Abstract
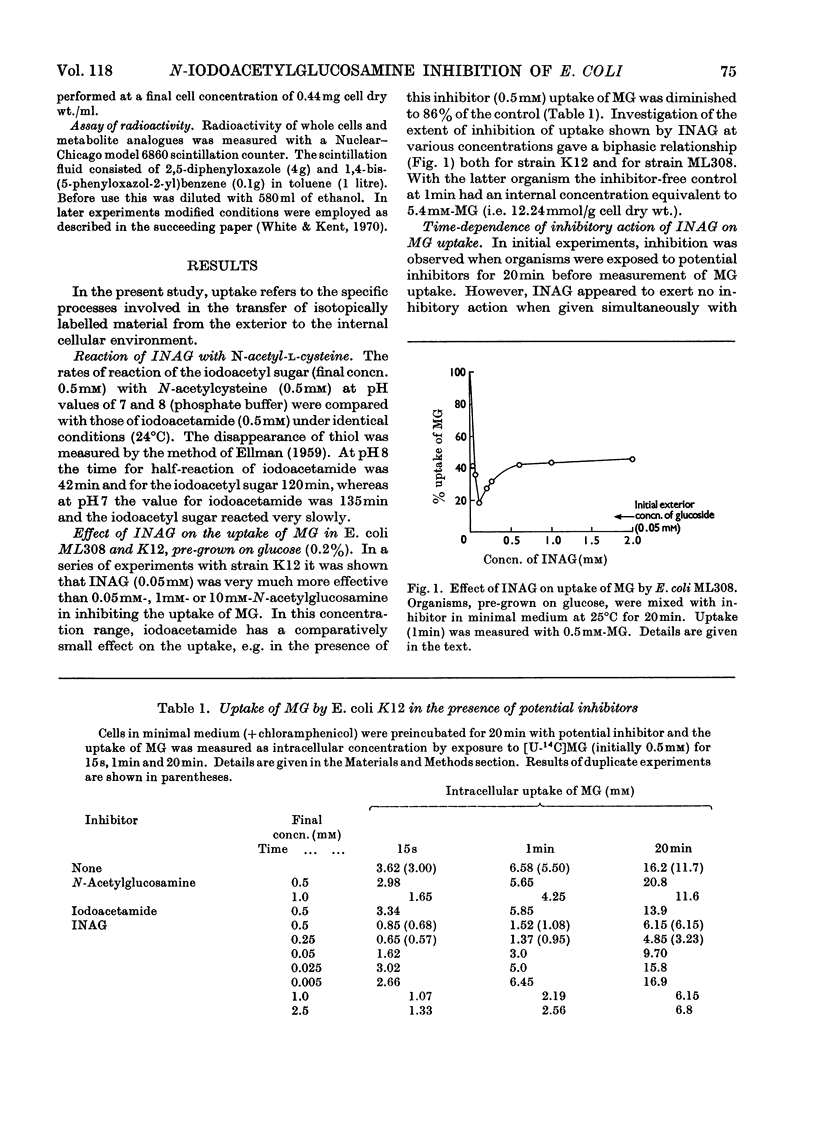
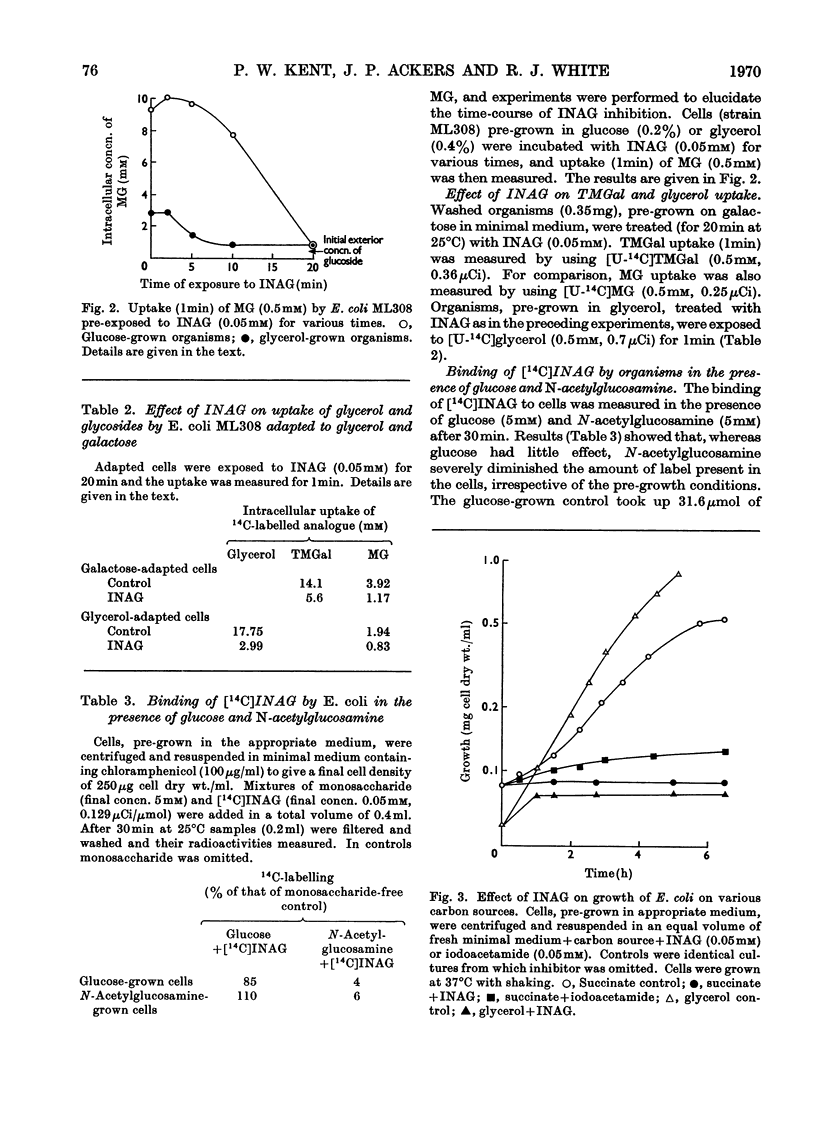
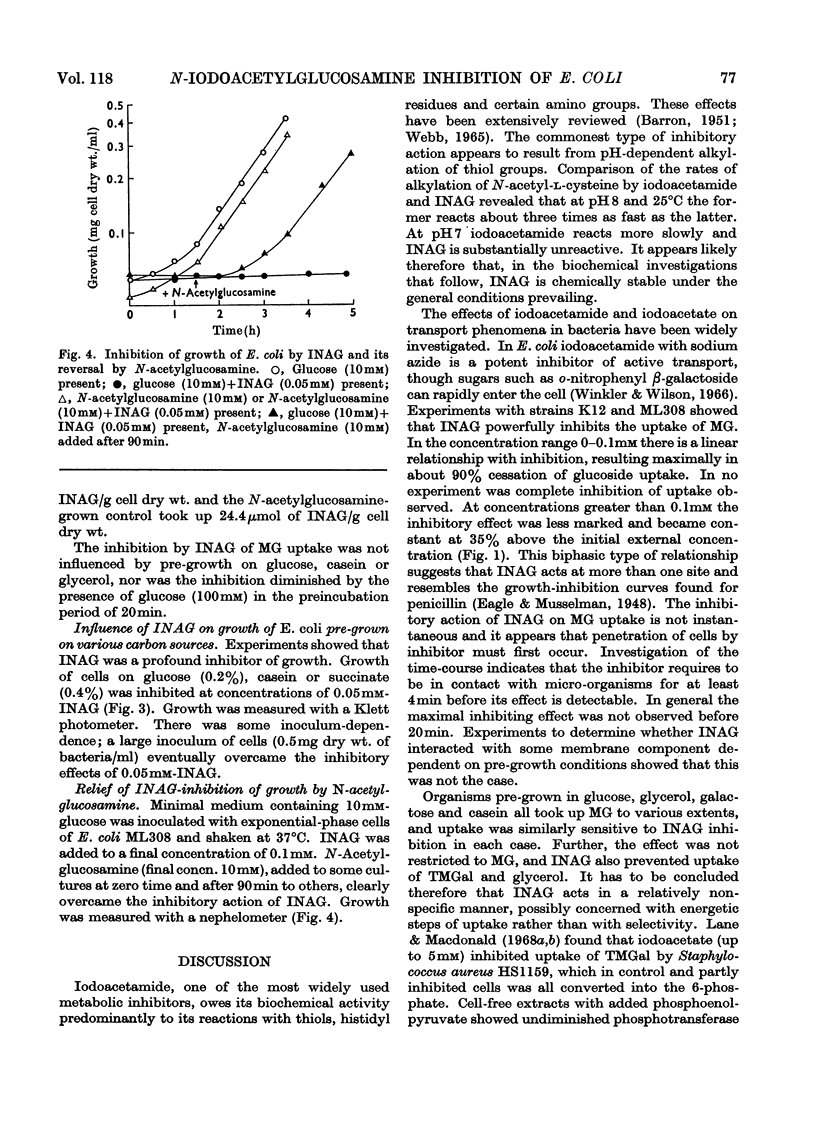
1. Synthesis of N-iodoacetyl-d-glucosamine and its N-iodo[1,2-14C2]acetyl form has been achieved from the tetra-O-acetyl amino sugar and iodoacetic acid in the presence of dicyclohexylcarbodi-imide followed by catalytic deacetylation. 2. N-Iodoacetylglucosamine (up to 0.1mm) linearly inhibits uptake (up to 1min) of methyl α-d-glucoside by Escherichia coli ML308 and K12. Uptake of methyl β-d-thiogalactoside and glycerol is also inhibited. 3. Growth of the organism (strain ML308) on glucose, succinate and glycerol is strongly inhibited by the iodoacetyl compound. The inhibition is relieved by N-acetylglucosamine. 4. The inhibitor has multiple effects, some of which are considered to be intracellular. 5. A separate transport pathway exists for N-acetylglucosamine by means of which the iodoacetyl analogue may enter the cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter J. R., Fox C. F., Kennedy E. P. Interaction of sugars with the membrane protein component of the lactose transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Jun;60(2):725–732. doi: 10.1073/pnas.60.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN O. M., RUTENBURG A. M. Possible usefulness of substituted amino acids for tumor growth inhibition. Proc Soc Exp Biol Med. 1950 Aug;74(4):764–766. doi: 10.3181/00379727-74-18040. [DOI] [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E., Needham D. M., Dewan J. G. Dismutations and oxidoreductions. Biochem J. 1937 Dec;31(12):2327–2352. doi: 