Abstract
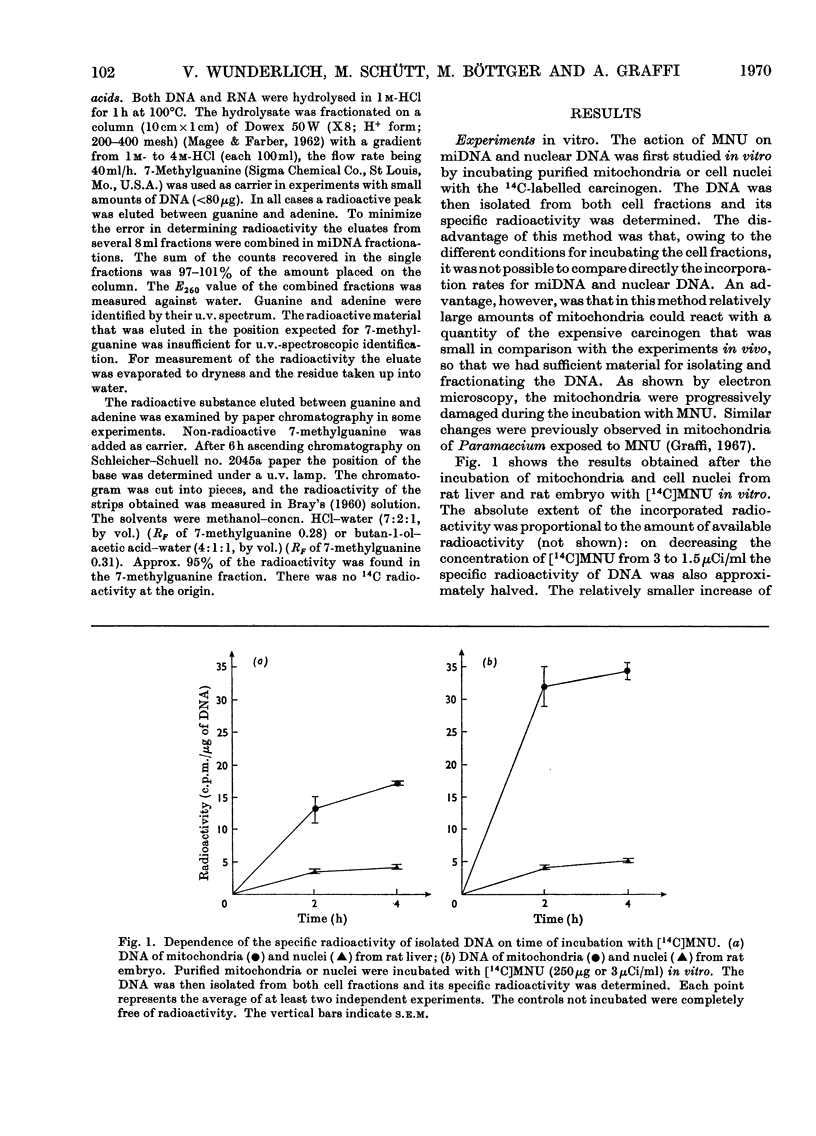
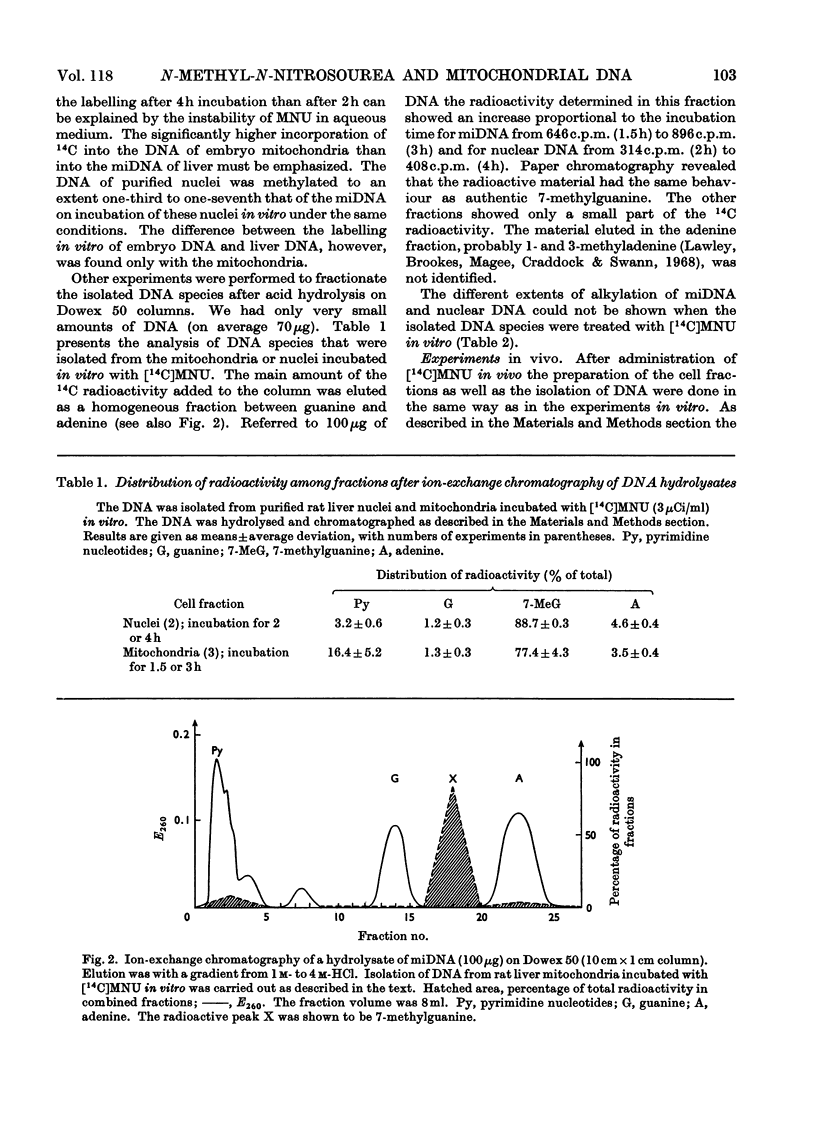
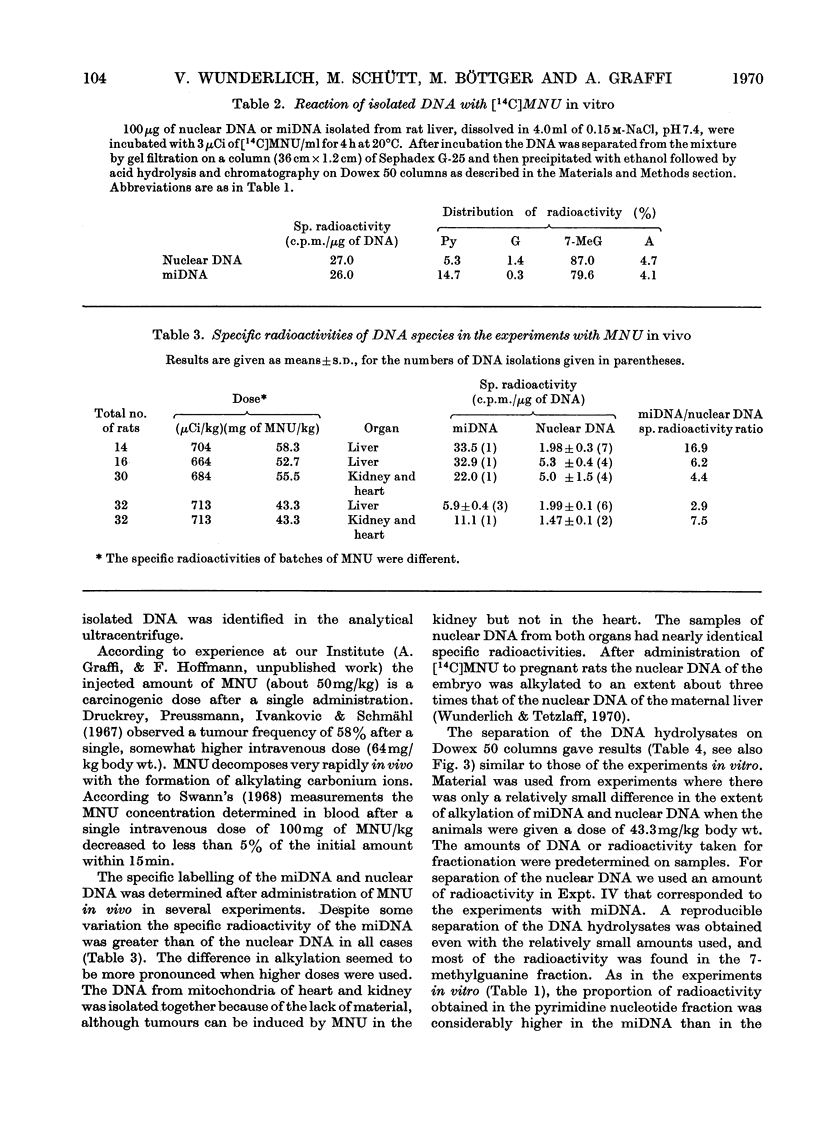
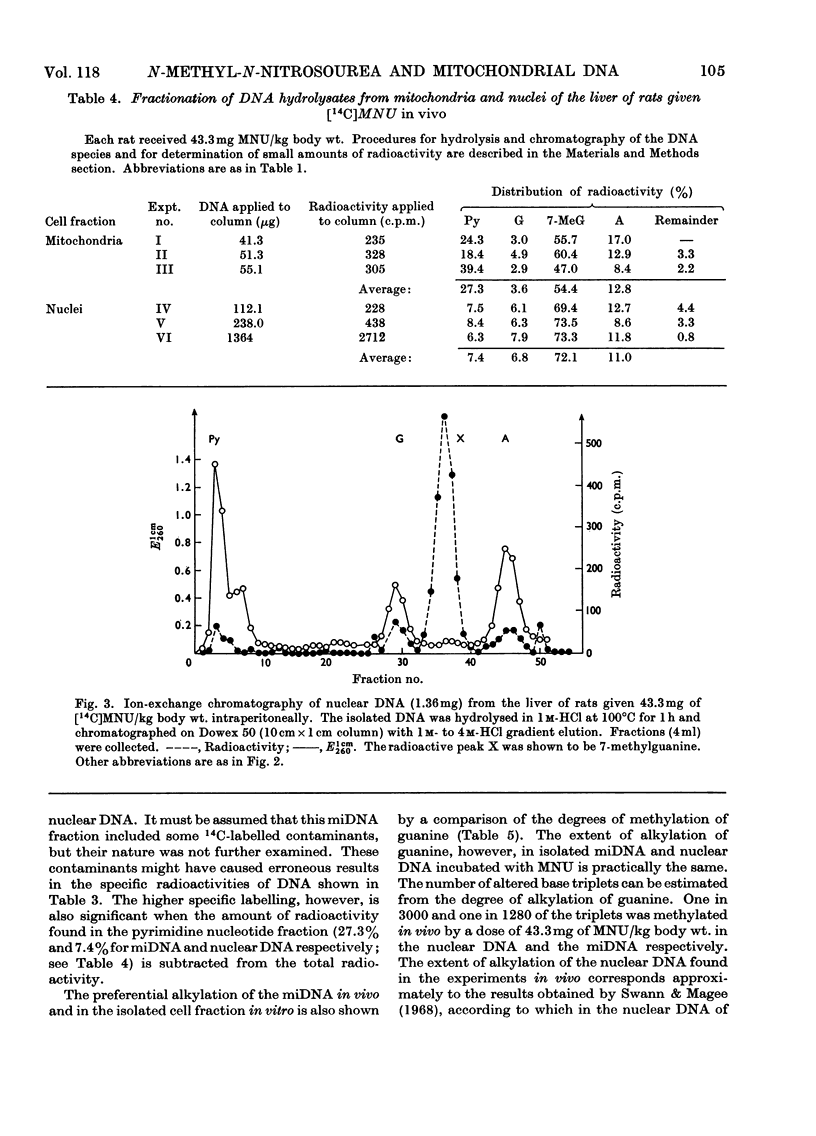
The reaction of the carcinogen N-methyl-N-nitrosourea with mitochondrial DNA from various rat tissues was examined in vivo and in vitro. After incubation of isolated mitochondria or cell nuclei with N[14C]-methyl-N-nitrosourea in vitro and subsequent isolation and purification of the DNA the specific radioactivity of the mitochondrial DNA was 3–7 times that of the nuclear DNA. The incorporation of 14C into embryonic mitochondrial DNA in vitro was about twice that into the liver mitochondrial DNA. Identical incorporation rates, however, were found for the reaction of isolated mitochondrial DNA or nuclear DNA respectively with N[14C]-methyl-N-nitrosourea. After intraperitoneal injection of 43.3–58.5mg of N[14C]-methyl-N-nitrosourea/kg body wt. to adult rats the labelling of the mitochondrial DNA was on average 5 times that of the nuclear DNA. A smaller specific labelling was observed for the ribosomal RNA, transfer RNA, and mitochondrial RNA as well as for the mitochondrial protein as compared with the mitochondrial DNA. After hydrolysis of the alkylated nucleic acids with hydrochloric acid, fractionation was carried out on Dowex 50 cation-exchange columns. In most experiments 70–80% of the input 14C radioactivity was eluted in the 7-methylguanine fraction. The preferential alkylation of the mitochondrial DNA by N-methyl-N-nitrosourea in situ is discussed in connexion with the cytoplasmic-mutation hypothesis of carcinogenesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Carnevali F., Nicolaieff A., Piperno G., Tecce G. Separation and characterization of a satellite DNA from a yeast cytoplasmic "petite" mutant. J Mol Biol. 1968 Nov 14;37(3):493–505. doi: 10.1016/0022-2836(68)90117-4. [DOI] [PubMed] [Google Scholar]
