Abstract
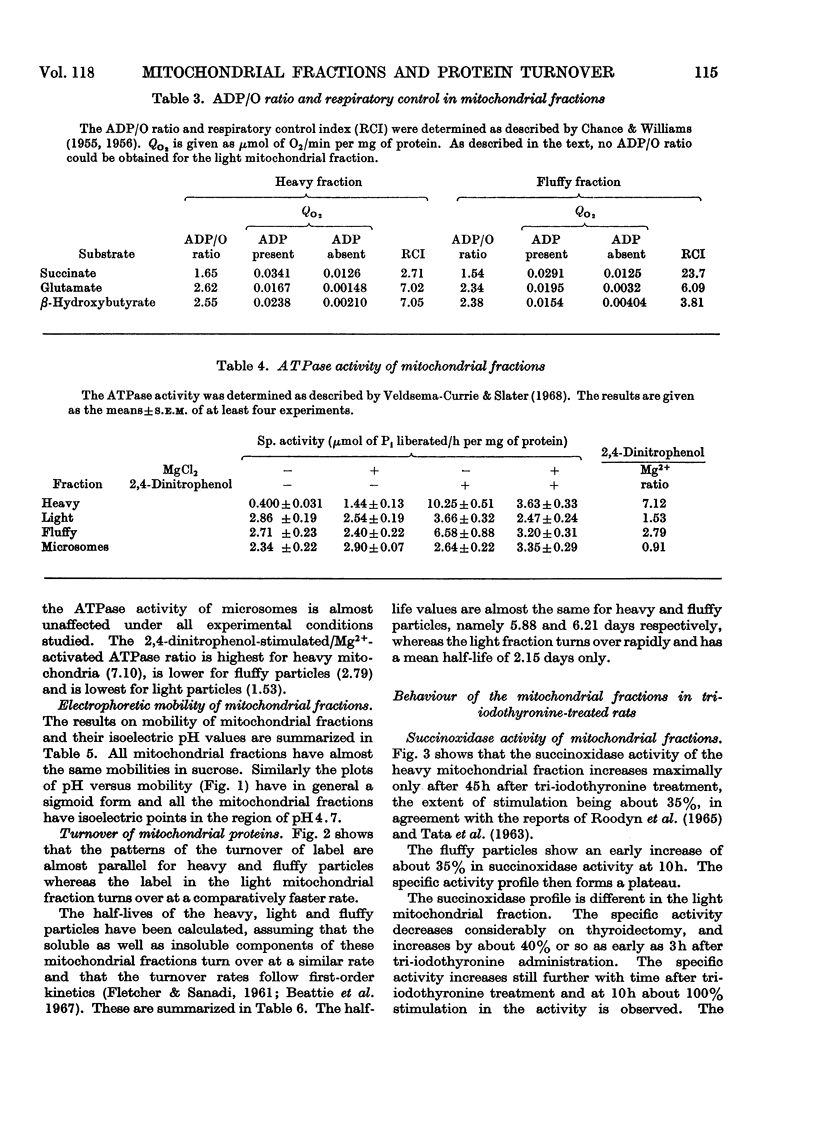
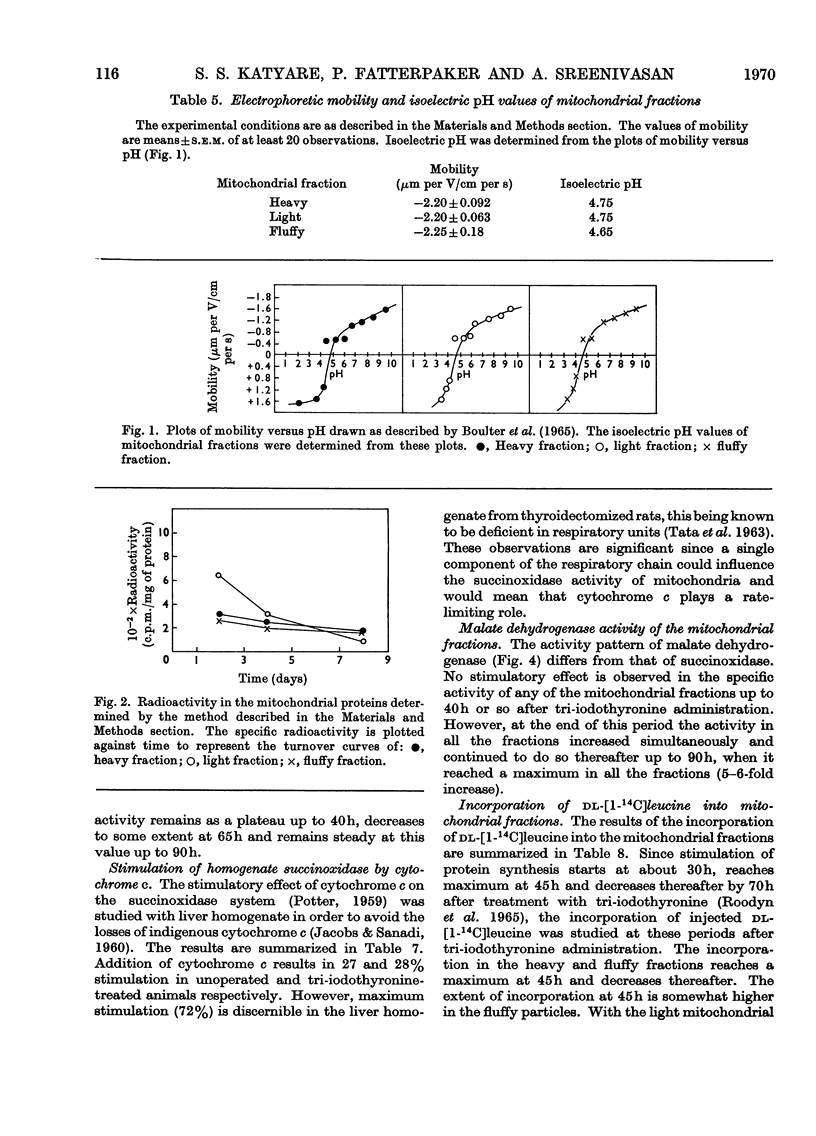
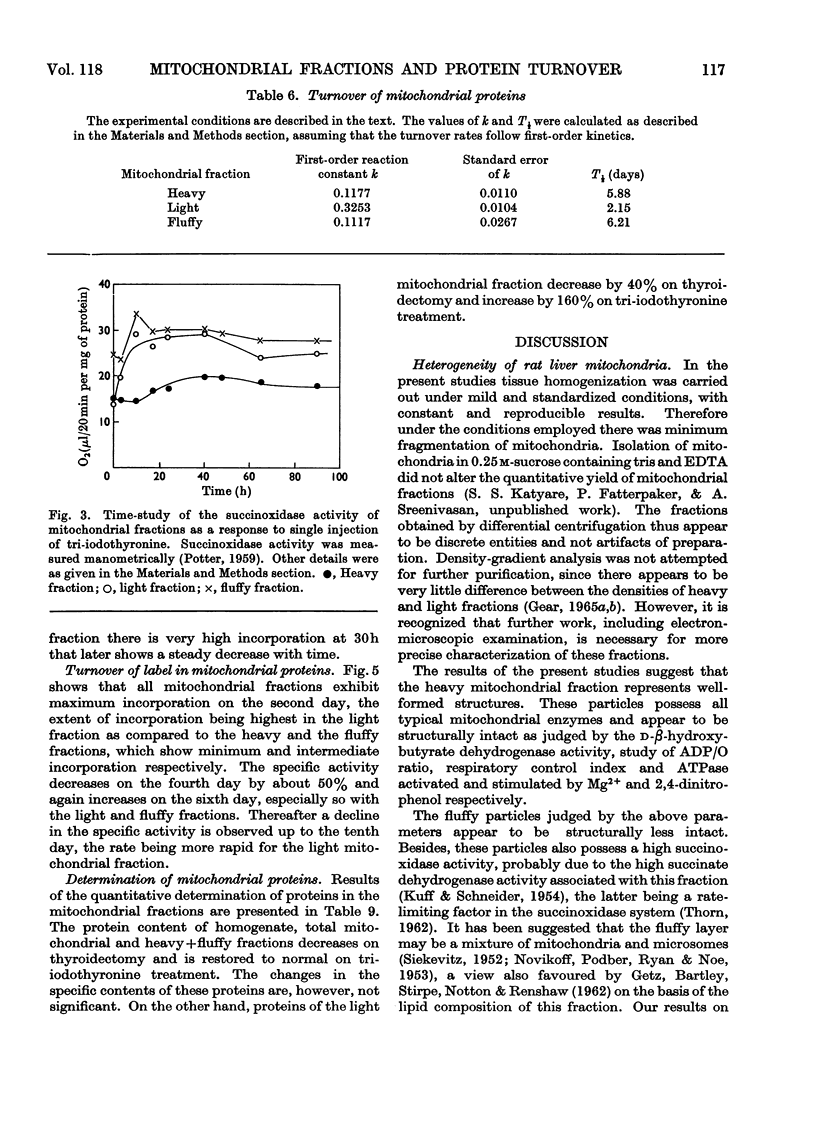
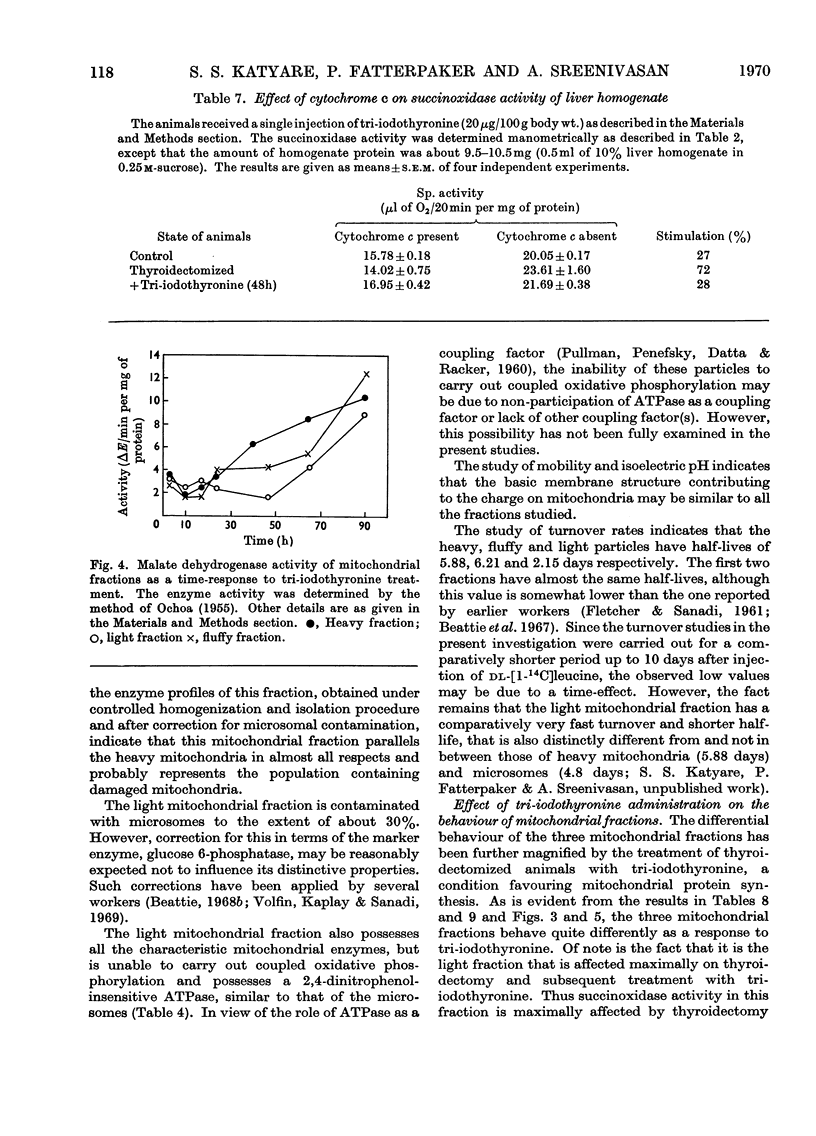
1. Rat liver mitochondria were separated into heavy, light and fluffy fractions by differential centrifugation under standard conditions. 2. All mitochondrial fractions possessed soluble as well as membrane-bound enzymes typical of mitochondria. 3. The heavy fraction represented the stable mitochondrial structures and the fluffy particles appear to be loosely coupled. 4. The light mitochondrial fraction lacked the ability of coupled phosphorylation. 5. A study of mobility and isoelectric pH indicated a similarity in the basic membrane structure of all the mitochondrial fractions. 6. The turnover rates of proteins in the heavy and fluffy particles were almost identical; however, this rate was rapid for the light mitochondrial fraction. 7. On treatment with 3,3′,5-tri-iodo-l-thyronine, succinoxidase activity was maximally stimulated much earlier in the light mitochondrial fraction than in the heavy fraction. The activity of the fluffy particles, however, remained almost unaffected. 8. Malate dehydrogenase activity in all the mitochondrial fractions was stimulated only at 40h after tri-iodothyronine treatment. 9. The pattern of incorporation of dl-[1-14C]leucine in vivo in the tri-iodothyronine-treated animals indicated a rapid initial incorporation and high synthetic ability of the light mitochondrial fraction. 10. The turnover pattern of proteins of the mitochondrial fractions from animals receiving repeated doses of tri-iodothyronine was remarkably different from the normal pattern and suggested that preformed soluble protein units may be incorporated in the light mitochondrial fraction during maturation to form the stable heavy mitochondria. 