur A, Konen E (2009) Acute myocarditis: noninvasive evaluation with cardiac MRI and transthoracic echocardiography. AJR Am J Roentgenol 192:254–258. 10.2214/AJR.08.1281 [DOI] [PubMed] [Google Scholar]
- 25.Gulley JL, Madan RA, Tsang KY, Jochems C, Marté JL, Farsaci B, Tucker JA, Hodge JW, Liewehr DJ, Steinberg SM, Heery CR, Schlom J (2014) Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res 2:133–141. 10.1158/2326-6066.CIR-13-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, Lyon AR, Wick W, Kostine M, Peters S, Jordan K, Larkin J, ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org (2022) Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol 33:1217–1238. 10.1016/j.annonc.2022.10.001 [DOI] [PubMed] [Google Scholar]
- 27.Herrmann J (2020) Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol 17:474–502. 10.1038/s41569-020-0348-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, Carver J, Dent S, Ky B, Lyon AR, López-Fernández T, Fradley MG, Ganatra S, Curigliano G, Mitchell JD, Minotti G, Lang NN, Liu JE, Neilan TG, Nohria A, O’Quinn R, Pusic I, Porter C, Reynolds KL, Ruddy KJ, Thavendiranathan P, Valent P (2022) Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J 43:280–299. 10.1093/eurheartj/ehab674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heusch G (2023) Cardioprotection in cardio-oncology: a case for concern? Cardiovasc Res 119:e144–e145. 10.1093/cvr/cvad111 [DOI] [PubMed] [Google Scholar]
- 30.Higano CS, Armstrong AJ, Sartor AO, Vogelzang NJ, Kantoff PW, McLeod DG, Pieczonka CM, Penson DF, Shore ND, Vacirca J, Concepcion RS, Tutrone RF, Nordquist LT, Quinn DI, Kassabian V, Scholz MC, Harmon M, Tyler RC, Chang NN, Tang H, Cooperberg MR (2019) Real-world outcomes of sipuleucel-T treatment in PROCEED, a prospective registry of men with metastatic castration-resistant prostate cancer. Cancer 125:4172–4180. 10.1002/cncr.32445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJM, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu J, Tian R, Ma Y, Zhen H, Ma X, Su Q, Cao B (2021) Risk of cardiac adverse events in patients treated with immune checkpoint inhibitor regimens: a systematic review and meta-analysis. Front Oncol 11:645245. 10.3389/fonc.2021.645245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu J-R, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, Lyon AR, Padera RF, Johnson DB, Moslehi J (2019) Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res 115:854–868. 10.1093/cvr/cvz026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Igarashi Y, Sasada T (2020) Cancer vaccines: toward the next breakthrough in cancer immunotherapy. J Immunol Res 2020:5825401. 10.1155/2020/5825401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jogalekar MP, Rajendran RL, Khan F, Dmello C, Gangadaran P, Ahn B-C (2022) CAR T-cell-based gene therapy for cancers: new perspectives, challenges, and clinical developments. Front Immunol 13:925985. 10.3389/fimmu.2022.925985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA, Anders RA, Sosman JA, Moslehi JJ (2016) Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375:1749–1755. 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.June CH, Sadelain M (2018) Chimeric antigen receptor therapy. N Engl J Med 379:64–73. 10.1056/NEJMra1706169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, Ibrahimi S, Mielke S, Mutsaers P, Hernandez-Ilizaliturri F, Izutsu K, Morschhauser F, Lunning M, Maloney DG, Crotta A, Montheard S, Previtali A, Stepan L, Ogasawara K, Mack T, Abramson JS, Investigators TRANSFORM (2022) Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet Lond Engl 399:2294–2308. 10.1016/S0140-6736(22)00662-6 [DOI] [PubMed] [Google Scholar]