- Brookes P. Quantitative aspects of the reaction of some carcinogens with nucleic acids and the possible significance of such reactions in the process of carcinogenesis. Cancer Res. 1966 Sep;26(9):1994–2003. [PubMed] [Google Scholar]
- Böttger M., Wunderlich V., Schütt M., Förster W., Graffi A. Uber das Sedimentationsverhalten mitochondrialer DNS in stabilisierenden D2O-Gradienten. Acta Biol Med Ger. 1968;21(5):587–593. [PubMed] [Google Scholar]
- Clayton D. A., Vinograd J. Circular dimer and catenate forms of mitochondrial DNA in human leukaemic leucocytes. Nature. 1967 Nov 18;216(5116):652–657. doi: 10.1038/216652a0. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Vinograd J. Complex mitochondrial DNA in leukemic and normal human myeloid cells. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1077–1084. doi: 10.1073/pnas.62.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacumakos E. G., Garnjobst L., Tatum E. L. A cytoplasmic character in Neurospora crassa. The role of nuclei and mitochondria. J Cell Biol. 1965 Aug;26(2):427–443. doi: 10.1083/jcb.26.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckrey H., Preussmann R., Ivankovic S., Schmähl D. Organotrope carcinogene Wirkungen bei 65 verschiedenen N-Nitroso-Verbindungen an BD-Ratten. Z Krebsforsch. 1967;69(2):103–201. [PubMed] [Google Scholar]
- Fukuhara H., Faures M., Genin C. Comparison of RNA's transcribed in vivo from mitochondrial DNA of cytoplasmic and chromosomal respiratory deficient mutants. Mol Gen Genet. 1969 Jul 3;104(3):264–281. doi: 10.1007/BF02539291. [DOI] [PubMed] [Google Scholar]
- Graffi A., Butschak G., Schneider E. J., Kuhn W. Uber die Proteinsynthese in vitro von Mitochondrien aus Normal- und Tumorgeweben. Acta Biol Med Ger. 1965;15(6):826–853. [PubMed] [Google Scholar]
- Graffi A., Hoffmann A., Schütt M. Nmethyl-N-nitrosourea as a strong topical carcinogen when painted on skin of rodents. Nature. 1967 May 6;214(5088):611–611. doi: 10.1038/214611a0. [DOI] [PubMed] [Google Scholar]