11. The amount of light-mitochondrial proteins decreased by 40% on thyroidectomy and increased by 160% on treatment with tri-iodothyronine. 12. The possible significance of these results is discussed in relation to mitochondrial genesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANGHAM A. D., HEARD D. H., FLEMANS R., SEAMAN G. V. An apparatus for microelectrophoresis of small particles. Nature. 1958 Sep 6;182(4636):642–644. doi: 10.1038/182642a0. [DOI] [PubMed] [Google Scholar]
- Beattie D. S., Basford R. E., Koritz S. B. Studies on the biosynthesis of mitochondrial protein components. Biochemistry. 1966 Mar;5(3):926–930. doi: 10.1021/bi00867a018. [DOI] [PubMed] [Google Scholar]
- Beattie D. S., Basford R. E., Koritz S. B. The turnover of the protein components of mitochondria from rat liver, kidney, and brain. J Biol Chem. 1967 Oct 25;242(20):4584–4586. [PubMed] [Google Scholar]
- Beattie D. S. Enzyme localization in the inner and outer membranes of rat liver mitochondria. Biochem Biophys Res Commun. 1968 Jun 28;31(6):901–907. doi: 10.1016/0006-291x(68)90537-8. [DOI] [PubMed] [Google Scholar]
- Beattie D. S. Studies on the biogenesis of mitochondrial protein components in rat liver slices. J Biol Chem. 1968 Aug 10;243(15):4027–4033. [PubMed] [Google Scholar]
- Beaufay H., Jacques P., Baudhuin P., Sellinger O. Z., Berthet J., De Duve C. Tissue fractionation studies. 