- 39.Korell F, Entenmann L, Romann S, Giannitsis E, Schmitt A, Müller-Tidow C, Frey N, Dreger P, Schmitt M, Lehmann LH (2024) Evaluation of all-cause mortality and cardiovascular safety in patients receiving chimeric antigen receptor T cell therapy: a prospective cohort study. EClinicalMedicine 69:102504. 10.1016/j.eclinm.2024.102504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraehenbuehl L, Weng C-H, Eghbali S, Wolchok JD, Merghoub T (2022) Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol 19:37–50. 10.1038/s41571-021-00552-7 [DOI] [PubMed] [Google Scholar]
- 41.Kuhnly NM, Coviello J (2022) Immune checkpoint inhibitor-related myocarditis: recognition, surveillance, and management. Clin J Oncol Nurs 26:54–60. 10.1188/22.CJON.54-60 [DOI] [PubMed] [Google Scholar]
- 42.Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, Przepiorka D, Farrell AT, Pazdur R (2018) FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 23:943–947. 10.1634/theoncologist.2018-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL (2014) Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188–195. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, Go WY, Eldjerou L, Gardner RA, Frey N, Curran KJ, Peggs K, Pasquini M, DiPersio JF, van den Brink MRM, Komanduri KV, Grupp SA, Neelapu SS (2019) ASTCT Consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 25:625–638. 10.1016/j.bbmt.2018.12.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefebvre B, Kang Y, Smith AM, Frey NV, Carver JR, Scherrer-Crosbie M (2020) Cardiovascular effects of CAR T cell therapy: a retrospective study. JACC CardioOncology 2:193–203. 10.1016/j.jaccao.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li B, Chan HL, Chen P (2019) Immune checkpoint inhibitors: basics and challenges. Curr Med Chem 26:3009–3025. 10.2174/0929867324666170804143706 [DOI] [PubMed] [Google Scholar]
- 47.Lin Y-K, Hsiao L-C, Wu M-Y, Chen Y-F, Lin Y-N, Chang C-M, Chung W-H, Chen K-W, Lu C-R, Chen W-Y, Chang S-S, Shyu W-C, Lee A-S, Chen C-H, Jeng L-B, Chang K-C (2023) PD-L1 and AKT overexpressing adipose-derived mesenchymal stem cells enhance myocardial protection by upregulating CD25+ T cells in acute myocardial infarction rat model. Int J Mol Sci 25:134. 10.3390/ijms25010134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, Tocchetti CG, Moslehi JJ, Groarke JD, Bergler-Klein J, Khoo V, Tan LL, Anker MS, von Haehling S, Maack C, Pudil R, Barac A, Thavendiranathan P, Ky B, Neilan TG, Belenkov Y, Rosen SD, Iakobishvili Z, Sverdlov AL, Hajjar LA, Macedo AVS, Manisty C, Ciardiello F, Farmakis D, de Boer RA, Skouri H, Suter TM, Cardinale D, Witteles RM, Fradley MG, Herrmann J, Cornell RF, Wechelaker A, Mauro MJ, Milojkovic D, de Lavallade H, Ruschitzka F, Coats AJS, Seferovic PM, Chioncel O, Thum T, Bauersachs J, Andres MS, Wright DJ, López-Fernández T, Plummer C, Lenihan D (2020) Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail 22:1945–1960. 10.1002/ejhf.1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH, ESC Scientific Document Group (2022) 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 43:4229–4361. 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 50.Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J (2018) Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol 19:e447–e458. 10.1016/S1470-2045(18)30457-1 [DOI] [PubMed] [Google Scholar]
- 51.Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG (2018) Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 71:1755–1764. 10.1016/j.jacc.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mellman I, Coukos G, Dranoff G (2011) Cancer immunotherapy comes of age. Nature 480:480–489. 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michel L, Helfrich I, Hendgen-Cotta UB, Mincu R-I, Korste S, Mrotzek SM, Spomer A, Odersky A, Rischpler C, Herrmann K, Umutlu L, Coman C, Ahrends R, Sickmann A, Löffek S, Livingstone E, Ugurel S, Zimmer L, Gunzer M, Schadendorf D, Totzeck M, Rassaf T (2022) Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur Heart J 43:316–329. 10.1093/eurheartj/ehab430 [DOI] [PubMed] [Google Scholar]