- Graffi A. Ist die URSACHE DER MALIGNEN Entartung eine Mutation der Mitochondrien? Zentralbl Gynakol. 1968 Jul 13;90(28):945–948. [PubMed] [Google Scholar]
- Graffi A. Untersuchungen zur Frage der Bedeutung der Mitochondrien bei der Kanzerisierung. Dtsch Gesundheitsw. 1967 Dec 7;22(49):2305–2312. [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Kroon A. M. Protein synthesis in mitochondria. 3. On the effects of inhibitors on the incorporation of amino acids into protein by intact mitochondria and digitonin fractions. Biochim Biophys Acta. 1965 Oct 11;108(2):275–284. doi: 10.1016/0005-2787(65)90012-2. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Brookes P., Magee P. N., Craddock V. M., Swann P. F. Methylated bases in liver acids from rats treated with dimethylnitrosamine. Biochim Biophys Acta. 1968 May 21;157(3):646–648. doi: 10.1016/0005-2787(68)90167-6. [DOI] [PubMed] [Google Scholar]
- Leaver D. D., Swann P. F., Magee P. N. The induction of tumours in the rat by a single oral dose of N-nitrosomethylurea. Br J Cancer. 1969 Mar;23(1):177–187. doi: 10.1038/bjc.1969.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijinsky W., Ross A. E. Alkylation of rat liver nucleic acids not related to carcinogenesis by N-nitrosamines. J Natl Cancer Inst. 1969 Jun;42(6):1095–1100. [PubMed] [Google Scholar]
- Linnane A. W., Saunders G. W., Gingold E. B., Lukins H. B. The biogenesis of mitochondria. V. Cytoplasmic inheritance of erythromycin resistance in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1968 Mar;59(3):903–910. doi: 10.1073/pnas.59.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- MAGEE P. N., FARBER E. Toxic liver injury and carcinogenesis. Methylation of rat-liver nucleic acids by dimethylnitrosamine in vivo. Biochem J. 1962 Apr;83:114–124. doi: 10.1042/bj0830114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGEE P. N., HULTIN T. Toxic liver injury and carcinogenesis. Methylation of