18. Resolution of mitochondrial fractions from rat liver into three distinct populations of cytoplasmic particles by means of density equilibration in various gradients. Biochem J. 1964 Jul;92(1):184–205. doi: 10.1042/bj0920184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. A simple and rapid assay of oxidative phosphorylation. Nature. 1955 Jun 25;175(4469):1120–1121. doi: 10.1038/1751120a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- CORWIN L. M., SCHWARZ K. An effect of vitamin E on the regulation of succinate oxidation in rat liver mitochondria. J Biol Chem. 1959 Jan;234(1):191–197. [PubMed] [Google Scholar]
- FLETCHER M. J., SANADI D. R. Turnover of rat-liver mitochondria. Biochim Biophys Acta. 1961 Aug 5;51:356–360. doi: 10.1016/0006-3002(61)90177-9. [DOI] [PubMed] [Google Scholar]
- FREEMAN K. B., ROODYN D. B., TATA J. R. Stimulation of amino acid incorporation into protein by isolated mitochondria from rats treated with thyroid hormones. Biochim Biophys Acta. 1963 May 28;72:129–132. [PubMed] [Google Scholar]
- FRISELL W. R., PATWARDHAN M. V., MACKENZIE C. G. QUANTITATIVE STUDIES ON THE SOLUBLE COMPARTMENTS OF LIGHT AND HEAVY MITOCHONDRIA FROM RAT LIVER. J Biol Chem. 1965 Apr;240:1829–1835. [PubMed] [Google Scholar]
- Freeman K. B., Haldar D., Work T. S. The morphological site of synthesis of cytochrome c in mammalian cells (Krebs cells). Biochem J. 1967 Dec;105(3):947–952. doi: 10.1042/bj1050947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEAR A. R. SOME FEATURES OF MITOCHONDRIA AND FLUFFY LAYER IN REGENERATING RAT LIVER. Biochem J. 1965 Apr;95:118–137. doi: 10.1042/bj0950118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GETZ G. S., BARTLEY W., STIRPE F., NOTTON B. M., RENSHAW A. The lipid composition of rat-liver mitochondria, fluffy layer and microsomes. Biochem J. 1962 Apr;83:181–191. doi: 