- 54.Mirabel M, Eslami A, Thibault C, Oudard S, Mousseaux E, Wahbi K, Fabre E, Terrier B, Marijon E, Villefaillot A, Fayol A, Dragon-Durey M-A, Le Louet AL, Bruno RM, Soulat G, Hulot JS (2024) Adverse myocardial and vascular side effects of immune checkpoint inhibitors: a prospective multimodal cardiovascular assessment. Clin Res Cardiol Off J Ger Card Soc. 10.1007/s00392-024-02462-x [DOI] [PubMed] [Google Scholar]
- 55.Naidoo J, Page DB, Wolchok JD (2014) Immune checkpoint blockade. Hematol Oncol Clin North Am 28:585–600. 10.1016/j.hoc.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 56.Nso N, Antwi-Amoabeng D, Beutler BD, Ulanja MB, Ghuman J, Hanfy A, Nimo-Boampong J, Atanga S, Doshi R, Enoru S, Gullapalli N (2020) Cardiac adverse events of immune checkpoint inhibitors in oncology patients: a systematic review and meta-analysis. World J Cardiol 12:584–598. 10.4330/wjc.v12.i11.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okazaki T, Okazaki I, Wang J, Sugiura D, Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T, Hioki K, Honjo T (2011) PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med 208:395–407. 10.1084/jem.20100466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panuccio G, Neri G, Macrì LM, Salerno N, De Rosa S, Torella D (2022) Use of Impella device in cardiogenic shock and its clinical outcomes: a systematic review and meta-analysis. Int J Cardiol Heart Vasc 40:101007. 10.1016/j.ijcha.2022.101007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pardoll D (2015) Cancer and the immune system: basic concepts and targets for intervention. Semin Oncol 42:523–538. 10.1053/j.seminoncol.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park J-E, Kim S-E, Keam B, Park H-R, Kim S, Kim M, Kim TM, Doh J, Kim D-W, Heo DS (2020) Anti-tumor effects of NK cells and anti-PD-L1 antibody with antibody-dependent cellular cytotoxicity in PD-L1-positive cancer cell lines. J Immunother Cancer 8:e000873. 10.1136/jitc-2020-000873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pathak R, Katel A, Massarelli E, Villaflor VM, Sun V, Salgia R (2021) Immune checkpoint inhibitor-induced myocarditis with myositis/myasthenia gravis overlap syndrome: a systematic review of cases. Oncologist 26:1052–1061. 10.1002/onco.13931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Porter DL, Levine BL, Kalos M, Bagg A, June CH (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365:725–733. 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006–2017. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Power JR, Alexandre J, Choudhary A, Ozbay B, Hayek SS, Asnani A, Tamura Y, Aras M, Cautela J, Thuny F, Gilstrap L, Arangalage D, Ewer S, Huang S, Deswal A, Palaskas NL, Finke D, Lehmann LH, Ederhy S, Moslehi J, Salem J-E (2022) Association of early electrical changes with cardiovascular outcomes in immune checkpoint inhibitor myocarditis. Arch Cardiovasc Dis 115:315–330. 10.1016/j.acvd.2022.03.003 [DOI] [PubMed] [Google Scholar]
- 65.Qu D, Liu J, Lau CW, Huang Y (2014) IL-6 in diabetes and cardiovascular complications. Br J Pharmacol 171:3595–3603. 10.1111/bph.12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, KEYNOTE-006 investigators (2015) Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med 372:2521–2532. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 67.Roth ME, Muluneh B, Jensen BC, Madamanchi C, Lee CB (2016) Left ventricular dysfunction after treatment with ipilimumab for metastatic melanoma. Am J Ther 23:e1925–e1928. 10.1097/MJT.0000000000000430 [DOI] [PubMed] [Google Scholar]
- 68.Rowshanravan B, Halliday N, Sansom DM (2018) CTLA-4: a moving target in immunotherapy. Blood 131:58–67. 10.1182/blood-2017-06-741033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahin U, Türeci Ö (2018) Personalized vaccines for cancer immunotherapy. Science 359:1355–1360. 10.1126/science.aar7112 [DOI] [PubMed] [Google Scholar]