proteins of rat-liver slices by dimethylnitrosamine in vitro. Biochem J. 1962 Apr;83:106–114. doi: 10.1042/bj0830106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIFUCHI I., MORITA T., YANAGIHARA Y., HOSOI M., NISHIDA M. INDUCTION OF RESPIRATION-DEFICIENT MUTANT OF SACCHAROMYCES CEREVISIAE BY CARCINOGENIC AGENT, 4-NITROQUINOLINE N-OXIDE AND THE EXISTENCE OF TOXOHORMONE-LIKE FACTOR IN THE MUTANT. Jpn J Microbiol. 1963 Aug;7:69–79. doi: 10.1111/j.1348-0421.1963.tb00244.x. [DOI] [PubMed] [Google Scholar]
- Magee P. N., Barnes J. M. Carcinogenic nitroso compounds. Adv Cancer Res. 1967;10:163–246. doi: 10.1016/s0065-230x(08)60079-2. [DOI] [PubMed] [Google Scholar]
- McCalla D. R. Reaction of N-methyl-N'-nitro-N-nitroguanidine and N-methyl-N-nitroso-p-toluenesulfonamide with DNA in vitro. Biochim Biophys Acta. 1968 Jan 29;155(1):114–120. doi: 10.1016/0005-2787(68)90341-9. [DOI] [PubMed] [Google Scholar]
- Mehrotra B. D., Mahler H. R. Characterization of some unusual DNAs from the mitochondria from certain "petite" strains of Saccharomyces cerevisiae. Arch Biochem Biophys. 1968 Dec;128(3):685–703. doi: 10.1016/0003-9861(68)90078-7. [DOI] [PubMed] [Google Scholar]
- Mounolou J. C., Jakob H., Slonimski P. P. Mitochondrial DNA from yeast "petite" mutants: specific changes in buoyant density corresponding to different cytoplasmic mutations. Biochem Biophys Res Commun. 1966 Jul 20;24(2):218–224. doi: 10.1016/0006-291x(66)90723-6. [DOI] [PubMed] [Google Scholar]
- Nagai S. Production of respiration-deficient mutants in yeast by a carcinogen, 4-nitroquinoline 1-oxide. Mutat Res. 1969 May-Jun;7(3):333–337. doi: 10.1016/0027-5107(69)90104-3. [DOI] [PubMed] [Google Scholar]
- Nass S. The significance of the structural and functional similarities of bacteria and mitochondria. Int Rev Cytol. 1969;25:55–129. doi: 10.1016/s0074-7696(08)60201-6. [DOI] [PubMed] [Google Scholar]