10.1042/bj0830181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear A. R. Observations on iron uptake, iron metabolism, cytochrome c content, cytochrome a content and cytochrome c-oxidase activity in regenerating rat liver. Biochem J. 1965 Nov;97(2):532–539. doi: 10.1042/bj0970532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Cadavid N. F., Campbell P. N. The biosynthesis of cytochrome c. Sequence of incorporation in vivo of [14C]lysine into cytochrome c and total proteins of rat-liver subcellular fractions. Biochem J. 1967 Nov;105(2):443–450. doi: 10.1042/bj1050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson R., Tata J. R., Lindberg O., Ernster L. The relationship between the structure and activity of rat skeletal muscle mitochondria after thyroidectomy and thyroid hormone treatment. J Cell Biol. 1965 Aug;26(2):555–578. doi: 10.1083/jcb.26.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS E. E., SANADI D. R. The reversible removal of cytochrome c from mitochondria. J Biol Chem. 1960 Feb;235:531–534. [PubMed] [Google Scholar]
- KIELLEY W. W., KIELLEY R. K. Myokinase and adenosinetriphosphatase in oxidative phosphorylation. J Biol Chem. 1951 Aug;191(2):485–500. [PubMed] [Google Scholar]
- KUFF E. L., SCHNEIDER W. C. Intracellular distribution of enzymes. XII. Biochemical heterogeneity of mitochondria. J Biol Chem. 1954 Feb;206(2):677–685. [PubMed] [Google Scholar]
- Kadenbach B. Synthesis of mitochondrial proteins. The synthesis of cytochrome c in vitro. Biochim Biophys Acta. 1967 May 30;138(3):651–654. doi: 10.1016/0005-2787(67)90574-6. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L., SUDDUTH H. C., WISE J. B. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J Biol Chem. 1960 Aug;235:2450–2455. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACLAGAN N. F., SHEAHAN M. M. The measurement of oxygen consumption in small animals by a closed circuit method. J Endocrinol. 1950 Jul;6(4):456–462. doi: 10.1677/joe.0.0060456. [DOI] [PubMed] [Google Scholar]
- Matile P., Bahr G. F. Biochemical and quantitative electron microscopic evidence for heterogeneity of mitochondria from Saccharomyces cerevisiae. Exp Cell Res. 1968 Oct;52(2):301–307. doi: 10.1016/0014-4827(68)90471-0. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., PODBER E., RYAN J., NOE E. Biochemical heterogeneity of the cytoplasmic particles isolated from rat liver homogenate. J Histochem Cytochem. 1953 Jan;1(1):27–46. doi: 10.1177/1.1.27. [DOI] [PubMed] [Google Scholar]
- PENEFSKY H. S., PULLMAN M. E., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. II. Participation of a soluble adenosine tolphosphatase in oxidative phosphorylation. J Biol Chem. 1960 Nov;235:3330–3336. [PubMed] [Google Scholar]
- Paltauf F., Schatz G. Promitochondria of anaerobicallly grown yeast. II. Lipid composition. Biochemistry. 1969 Jan;8(1):335–339. doi: 10.1021/bi00829a046. [DOI] [PubMed] [Google Scholar]
- Plattner H., Schatz G. Promitochondria of anaerobically grown yeast. 3. Morphology. Biochemistry. 1969 Jan;8(1):339–343. doi: 10.1021/bi00829a047. [DOI] [PubMed] [Google Scholar]
- RECKNAGEL R. O., LOMBARDI B. Studies of biochemical changes in subcellular particles of rat liver and their relationship to a new hypothesis regarding the pathogenesis of carbon tetrachloride fat accumulation. J Biol Chem. 1961 Feb;236:564–569. [PubMed] [Google Scholar]