10.1042/bj0312327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Scarborough G. A. Mechanism of hydrolysis of O-nitrophenyl-beta-galactoside in Staphylococcus aureus and its significance for theories of sugar transport. Proc Natl Acad Sci U S A. 1967 Jul;58(1):225–228. doi: 10.1073/pnas.58.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent P. W., Allen A. The biosynthesis of intestinal mucins. The effect of salicylate on glycoprotein biosynthesis by sheep colonic and human gastric mucosal tissues in vitro. Biochem J. 1968 Feb;106(3):645–658. doi: 10.1042/bj1060645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber A. R., Stein W. D. Identification of a component of a transport 'carrier' system: isolation of the permease expression of the LAC operon of Escherichia coli. Nature. 1966 Feb 12;209(5024):691–694. doi: 10.1038/209691a0. [DOI] [PubMed] [Google Scholar]
- Kolber A. R., Stein W. D. The isolation of a membrane-bound protein associated with the beta-galactoside-permease of Escherichia coli. Curr Mod Biol. 1967 Nov;1(4):244–248. doi: 10.1016/0303-2647(67)90002-0. [DOI] [PubMed] [Google Scholar]
- Laue P., MacDonald R. E. Identification of thiomethyl-beta-D-galactoside 6-phosphate accumulated by Staphylococcus aureus. J Biol Chem. 1968 Feb 10;243(3):680–682. [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkine L. Sulphydryl groups and enzymic oxido-reduction. Biochem J. 1938 Oct;32(10):1729–1739. doi: 10.1042/bj0321729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Levinthal M., Kundig F. D., Kundig W., Anderson B., Hartman P. E., Roseman S. Genetic evidence for the role of a bacterial phosphotransferase system in sugar transport. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1963–1970. doi: 10.1073/pnas.58.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Smith M. F., Roseman S. Resolution of a staphylococcal phosphotransferase system into four protein components and its relation to sugar transport. Biochem Biophys Res Commun. 1968 Jun 10;31(5):804–811. doi: 10.1016/0006-291x(68)90634-7. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Lin E. C. Two classes of pleiotropic mutants of Aerobacter aerogenes lacking components of a phosphoenolpyruvate-dependent phosphotransferase system. Proc Natl Acad Sci U S A. 1967 Apr;57(4):913–919. doi: 10.1073/pnas.57.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenfels K., Eisele B. Stereospecific alkylation with asymmetric reagents. Eur J Biochem. 1968 Jan;3(3):267–275. doi: 10.1111/j.1432-1033.1968.tb19526.x. [DOI] [PubMed] [Google Scholar]
- White R. J. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem J. 1968 Feb;106(4):847–858. doi: 10.1042/bj1060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Kent P. W. An examination of the inhibitory effects of N-iodoacetylglucosamine on Escherichia coli and isolation of resistant mutants. Biochem J. 1970 Jun;118(1):81–87. doi: 10.1042/bj1180081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Pasternak C. A. The purification and properties of N-acetylglucosamine 6-phosphate deacetylase from Escherichia coli. Biochem J. 1967 Oct;105(1):121–125. doi: 10.1042/bj1050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]