- 70.Salem J-E, Bretagne M, Abbar B, Leonard-Louis S, Ederhy S, Redheuil A, Boussouar S, Nguyen LS, Procureur A, Stein F, Fenioux C, Devos P, Gougis P, Dres M, Demoule A, Psimaras D, Lenglet T, Maisonobe T, De Chambrun MP, Hekimian G, Straus C, Gonzalez-Bermejo J, Klatzmann D, Rigolet A, Guillaume-Jugnot P, Champtiaux N, Benveniste O, Weiss N, Saheb S, Rouvier P, Plu I, Gandjbakhch E, Kerneis M, Hammoudi N, Zahr N, Llontop C, Morelot-Panzini C, Lehmann L, Qin J, Moslehi JJ, Rosenzwajg M, Similowski T, Allenbach Y (2023) Abatacept/ruxolitinib and screening for concomitant respiratory muscle failure to mitigate fatality of immune-checkpoint inhibitor myocarditis. Cancer Discov 13:1100–1115. 10.1158/2159-8290.CD-22-1180 [DOI] [PubMed] [Google Scholar]
- 71.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M (2012) Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 5:596–603. 10.1161/CIRCIMAGING.112.973321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saxena M, van der Burg SH, Melief CJM, Bhardwaj N (2021) Therapeutic cancer vaccines. Nat Rev Cancer 21:360–378. 10.1038/s41568-021-00346-0 [DOI] [PubMed] [Google Scholar]
- 73.Sh Ahmed O, Mahadevia H, Manochakian R, Zhao Y, Salinas M, Khoor A, LeGout J, Lou Y (2022) A case of full recovery from prolonged cardiac arrest after infusion with paclitaxel and pembrolizumab. Case Rep Oncol 15:1063–1073. 10.1159/000527205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shalabi H, Sachdev V, Kulshreshtha A, Cohen JW, Yates B, Rosing DR, Sidenko S, Delbrook C, Mackall C, Wiley B, Lee DW, Shah NN (2020) Impact of cytokine release syndrome on cardiac function following CD19 CAR-T cell therapy in children and young adults with hematological malignancies. J Immunother Cancer 8:e001159. 10.1136/jitc-2020-001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M, IMpower150 Study Group (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288–2301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 76.Sterner RC, Sterner RM (2021) CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 11:69. 10.1038/s41408-021-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taneja V, Behrens M, Cooper LT, Yamada S, Kita H, Redfield MM, Terzic A, David C (2007) Spontaneous myocarditis mimicking human disease occurs in the presence of an appropriate MHC and non-MHC background in transgenic mice. J Mol Cell Cardiol 42:1054–1064. 10.1016/j.yjmcc.2007.03.898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taneja V, David CS (2009) Spontaneous autoimmune myocarditis and cardiomyopathy in HLA-DQ8.NODAbo transgenic mice. J Autoim 33:260–269. 10.1016/j.jaut.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, Wu W, Han L, Wang S (2022) The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol 13:964442. 10.3389/fimmu.2022.964442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, Rutkowski P, Gogas HJ, Lao CD, De Menezes JJ, Dalle S, Arance A, Grob J-J, Srivastava S, Abaskharoun M, Hamilton M, Keidel S, Simonsen KL, Sobiesk AM, Li B, Hodi FS, Long GV, RELATIVITY-047 Investigators (2022) Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 386:24–34. 10.1056/NEJMoa2109970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomas S, Prendergast GC (2016) Cancer vaccines: a brief overview. Methods Mol Biol Clifton NJ 1403:755–761. 10.1007/978-1-4939-3387-7_43 [DOI] [PubMed] [Google Scholar]
- 82.Tocchetti CG, Galdiero MR, Varricchi G (2018) Cardiac toxicity in patients treated with immune checkpoint inhibitors: it is now time for cardio-immuno-oncology. J Am Coll Cardiol 71:1765–1767. 10.1016/j.jacc.2018.02.038 [DOI] [PubMed] [Google Scholar]
- 83.Totzeck M, Michel L, Lin Y, Herrmann J, Rassaf T (2022) Cardiotoxicity from chimeric antigen receptor-T cell therapy for advanced malignancies. Eur Heart J 43:1928–1940. 10.1093/eurheartj/ehac106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T (2019) Cardio-oncology—strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol 280:163–175. 10.1016/j.ijcard.2019.01.038 [DOI] [PubMed] [Google Scholar]