- Neubert D. Vergleichende Untersuchungen über die Nucleinsäuresynthese in Zellkernen und Mitochondrien und ihre Beeinflussbarketi durch Pharmaka. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1966;253(1):152–176. [PubMed] [Google Scholar]
- Nordström K. Induction of the petite mutation in Saccharomyces cerevisiae by N-methyl-N'-nitro-N-nitrosoguanidine. J Gen Microbiol. 1967 Aug;48(2):277–281. doi: 10.1099/00221287-48-2-277. [DOI] [PubMed] [Google Scholar]
- Olson A. O., Baird K. M. Single-strand breaks in Escherichia coli DNA caused by treatment with nitrosoguanidine. Biochim Biophys Acta. 1969 Apr 22;179(2):513–514. doi: 10.1016/0005-2787(69)90063-x. [DOI] [PubMed] [Google Scholar]
- Reich E., Luck D. J. Replication and inheritance of mitochondrial DNA. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1600–1608. doi: 10.1073/pnas.55.6.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. C., Kuff E. L. The isolation and some properties of rat liver mitochondrial deoxyribonucleic acid. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1650–1658. doi: 10.1073/pnas.54.6.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaier R. Induktion von cytoplasmatischen und genischen Atmungsdefekt-Mutanten durch das carcinogene Nitrosamid l-Nitroso-imidazolidon-2 an Saccharomyces cerevisiae. Arzneimittelforschung. 1969 Jul;19(7):1050–1052. [PubMed] [Google Scholar]
- Schwaier R., Nashed N., Zimmermann F. K. Mutagen specificity in the induction of karyotic versus cytoplasmic respiratory deficient mutants in yeast by nitrous acid and alkylating nitrosamides. Mol Gen Genet. 1968;102(4):290–300. doi: 10.1007/BF00433720. [DOI] [PubMed] [Google Scholar]
- Serebrianyi A. M., Smotriaeva M. A., Krugliakova K. E., Kostianovskii R. G. Karbamoilirovanie DNK N-nitrozo-N-metilmochevinoi. Dokl Akad Nauk SSSR. 1969 Apr 1;185(4):847–849. [PubMed] [Google Scholar]