- ROODYN D. B., FREEMAN K. B., TATA J. R. THE STIMULATION BY TREATMENT IN VIVO WITH TRI-IODOTHYRONINE OF AMINO ACID INCORPORATION INTO PROTEIN BY ISOLATED RAT-LIVER MITOCHONDRIA. Biochem J. 1965 Mar;94:628–641. doi: 10.1042/bj0940628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEKEVITZ P. Uptake of radioactive alanine in vitro into the proteins of rat liver fractions. J Biol Chem. 1952 Apr;195(2):549–565. [PubMed] [Google Scholar]
- TATA J. R., ERNSTER L., LINDBERG O., ARRHENIUS E., PEDERSEN S., HEDMAN R. The action of thyroid hormones at the cell level. Biochem J. 1963 Mar;86:408–428. doi: 10.1042/bj0860408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORN M. B. Activation of succinate dehydrogenase in heart-muscle preparations. Biochem J. 1962 Oct;85:116–127. doi: 10.1042/bj0850116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldsema-Currie R. D., Slater E. C. Inhibition by anions of dinitrophenol-induced ATPase of mitochondria. Biochim Biophys Acta. 1968 Oct 1;162(3):310–319. doi: 10.1016/0005-2728(68)90117-5. [DOI] [PubMed] [Google Scholar]
- Volfin P., Kaplay S. S., Sanadi D. R. Early effect of thyroxine in vi on rapidly labeled mitochondrial protein fractions and respiratory control. J Biol Chem. 1969 Oct 25;244(20):5631–5635. [PubMed] [Google Scholar]
- WERKHEISER W. C., BARTLEY W. The study of steady-state concentrations of internal solutes of mitochondria by rapid centrifugal transfer to a fixation medium. Biochem J. 1957 May;66(1):79–91. doi: 10.1042/bj0660079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work T. S., Coote J. L., Ashwell M. Biogenesis of mitochondria. Fed Proc. 1968 Sep-Oct;27(5):1174–1179. [PubMed] [Google Scholar]