- 85.Varricchi G, Galdiero MR, Marone G, Criscuolo G, Triassi M, Bonaduce D, Marone G, Tocchetti CG (2017) Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2:e000247. 10.1136/esmoopen-2017-000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vdovenko D, Eriksson U (2018) Regulatory role of CD4+ T cells in myocarditis. J Immunol Res 2018:4396351. 10.1155/2018/4396351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, Bergmann T, Bockmeyer CL, Eigentler T, Fluck M, Garbe C, Gutzmer R, Grabbe S, Hauschild A, Hein R, Hundorfean G, Justich A, Keller U, Klein C, Mateus C, Mohr P, Paetzold S, Satzger I, Schadendorf D, Schlaeppi M, Schuler G, Schuler-Thurner B, Trefzer U, Ulrich J, Vaubel J, von Moos R, Weder P, Wilhelm T, Göppner D, Dummer R, Heinzerling LM (2013) The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS ONE 8:e53745. 10.1371/journal.pone.0053745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB (2018) Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 4:1721–1728. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang F, Yang L, Xiao M, Zhang Z, Shen J, Anuchapreeda S, Tima S, Chiampanichayakul S, Xiao Z (2022) PD-L1 regulates cell proliferation and apoptosis in acute myeloid leukemia by activating PI3K-AKT signaling pathway. Sci Rep 12:11444. 10.1038/s41598-022-15020-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Westin JR, Oluwole OO, Kersten MJ, Miklos DB, Perales M-A, Ghobadi A, Rapoport AP, Sureda A, Jacobson CA, Farooq U, van Meerten T, Ulrickson M, Elsawy M, Leslie LA, Chaganti S, Dickinson M, Dorritie K, Reagan PM, McGuirk J, Song KW, Riedell PA, Minnema MC, Yang Y, Vardhanabhuti S, Filosto S, Cheng P, Shahani SA, Schupp M, To C, Locke FL, ZUMA-7 Investigators, Kite Members (2023) Survival with axicabtagene ciloleucel in large B-cell lymphoma. N Engl J Med 389:148–157. 10.1056/NEJMoa2301665 [DOI] [PubMed] [Google Scholar]
- 91.Xu C, Chen Y-P, Du X-J, Liu J-Q, Huang C-L, Chen L, Zhou G-Q, Li W-F, Mao Y-P, Hsu C, Liu Q, Lin A-H, Tang L-L, Sun Y, Ma J (2018) Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ 363:k4226. 10.1136/bmj.k4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yun S, Vincelette ND, Mansour I, Hariri D, Motamed S (2015) Late onset ipilimumab-induced pericarditis and pericardial effusion: a rare but life threatening complication. Case Rep Oncol Med 2015:794842. 10.1155/2015/794842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhan X, Wang B, Wang Y, Chen L, Peng X, Li J, Wu M, Zhang L, Tang S (2020) Phase I trial of personalized mRNA vaccine encoding neoantigen in patients with advanced digestive system neoplasms. J Clin Oncol 38:e15269–e15269. 10.1200/JCO.2020.38.15_suppl.e15269 [Google Scholar]
- 94.Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, Zlotoff DA, Murphy SP, Stone JR, Golden DLA, Alvi RM, Rokicki A, Jones-O’Connor M, Cohen JV, Heinzerling LM, Mulligan C, Armanious M, Barac A, Forrestal BJ, Sullivan RJ, Kwong RY, Yang EH, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Moslehi JJ, Coelho-Filho OR, Ganatra S, Rizvi MA, Sahni G, Tocchetti CG, Mercurio V, Mahmoudi M, Lawrence DP, Reynolds KL, Weinsaft JW, Baksi AJ, Ederhy S, Groarke JD, Lyon AR, Fradley MG, Thavendiranathan P, Neilan TG (2020) Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J 41:1733–1743. 10.1093/eurheartj/ehaa051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, Zhu L, Zhang H, Chen S, Xiao Y (2022) CAR-T cell therapy in hematological malignancies: current opportunities and challenges. Front Immunol 13:927153. 10.3389/fimmu.2022.927153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y, Zheng J (2020) Functions of immune checkpoint molecules beyond immune evasion. Adv Exp Med Biol 1248:201–226. 10.1007/978-981-15-3266-5_9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.