- Simpson L. Effect of acriflavin on the kinetoplast of Leishmania tarentolae. Mode of action and physiological correlates of the loss of kinetoplast DNA. J Cell Biol. 1968 Jun;37(3):660–682. doi: 10.1083/jcb.37.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann P. F., Magee P. N. Nitrosamine-induced carcinogenesis. The alklylation of nucleic acids of the rat by N-methyl-N-nitrosourea, dimethylnitrosamine, dimethyl sulphate and methyl methanesulphonate. Biochem J. 1968 Nov;110(1):39–47. doi: 10.1042/bj1100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann P. F. The rate of breakdown of methyl methanesulphonate, dimethyl sulphate and N-methyl-N-nitrosourea in the rat. Biochem J. 1968 Nov;110(1):49–52. doi: 10.1042/bj1100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K. K., Vötsch W., Mahler H. R., Mackler B. Biochemical correlates of respiratory deficiency. VI. Mitochondrial DNA. J Mol Biol. 1966 Oct;20(3):453–481. doi: 10.1016/0022-2836(66)90003-9. [DOI] [PubMed] [Google Scholar]
- Thomas D. Y., Wilkie D. Recombination of mitochondrial drug-resistance factors in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1968 Feb 26;30(4):368–372. doi: 10.1016/0006-291x(68)90753-5. [DOI] [PubMed] [Google Scholar]
- Tuppy H., Swetly P., Wolff I. Structural protein of wild-type (rho+) and respiration-deficient (rho-) yeast mitochondria. A comparative study using electrophoretic and immunological methods. Eur J Biochem. 1968 Aug;5(3):339–345. doi: 10.1111/j.1432-1033.1968.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. A procedure for the isolation of enzymically active rat-liver nuclei. Biochem J. 1964 Aug;92(2):313–317. doi: 10.1042/bj0920313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintersberger E., Viehhauser G. Function of mitochondrial DNA in yeast. Nature. 1968 Nov 16;220(5168):699–702. doi: 10.1038/220699b0. [DOI] [PubMed] [Google Scholar]
- Wunderlich V., Tetzlaff I. Alkylierung der Kern-DNS verschiedener Organe der Ratte drch Nitrosomethylharnstoff in vivo. Arch Geschwulstforsch. 1970;35(3):251–258. [PubMed] [Google Scholar]


